Abstract
Colony size is an important predictor of annual survival and reproduction in social insects. By tracking monthly forager turn over, we measured the size-specific production rates necessary to counteract forager mortality in wild Veromessor pergandei colonies. Between 31,180 and 237,980 individuals appeared as foragers annually, representing an eightfold difference in production between neighboring nests. Highly productive colonies housed 26,000 foragers at one time, turned over 34,000 foragers in a single month, and produced 470 g of worker biomass during the year. Each forager population turned over approximately 20 times during the year, cumulatively returning 3.4 kg of biomass per hectare (with 14 nests per hectare). Forager longevity was not influenced by forager number, season, or pressure from neighboring conspecifics. Genotyping at three microsatellite loci revealed that all colonies contained a single queen and one to seven patrilines (mean m observed = 3.8; m e = 2.56). The most productive colonies had significantly fewer patrilines, a larger peak forager population, and a larger annual foraging range. Colonies varied in their ability to replace lost workers, adding anywhere from 2 to 42 new foragers per death during peak forager allocation. Seasonal increases in forager population size corresponded to decreases in worker body size, suggesting a trade-off during production earlier in the year. Together, these findings demonstrate how the combination of individual colony-member characteristics influence whole colony survival across multiple years.
Significance statement
The survival of an animal society can depend on its size and stability. We measured the seasonal relationships between mortality and production of new colony members, with respect to foraging range size, paternity, neighbor pressure, and body size in a desert seed harvesting ant. Mortality rate was stable across seasons, but the ability of colonies to replace dead individuals corresponded to increased foraging range size, decreased patriline number, and seasonal decreases in body size. Our findings reveal the characteristics that allow colonies to respond to worker mortality on an annual scale.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
To avoid losses in colony size, social insect societies must balance worker mortality and new worker production during each annual cycle. Colony size is tied to important life history traits including survival across years (Keeler 1993; Wiernasz and Cole 1995; Tschinkel 2017), the onset of reproductive maturity (Johnston and Wilson 1985; Smith and Tschinkel 2006; Smith et al. 2014), the likelihood of annual reproduction (Fowler 1986; Elmes 1987; Cole and Wiernasz 2000), relative investment in growth versus reproduction (Tschinkel 2011; Kwapich and Tschinkel 2013), and the size of offspring produced (Shik 2008). Compared to small colonies, large colonies tend to have larger workers (Wilson 1983; Rissing 1987; Tschinkel 1988a), expend less energy per capita (Fewell and Harrison 2016), defend larger territories (Hölldobler and Lumsden 1980; Tschinkel et al. 1995; Adams 2016), and more rapidly exploit food resources (Hölldobler 1986; Cerdá et al. 2002).
Despite the importance of colony size, size-specific differences in worker mortality rate and the corresponding production rates necessary for maintenance at different sizes are seldom reported (Kramer et al. 2015). For instance, colonies experiencing low forager mortality may accumulate workers over time, despite a low production rate, while colonies experiencing exceptionally high forager mortality would need to be highly productive to counteract mortality, despite being small at any given time. In the current study, we examine the basis of size-specific differences in worker mortality and production in colonies of a group foraging, seed harvesting ant, Veromessor pergandei.
Increasing worker production
Queens have been demonstrated to modify egg laying rate in response to the abundance of successfully pupating larvae in a colony (Tschinkel 1988b ). In productive, mature colonies, the most basic way to increase worker production is to increase the number of egg-laying queens present (Hölldobler and Wilson 1977; Boulay et al. 2014). In facultatively polygynous species, colonies with multiple queens reach larger sizes than monogyne conspecifics (Fletcher et al. 1980; Mercier et al. 1985) are more likely to occupy large territories (Adams 2016), and to occur at higher population densities (Macom and Porter 1996). Therefore, while within colony relatedness may be low for colonies with multiple mothers, their size may allow them to out-compete monogyne colonies, or colonies with fewer queens.
Increased genetic diversity may also influence colony size by producing an adaptive, colony-level phenotype. In the harvester ant Pogonomyrmex occidentalis, lineage specific foraging preferences result in more foraging time overall, and increased growth rates for colonies with more fathers (Cole and Wiernasz 1999; Cole and Wiernasz 2000). Likewise, genetic diversity may enhance disease resistance (William and Boomsma 2004; Simone-Finstrom et al. 2016) or improve division of labor through the production of morphologically distinct worker castes (Hughes et al. 2003; Smith et al. 2008).
An additional way to increase worker number is simply to reduce the size of workers. During founding, queens maximize labor by producing tiny workers, which increase in size in successive worker generations (Hölldobler and Wilson 1990; Johnson 2006a). In polymorphic species, the size frequency distribution of workers changes with increasing colony size, presumably in an adaptive way (Wilson 1983; Rissing 1987; Tschinkel 1988a). Although investing in small workers may increase labor at the colony level, the foraging tenure of small workers may be shorter due to increased risk or desiccation resistance and lower absolute body fat stores. Yet, evidence from several ant species indicates that foragers die well before fat stores are depleted, and have a potential life span that is much longer that the life span imposed by extrinsic risks, unlike honeybees (Giraldo and Traniello 2014; Giraldo et al. 2016; Kwapich and Tschinkel 2016).
Finally, decreases in colony size may occur if the behavioral development rates of younger workers do not anticipate losses in the forager population. In healthy colonies, the appearance of new foragers appears to be well tuned to natural forager mortality rates. For example, Cataglyphis bicolor maintains a stable forager by replacing 16.4% of foragers per day in (Schmid-Hempel 1982; Schmid-Hempel and Schmid-Hempel 1984), Pogonomyrmex badius replaces 3.7% per day (Kwapich and Tschinkel 2013, 2016), and Solenopsis invicta turns over up to 50% of the entire adult population of the nest per month (Tschinkel and Porter 1988; Tschinkel 2006). However, ant colonies may be less robust to departures from typical mortality rate, and unable to replace lost foragers over a certain threshold. In P. badius, forager replacement begins to fail when mortality rates exceed 5% per day, and larvae die above 50% (Kwapich and Tschinkel 2016).
Study organism
Veromessor pergandei is a conspicuous, seed-harvester ant of the Sonoran and Mojave deserts in the western USA. It is an ideal ant for the study the factors that influence variation in colony size and worker production, because adult colonies differ in queen and patriline number (Helms Cahan 2001), exhibit seasonal worker polymorphism (Gordon 1978; Rissing and Pollock 1984), have a distinct annual reproductive period (Helms Cahan 2001), and expose large numbers of foragers to sources of mortality outside the nest during group foraging.
Each morning, foragers depart en masse one to two discrete foraging columns. Foraging activity continues year-round, but is restricted to the coolest hours of the day, beginning well before dawn in summer months. The remarkable group-foraging columns of V. pergandei can reach 40 m in length and contain up to 35,000 individuals (Went et al. 1972; Wheeler and Rissing 1975). Foraging column formation, orientation, temporal rotation, relation to food resources, and avoidance of neighboring foragers have all been the topic of extensive study in this species (Plowes et al. 2013), though seasonal estimates of forager longevity have not been made. Because the final job undertaken by worker is that of foraging, it is possible to determine the number of adults that eclose in a colony by repeatedly measuring the turnover of foragers in individual colonies.
In the current study, annual worker production and biomass were estimated by monitoring V. pergandei forager turnover and replacement across one calendar year. The relationship between colony size, mortality, and worker production were considered with respect to forager population size, queen number, patriline number, neighboring forager pressure, seasonal foraging trajectory, worker dry weight, worker body size, and annual foraging range.
Methods
Site description
Studies were conducted in Sonoran desert habitat at South Mountain Regional Park, in Phoenix, AZ, where V. pergandei colonies occurred at an average density of 14.7 nests per hectare (4 ha were surveyed exhaustively). Surrounding vegetation included creosote bush (Larrea tridentate), Ambrosia deltoidea, Euphorbia albomarginata, occasional ocotillo (Fouquieria splendens), palo verde (Parkinsonia microphylla), compass barrel cactus (Ferocactus cylindraceus), and numerous other species. Co-occurring ants included Novomessor cockerelli, Pogonomyrmex rugosus, Forelius mccooki, Myrmecocystus placodops, and various Pheidole species.
The selected sites, San Gabriel Rd. and War Paint Rd., were spaced 1.8 km apart, and contained a cluster of eight neighboring colonies and six neighboring colonies, respectively. At the San Gabriel Rd. site, forager population size and forager mortality rate were measured at multiple points between December 2014 and November 2015 to estimate the total number of foragers produced across one annual cycle (n = 8 colonies). Six additional colonies were monitored from May to August only at War Paint Rd. to increase our sample sizes of mortality rate and peak forager number in the population.
Estimating forager population size
In social insect societies, allocation to foraging occurs at two distinct levels. Measures of forager activity describe the number of trips taken by individual foragers, or the number of foragers active under specific conditions; including time of day, during weather events, or following recruitment to food sources. In contrast, forager population size describes the total number of adults in a colony that are available to forage. Previous work has demonstrated that the forager population is a relatively stable, developmentally correlated subset of the larger adult population in age-structured colonies (Gentry 1974; Porter and Jorgensen 1980; Kwapich and Tschinkel 2013; Mersch et al. 2013; Kwapich and Tschinkel 2013).
In the current study, we estimated forager population size using the Lincoln index mark-release-recapture method. V. pergandei foragers were defined operationally as individuals that were active in the foraging column established each morning. Each estimate was made over a 24-h period. On day 1, foragers were captured, counted marked, and released. On day 2, foragers were captured, counted, and inspected for marks. Estimates assumed that the proportion of the total forager population (\( \widehat{N} \)) that was marked in an initial sample (m) was equivalent to the proportion of marked individuals (r) present in a second sample (n) collected 24 h later (Lincoln 1930)
The Lincoln index method assumed that foragers mixed randomly overnight, were equally catchable during mark and recapture in a 24-h period, and did not lose or transfer their marks. All mark-recapture estimates took place over 24-h, and forager sample size on day 1 was a significant predictor of sample size on day 2 (y = 233 + 0.67*x; r = 0.69, p = 0.0000; r 2 = 0.47). Later, estimates of forager longevity were made between two mark-recapture events spaced 10 days apart. While some colonies showed a dramatic increase in forager number after 10 days, the new, 10-day estimate was made over a single, 24-h activity period as well. The positive relationship between samples on day 1 and day 2 of any sampling event indicates that foraging activity was similar during any 24-h (mark-release-recapture) sampling period.
Each mark-recapture event involved three photographs of captured workers (day 1 total, day 2 total, UV photo of ants marked in day 2’s sample). A recapture sample containing a minimum of 10% of marked individuals is necessary to make a reasonable estimate of an animal population with 20 individuals or more (Bailey 1952). In the current study, estimates of forager number were only considered in our analysis if the proportion of individuals marked exceeded 14% of the recapture sample (mean recaptured = 30% SD + −20%, n = 80) or 14% of the total marked (mean = 28% SD + −17%, n = 80). For each estimate of forager population size, 95% confidence intervals were calculated from the variance associate with the percent of marked foragers recaptured (\( \mathit{\operatorname{var}}\ \widehat{N}=\frac{m^2 n\left( n- r\right)}{r^3}; \) \( 95\% CI=\widehat{N}\pm 1.96\left( SE\sqrt{Var\ \widehat{N}}\right) \)) (Bailey 1952).
During recapture, unmarked workers were also painted to increase catchability of survivors after 10 days. The total proportion of foragers marked averaged 54% (SD 20%) after the initial, 2-day mark-recapture event.
Capture, marking, and counting of foragers
On the first morning of each 24-h mark-recapture event, foragers were collected beginning 30 to 60 min after foraging trails were active in both directions. Foragers were captured by pitfall trapping in a sandwich box buried directly in the path of the established foraging trail, 20 cm from the margin of the nest mound. After planting the trap, traffic was allowed to reestablish over the lid and flat rocks were angled to direct traffic towards the desired route. Then, the lid was removed and outbound and nest-bound foragers were captured as they walked directly into the box (Supplementary video 1). Every 15 min, the lid was replaced on the box until foraging began to decline after 2 to 3 h (as in surrounding, non-focal colonies). There was no alarm or excitement at the nest entrance that would suggest that individuals entered the trail for purposes other than foraging, and group foraging continued for the typical period (several hours) after installing the forager traps.
Video of pitfall trapping method used for passive capture of foragers (MP4 102650 kb)
Captured foragers were photographed in a white tray, then marked with fluorescent printer’s ink suspended in ether using a perfume sprayer (GANS Ink & Supply Co.). Foragers were allowed to dry and groom for 2 min and returned to their nest mound. The nest mound was temporarily shaded and worker condition was inspected. Most workers immediately returned to foraging or re-entered the nest. Nest-mates behaved normally towards marked ants, and marked and un-marked individuals survived >40 days in the lab, as in all other studies where this ink has been applied (Porter and Jorgensen 1980; Tschinkel 2011; Kwapich and Tschinkel 2013; Kwapich and Tschinkel 2016a). Data were not collected blind, because colony identity was known in the field.
On the second day of each mark-recapture event, the total number of foragers captured was determined by counting individuals photographed in a white tray. The proportion of fluorescently marked individuals recaptured was determined by counting marked ants under UV light. Ants were spread out as evenly as possible so that stacking of individuals was minimized. Numerous photos were taken of each tray, and the best photo was chosen for counting.
We made an effort to look for ant heads when conducting our counts to standardize the search method. Each forager was tagged with a single dot in Microsoft PAINT (Microsoft, Windows, version 1607), and the number of dots was counted using the program DotCount (Martin Reuter, v1.2; Supplementary 2 and 3). A dot was placed on each ant in each photo by one observer, then checked by a second observer for missed ants or errors (CLK or YD). The ability of the “Dot Count” program was assessed by comparing dots counted with a click counter. The accuracy of individual observers was tested initially by having two people mark the same photos and then having the program count the dots. In total, 288,780 ants were individually scored.
Forager mortality and replacement
Forager survival in the current study was estimated between consecutive 24-h samples spaced 10 days apart. A unique ink color was used on each date (orange, green, or blue), so that the number of foragers surviving 10 days could be determined from single- and doubled-marked individuals in the second forager population estimate. Forager survival (\( {\widehat{N}}_{surv}\Big) \)was calculated from the total number of individuals bearing the initial mark on the first day of the 10-day sampling event (m 1m2), the total number of individuals bearing the initial mark on the second day of the 10-day sampling event (m 1n2 ), and number of doubled-marked foragers recaptured on the second day of the day-10 sampling event (d n2). The number of marked foragers surviving was expressed as a proportion of the total marked in the initial sampling event (∑m 1), and multiplied by the total forager population size estimated during the initial sampling event (\( \widehat{N_1} \)).
The proportion of the initial forager population dead after 10 days was converted into a daily rate by assuming that an equal fraction of foragers died per day. Using the daily death rate, it was possible to approximate (1) number of days necessary for the forager population to turn over completely, (2) the number of times the forager population turned over per month, and (3) the maximum longevity of an individual forager. Likewise, the number of new foragers present on day 10 (\( {\widehat{N}}_{new\ }\Big) \) was determined by subtracting the number of foragers surviving after 10 days (\( {\widehat{N}}_{surv}\Big) \) from the total forager population size on day 10 (\( {\widehat{N}}_2\Big) \). While these calculations do not consider workers that entered and died in the forager population before being sampled on day 10, they provide a conservative estimate of flux in the forager population.
Annual worker production
Total annual worker production was estimated for seven of eight San Gabriel Rd. colonies with sufficient sampling across the annual cycle. For each colony, an estimate of the number of new foragers added over 10 days was made approximately every 2 months, and then multiplied across the entire ~60-day period. The annual number of foragers produced in each colony was calculated by adding together all bimonthly estimates. Forager population size was not measured in September or February to avoid interfering with mating activity and nest excavation following rains. In any months with missing data, a colony’s lowest recorded rate of forager production was supplemented (March/April sample). Supplementing lowest annual production in these months is reasonable, because colonies tended to maintain the same forager number, death rate, body size, and replacement rate in spring, autumn, and winter (see the “Results” section).
Annual biomass and seasonal body size
To calculate annual biomass production, 30–50 workers were collected from each colony following April and June mark-recapture events. Workers were dried at 60 °C, counted, and weighed on an electronic balance. The average dry weight per worker was calculated from the group’s dry weight, then multiplied by the annual number of workers produced (above). An actual and per hectare estimate of V. pergandei biomass production was calculated by summing each colony’s biomass and dividing by the area of a polygon encompassing the outer boundaries of the neighborhood foraging range. The seven of eight San Gabriel Rd. colonies with known annual production rates occupied a 0.49-ha space.
Workers of V. pergandei are polymorphic, and body size follows a strong, ontogenetic, and seasonal pattern (Rissing 1987). Small workers produced during the winter “triple crunch” of low seed availability, reduced foraging time, and alate production appear as foragers in the late spring and early summer (Gordon 1978; Rissing and Pollock 1984). In the current study, trade-offs between forager number and forager body size were explored by measuring seasonal variation in worker head width in April (intraocular distance). For each San Gabriel Rd. colony (n = 8), 50 workers were randomly selected from photographs taken in April, June, August, and November (1600 ants total). The relationship between forager number and body size was determined across seasons, and measurements without knowledge of colony identity (blind).
Neighbors and foraging range
To determine if neighboring conspecifics influenced forager longevity in focal nests, neighbor indices were calculated in each season by taking the sum of each neighbor’s forager population size divided by its squared distance from the focal nest. Colony locations were mapped with a Garmin 60Cx handheld GPS unit, and distances were measured by taking the shortest linear length between the centers of each nest-mound in Google Earth (v7.1.7.2602). At the San Gabriel Rd. site, all colonies within 100 m of focal nests were included for every neighbor index, with the exception of one nest located 84 m from the nearest focal colony, in an adjacent wash. In the War Paint Rd. site, only summer forager allocation was measured.
During each mark-recapture event, foraging column length (up to the foraging fan) and direction were measured. Foraging trails were plotted in Google Earth as lines extending from each focal nest. Annual forager range was calculated by connecting the tips of adjacent radii and taking the area inside the polygon. Many colonies repeatedly used the same foraging trails, or only formed short foraging fans in certain directions. Map design was performed in QGIS (2.18.2).
Queen and patriline number
For nine focal colonies, queen and patriline number were inferred from the genotypes of 30 workers collected in May, or May and September of 2015 (to determine if parentage varied seasonally). Additional male and female alates were collected from nests during the following mating season, although no mating flights occurred at South Mountain Regional Park during the course of our study. No alates were found in association with Vp30, Vp23, or Vp25.
We tested four microsatallite loci (LXAGT1, Myrt3, PPAL12, Po3) that were previously demonstrated by Helms and Helms Cahan (2012) to exhibit significant polymorphism and Mendelian inheritance patterns in numerous V. pergandei populations. Three loci, LXAGT1, MYRT3, and Po3, proved to be informative in our study population.
Ants were killed by freezing at −30 °C. Each ant’s gaster was removed, so that only the head and mesosoma were extracted. To extract DNA, each body was homogenized in 150 μl of 5% chelex resin (in TE, pH 8.0). One microliter of proteinase K was added before incubating at 57 °C overnight, then rapidly heating the sample at 95 °C for 5 min. Following extraction, each sample was centrifuged and the supernatant was separated into a fresh vial. PCR was performed separately for each dye-labeled primer by heating samples at 94 °C (6 min), followed by 30 cycles of 94 °C (30 s), 54 °C (30 s), and 72 °C (1.5 min), with a final period of 72 °C (10 min) (adapted from Helms and Helms Cahan 2012). Samples for LXAGT1 and MYRT3 were multiplexed, while Po3 was run independently on an ABI 3730 capillary sequencer (ASU molecular core facility).
The program GeneMapper (Applied Biosystems, v3.7) was used to identify allele peaks. Each peak was inspected manually to ensure correct scoring. Population-level allele frequencies were calculated with the online program GenePop, option 5 (v4.2). Monogyny, patriline number, and effective mating frequency were determined using MateSoft 2.0 (Moilanen et al. 2004). When available, the haploid genotypes of males collected during the mating season were used to choose among alternative queen genotypes. Note that males are produced parthenogenetically by the queen and hence allow the reconstruction of a queen’s genotype.
Analysis
We examined the fixed and the interactive effects of forager population size and neighbor index on maximum forager longevity using a gamma GLMM with a log-link function to account for variance structure and a right skewed distribution. Colony was included as a random effect (n = 25 samples, 8 colonies, 4 dates). The influence of season on maximum forager longevity was examined across both sites, with colony nested within site as a random effect (gamma GLMM, n = 32 samples, 14 colonies, 2 sites, 4 dates).
To generate size-free patterns in forager allocation for comparison, each colony’s maximum monthly forager population size was expressed as a ratio to the minimum forager population size recorded between March and April. The March/April sample was chosen to anchor the trajectories because variation between colonies was lowest, differing by only 2000 ants between colonies, and because forager number was at its annual minimum for most colonies.
Relative changes in forager allocation between seasons were assessed with a gamma-distributed generalized linear mixed model with a log-link function, with colony as a random effect (GLMM, n = 27 samples, 8 colonies, 4 dates). Colonies were assigned to groups using a longitudinal cluster analysis that compared size-free foraging trajectories across months (n = 8, R 3.3.2, package, KML). The optimal number of clusters was determined by maximizing Calinski Harabatz scores, while maintaining a minimum of two colonies per cluster (Genolini and Falissard 2011).
The influence of season on forager body size was assessed with a linear mixed model (LMM) that included colony identity as a random effect (n = 1600 workers, 8 colonies, 4 dates). Sample sizes were equal for each colony and month group, and adherence to assumptions of normality, linearity, and homogeneity of variance of residuals were checked by visual inspection of residual plots.
Differences between the observed and expected number of offspring sired by individual males were evaluated using a chi-squared test, with the expectation that patrilines were represented equally among offspring in each colony. Linear regressions were used to determine the relationship between patriline number annual worker production, and other size-related variables. The datasets collected and analyzed during the current study are available from the corresponding author upon reasonable request.
Results
Annual production
The annual number of foragers appearing per colony ranged from 31,180 to 237,980 individuals, representing an eightfold difference in production between neighboring nests (n = 7; Fig. 1). The most productive colonies in the study also had a larger maximum forager population size (linear regression, r = 0.81, r 2 = 0.66, p = 0.025), marginally larger annual foraging ranges (r = 0.74, r 2 = 0.54, p = 0.059) and produced alates during the study. Individual colonies housed up to 26,000 foragers at a single time, added as many as 34,148 new foragers during the peak month of forager allocation (mean = 20,254, SD 10,033, n = 14), and produced nearly half a kilogram (470 g) of worker biomass in a single year.
Plots show changes in forager population for neighboring colonies followed across one annual cycle (n = 8, San Gabriel Rd.). Gray bands represent 95% confidence intervals calculated from the success of each Lincoln index mark-recapture event. Foraging followed a seasonal pattern, with an annual peak in forager number in June or July. The total number of foragers produced per year (upper right corner) was calculated from the number of new foragers that appeared during multiple 10-day sampling events across months. Dark bars at the top of plots indicate points where forager mortality was measured between two mark-recapture events, spaced 10 days apart
If production were distributed evenly across the year, the most productive V. pergandei queen in our study would produce 652 successful eggs per day, or 27 eggs per hour. The cumulative annual, dry biomass of V. pergandei foragers from seven San Gabriel Rd. colonies was 1.7 kg (in 0.49 ha). At the observed density of 14 nests per hectare, colonies of a similar size distribution would return 3.4 kg of forager biomass per hectare of suitable habitat, annually.
Seasonal forager mortality and allocation
Forager longevity did not differ significantly between months (Fig. 2 and Table 1), and forager population size was not a significant predictor of individual forager longevity across the year (Table 2). Importantly, foragers from large and small colonies turned over at the same rate. Throughout the year, approximately 6.4% (SD 1.7%) of each marked forager population died per day, so that maximum forager longevity averaged just 18 days (SD 8.6). At this rate, each colony’s forager population turned over approximately 1.7 times per month, or 20 times per year (n = 14 colonies, maximum foraging = 2585 to 25,869 ants, median = 10,337).
Forager longevity averaged 18 days, and did not differ significantly between months. On average, a colony’s forager population turned over 1.7 times per month. The graph above shows bi-monthly means with standard error bars, mapped over raw data (one outlier was removed, n = 4 to 14 colonies per month)
Allocation to foraging increased significantly during summer months (GLMM; May–June t = 5.17, p < 0.0000; July–August t = 1.24, p < 0.0000; Table 3), though timing of peak forager allocation was staggered among neighboring nests, so that 57% of colonies reached their annual maximum in June, while the remaining proportion peaked in July (n = 14). At the peak of foraging, the number of new foragers added over a 10-day period was 6.8 (SD 5.3) times higher than the annual minimum.
Colonies varied considerably in their allocation of new foragers at the peak of foraging, adding between 2 and 42 new foragers per death. Together, these findings suggest that forager mortality and the addition of new foragers are decoupled processes in V. pergandei. In our population, forager population size did not increase as a result of lowered mortality rate, but instead resulted the ability of some colonies to add more new foragers per death.
Neighbors and mortality
The population-wide increase in forager allocation during summer months meant that neighbor indices jumped from an average of 16 (SD 7.4) in the spring, to 85 (SD 45) in early summer, then 54 (SD 30) in late summer, and finally decreased to 24 (SD 16) in autumn (n = 8 focal colonies). These values illustrate the seasonal variation in the effective size of neighboring colonies (due to changing forager population size) and the potential for neighbor interactions. Yet, despite increased pressure from neighbors in summer, forager mortality rate was not related to neighborhood index alone or to the interaction of forager population size and neighbor index (Table 2). This may be because foraging trails and foraging ranges were generally discrete and avoided one another, and all colonies increased allocation during summer months. Exceptions to this observation included a very small colony which was directly attacked and killed by VP200 in early May of 2015, and colony Vp30, which died in mid-2016 and frequently foraged on the cusp of Vp29’s foraging range (Fig. 3).
Size free changes in forager allocation were expressed relative to forager number in March/April (solids circles and lines). When forager allocation was low, foragers were large-bodied (dashed lines and open circles with SE of the mean). Likewise, dramatic increases in forager number corresponded to a reduction in body size during summer months (r 2 = 0.97, p = 0.000060)
Annual trajectory and production
Three main foraging trajectories were identified using a longitudinal cluster analysis of size-free changes in allocation between months. The annual pattern of forager allocation corresponded with colony productivity. The most productive colonies fell into clusters A and B, and averaged an 11-fold increase in forager allocation in either June (cluster A) or July (cluster B, S4 graph). Cluster C was defined by colonies with fewer than 8000 foragers at the peak of allocation, foraging ranges averaging only 70 m2, the absence of alate production, and a forager population size that grew by less than a factor of 5 at peak allocation (Vp23 and Vp30).
Seasonal trade-off in body size and forager number
Foragers that appeared at the peak of forager allocation during the summer were significantly smaller than those in spring (LMM, t = 10.07, p < 0.000, n = 8 colonies, 1600 workers) or autumn (t = 2.78, p = 0.0055; Fig. 4). Body size did not differ significantly between May and August during the peak of forager allocation (t = 0.52, p = 0.60; Table 4). Colonies with the most dramatic increases in worker number also experienced the greatest relative decrease in worker body size, suggesting that there is a trade-off between worker body size and worker number between seasons (y = 0.53 + 0.0001x, r = 0.98, r 2 = 0.97, p = 0.000060, n = 7; Vp200 removed as an outlier).
The map above shows the San Gabriel Rd. neighborhood only. For each colony, annual foraging range (gray polygons) was determined from foraging trails observed during mark-recapture events across the annual cycle (white radii). Annual foraging areas ranged from 31 to 252 m2. Colonies occurred between narrow washes (light gray lines). Black cross represents incipient colonies that died or were killed by March of 2015. Colony Vp30 did not survive in 2016
Average head width ranged from 0.9 to 1.1 mm when at peak foraging, and from 1.0 to 1.3 mm during the annual minimum. Overall, forager population size was not a significant predictor of average maximum head width (r = −0.29, p = 0.49; r 2 = 0.083, n = 8), or average minimum head width (r = −0.29, p = 0.48; r 2 = 0.084, n = 8). Therefore, body size within colonies was related change in forager population size rather than absolute forager population size.
Matriline and patriline number
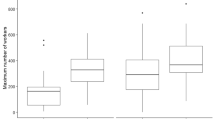
A total of 242 females (worker and alates) and 13 males from 9 colonies were successfully genotyped at 3 microsatellite loci (Table 5). All 9 colonies were found to be monogyne, despite an ~18,000 ant difference in peak forager addition over 10 days. A minimum of 1 to 7 patrilines were observed per colony, with an average observed mating frequency of 3.8 males per queen, and average effective mating frequency of 2.56 (the number of males that would produce the same average relatedness among workers if each contributed equally; Table 6). These measures are reasonable, given a mating frequency of 3.2 male per females recorded during flights (Ode and Rissing 2002).
There was a significant, inverse relationship between maximum patriline number and maximum forager population size (r = −0.93, r 2 = 0.87, p < 0.0000, n = 9; Fig. 5a), as well as minimum effective mating frequency (r = −0.88, r 2 = 0.78, p < 0.0000, n = 9; Fig. 5b). Patriline number was also negatively correlated with other size-associated factors including the maximum number of new foragers each colony added over a 10-day period (r = −0.88, r 2 = 0.77, p < 0.0040, n = 9), and annual worker production (r = −0.76, r 2 = 0.58, p = 0.040, n = 7).
In colonies with four or more patrilines, a single male sired an average of 44% (SD 15%) of offspring or 21% (SD 1.4) more than expected if reproduction were divided evenly among all males (only offspring that could be assigned unambiguously to a single male were included in the analysis). Workers from the dominant patriline were therefore 1.9 (SD 0.54) more common than expected (x 2 = 38.4, p < 0.0000). No novel patrilines or matrilines appeared in alate samples or among workers sampled from the same colonies in both May and September.
Discussion
In our study of V. pergandei, forager turnover rate was not related to forager population size, neighbor pressure, or season. Despite a stable mortality rate, colonies varied in their ability to replace lost workers, adding anywhere from 2 to 42 new foragers per death, ultimately resulting in an eightfold difference in annual production between neighboring nests. Increases in forager population size stemmed from the ability of some colonies to add more new foragers per death, not from worker accumulation due to lower mortality rates.
The colonies that produced the most workers on an annual scale did so by reducing worker body size during peak foraging months (though their annual biomass was still greater than that of less productive colonies). In contrast to our predictions, all colonies were headed by a single queen and colonies with more patrilines (greater genetic diversity) were less productive than colonies with fewer patrilines. Despite variation in worker production, all but one colony (Vp30) survived the year of our study and maintained a forager population size equivalent to that at the beginning of the study.
Productivity and colony size
V. pergandei colonies with a large forager force produced more workers, generated more worker biomass, held a larger annual foraging range, and reproduced during the year of our study. In contrast, colonies with a small forager population maintained a smaller foraging range, produced less workers and biomass, and did not reproduce. Together, these characteristics indicate that relative forager population size likely corresponds to colony size in V. pergandei, as in other ant species (Tschinkel 2011; Kwapich and Tschinkel 2013; Adams 2016). While we did not see differences in average worker body size across colonies, evidence from other studies of V. pergandei suggest that body size may only increase during the first year of growth (Rissing 1987), and can be influenced by competitive environment (Davidson 1978).
Worker life history
Allocation to foraging varied considerably across colonies. Three pieces of evidence suggest that forager allocation was related to the appearance of age cohorts of varying size, rather than a flexible response to changing environmental conditions in V. pergandei. (1) Forager body size differed seasonally, suggesting that foragers appearing at any one time were reared under similar nutritional conditions, as an age cohort. Because V. pergandei workers take an estimated 60 days to mature from eggs to new adults (Wheeler and Wheeler 1976), it is likely that the summertime reduction in worker size resulted from investment in sexual production earlier in the same year (Rissing 1987). (2) Mortality rate was stable, but the introduction of new foragers varied dramatically across colonies. In other words, new foragers were not allocated in proportion to the number of foragers that died, as might be expected if an absence of labor drove replacement via “foraging for work” (Franks and Tofts 1994). (3) The uncoordinated timing of peak forager allocation across neighboring colonies suggests that rapid increases in forager population size were not a response to the sudden appearance of a food resource.
While we did not manipulate the forager population size in V. pergandei, the natural differences in responsiveness to a stable mortality rate imply that this species also shows limited or directional flexibility based on intrinsic developmental processes. In the harvester ant P. badius, allocation to foraging is also strongly related to developmental processes. Age at first foraging in P. baidus varies by orders of magnitude between seasons, but is not related to seasonal or experimental changes in forager mortality, or the experimental addition of larvae (Kwapich and Tschinkel 2013). It would therefore be instructive to remove more than the observed 6.4% of foragers lost per day from V. pergandei colonies to determine the maximum mortality rate colonies can tolerate.
In ant species, worker life histories differ markedly from those of solitary insects because reproduction is typically absent, risk of mortality is low early in life, and death imposed by extrinsic sources is decoupled from senescence (Giraldo and Traniello 2014; Giraldo et al. 2016; Kwapich and Tschinkel 2016b). Differences in the potential and realized life span of V. pergandei workers have not been described, but studies of other ants suggest that unlike honeybees, mortality related to extrinsic sources, without programmed death (i.e., age-dependent senescence). This may be why workers of different body sizes did not differ in their foraging tenure across seasons or with respect to the size of their colony. V. pergandei foragers travel extraordinary distances in desert habitat (up to 40 m). Extrinsic risks such as desiccation, predation, fights with conspecifics, disorientation, and depletion of resources likely contribute to their brief (18 day) life span in the forager population.
Productive colonies have fewer fathers
Allelic diversity resulting from the presence of multiple patrilines has been shown to have positive effects at colony level, including the probability of producing major workers in Acromyrmex echinatior (Hughes et al. 2003) and P. badius (Smith et al. 2008), and increased variation in temporal foraging preferences in Pogonomyrmex occidentalis (Cole and Wiernasz 1999; Wiernasz et al. 2008). Why then do larger, more productive V. pergandei colonies contain fewer patrilines? If genetic diversity were related to any behavioral variation in this species, then it would appear to have a negative effect on colony productivity. In terms of foraging time, only one colony consistently began foraging later than others in the study, and it had moderate productivity and an intermediate number of patrilines.
It is tempting to suggest that conflict among workers over care for less related brood may be to blame, but this is not the expectation for a derived, myrmicine ant. Perhaps small or low-quality queens are over-powered by more males in mating swarms than large, high-quality queens, or colony size is related to colony age, and the availability of males was simply lower in the years that the older colonies were established in the population (Pollock and Rissing 1985). Another alternative is that queens that mate with more males suffer from genetic incompatibilities between patriline genes and queen genes (Cook and Crozier 1995). Under these conditions, queens mated to multiple males have a better chance of mating with compatible males. Queens that mate with few males would chance failing outright due to incompatibility, or could ultimately be more productive by avoiding incompatible mates altogether.
As an alternative, if males deliver sperm of unequal abundance, over time old queens may be left with the sperm of only one or two partners that invested substantially more during copulation. This explanation supported by the observation that reproduction was distributed unequally among males in polyandrous colonies. Based on the high number of patrilines and unequal contributions of fathers, small colonies could represent young colonies. This hypothesis would only hold true if dying colonies or sperm-limited queens declined rapidly, and did not remain in the population as low-producing intermediates. Because the observed colonies in the study remained productive for more than a year, it is unlikely that we sampled any dying colonies. Further investigation of age, productivity, and patriline number is needed to untangle to possible sources of observed variation.
Annual production, stored sperm, and colony longevity
Monogyne colonies of S. invicta house as many as 268,000 workers at one time, and turn over >600,000 workers annually (Tschinkel and Porter 1988; Tschinkel 1993). In contrast, P. badius reaches a maximum colony size of 11,000, producing up to 33,000 workers per year from a single queen (Kwapich and Tschinkel 2016a, b). At a maximum production rate of 238,000 workers per year, monogyne V. pergandei colonies fall squarely in the middle of the aforementioned species, both in terms of productivity and average worker body size.
V. pergandei queens store approximately 2.9 million sperm in their spermatheca (n = 2 queens; Robert A. Johnson, personal communication). While we did not measure fertilization efficiency in V. pergandei, the number of sperm cells expended per fertilization event was determined to be 2.6 to 3.2 in another highly productive myrmicine, Solenopsis invicta (Tschinkel and Porter 1988). Assuming an average efficiency of three sperm per worker fertilization, a single V. pergandei queen could produce close to 1 million offspring in her lifetime. Ignoring alate production and colony ontogeny, the average queen in the current study would run out of sperm 6.5 years after colony founding if she maintained the same rate of production measured in the year of our study. If the lowest productivity queen maintained her annual production rate, she could produce workers for as many as 34.7 years, while the highest producing queens would run out of sperm in 4.2 years.
In actuality, worker production per annum is not static, and young colonies are likely to spend several years with a relatively small size before reaching the minimum worker number necessary for reproduction. V. pergandei colonies have been observed occupying the same area for as long as 12 years (Robert A. Johnson, personal communication), but no detailed census has been made of a population over time.
Summary
The bodies of complex organisms are maintained by the continuous replacement of elementary parts, such as somatic cells. Likewise, eusocial insect colonies that persist across years or decades are maintained by multiple generations of short-lived workers. This study describes the pace of production necessary balance losses of 6.4% of the forager population per day, emphasizes the idiosyncratic responses of colonies faced with the same mortality rate, and reveals the exceptional fecundity of individual V. pergandei queens. Annual patterns of allocation and seasonal trade-offs in worker size were positively associated with worker production, and likely corresponded to large colony size. In contrast to other studies, increased genetic diversity within V. pergandei colonies was associated with poor performance on an annual scale.
Like the body of a traditional organism, an ant colony is selected to build and replace its most basic unit, the worker, on an adaptive schedule. Given their density, longevity, and productivity, V. pergandei colonies contribute substantial biomass to the Sonoran and Mojave desert ecosystems where they occur. Together, our findings offer an alternative perspective on the nature of colony size by considering the production necessary for perennial survival and growth. With these findings, the relationship between annual biomass, whole colony metabolism, and necessary income of seeds can be explored.
References
Adams ES (2016) Territoriality in ants (Hymenoptera: Formicidae): a review. Myrmecol News 23:101–118
Bailey NTJ (1952) Improvements in the interpretation of recapture data. J Anim Ecol 21:120–127. doi:10.2307/1913
Boulay R, Arnan X, Cerdá X, Retana J (2014) The ecological benefits of larger colony size may promote polygyny in ants. J Exp Biol 27:2856–2863. doi:10.1111/jeb.12515
Cerdá X, Dahbi A, Retana J (2002) Spatial patterns, temporal variability, and the role of multi-nest colonies in a monogynous Spanish desert ant. Ecolo Entomol 27:7–15. doi:10.1046/j.0307-6946.2001.00386.x
Cole BJ, Wiernasz DC (1999) The selective advantage of low relatedness. Science (Washington, DC) 285:891-893 doi: doi:10.1126/science.285.5429.891
Cole BJ, Wiernasz DC (2000) Colony size and reproduction in the western harvester ant, Pogonomyrmex occidentalis. Insect Soc 47:249–255. doi:10.1007/PL00001711
Cook JM, Crozier RH (1995) Sex determination and population biology in the hymenoptera. Trends Ecol Evol 10:281–286
Davidson DW (1978) Size variability in the worker caste of a social insect (Veromessor pergandei Mayr) as a function of the competitive environment. Am Nat 112:523–532. doi:10.1086/283294
Elmes GW (1987) Temporal variation in colony populations of the ant Myrmica sulcinodis. Ii. Sexual production and sex ratios. J Anim Ecol 56:573–583
Fewell JH, Harrison JF (2016) Scaling of work and energy use in social insect colonies. Behav Ecol Sociobiol 70:1047–1061. doi:10.1007/s00265-016-2097-z
Fletcher DJC, Blum MS, Whitt TV, Temple N (1980) Monogyny and polygyny in the fire ant, Solenopsis invicta. Ann Entomol Soc Am 73:658–661
Fowler HG (1986) Polymorphism and colony ontogeny in North American carpenter ants (Hymenoptera: Formicidae: Camponotus pennsylvanicus and Camponotus ferrugineus). Zool Jahrb Abt Allg Zool Physiol Tiere 90:297–316
Franks NR, Tofts C (1994) Foraging for work: how tasks allocate workers. Anim Behav 48:470–472
Genolini C, Falissard B (2011) Kml: a package to cluster longitudinal data. Comput Methods Prog Biomed 104:e112–e121. doi:10.1016/j.cmpb.2011.05.008
Gentry JB (1974) Response to predation by colonies of the Florida harvester ant, Pogonomyrmex badius. Ecology 55:1328–1338
Giraldo YM, Traniello JFA (2014) Worker senescence and the sociobiology of aging in ants. Behav Ecol Sociobiol 68:1901–1919. doi:10.1007/s00265-014-1826-4
Giraldo YM et al (2016) Lifespan behavioural and neural resilience in a social insect. Proc R Soc Lond [Biol] 283. doi:10.1098/rspb.2015.2603
Gordon SH (1978) Food and foraging ecology of a desert harvester ant, Veromessor pergandei (Mayr). Dissertation, UC Berkeley
Helms Cahan S (2001) Ecological variation across a transition in colony-founding behavior in the ant Messor pergandei. Oecol 129:629–635. doi:10.1007/s004420100761
Helms KR, Helms Cahan S (2012) Large-scale regional variation in cooperation and conflict among queens of the desert ant Messor pergandei. Anim Behav 84:499–507. doi:10.1016/j.anbehav.2012.05.019
Hölldobler B (1986) Food robbing in ants, a form of interference competition. Oecolo 69:12–15. doi:10.1007/BF00399031
Hölldobler B, Lumsden CJ (1980) Territorial strategies in ants. Science 210:732–739. doi:10.1126/science.210.4471.732
Hölldobler B, Wilson EO (1977) The number of queens: an important trait in ant evolution. Sci Nat 64:8–15. doi:10.1007/BF00439886
Hölldobler B, Wilson EO (1990) The ants. Harvard University Press, Cambridge
Hughes WOH, Sumner S, Van Borm S, Boomsma JJ (2003) Worker caste polymorphism has a genetic basis in Acromyrmex leaf-cutting ants. Proc Nat Acad Sci 100:9394–9397. doi:10.1073/pnas.1633701100
Johnson RA (2006a) Capital and income breeding and the evolution of colony founding strategies in ants. Insect Soc 53:316–322. doi:10.1007/s00040-006-0874-9
Johnson RA (2006b) Biogeographical parallels between plants and ants in North American deserts (Hymenoptera: Formicidae; Spermatophyta). Myrmecol Nachr 8:209–218
Johnson RA, Kaiser A, Quinlan M, Sharp W (2011) Effect of cuticular abrasion and recovery on water loss rates in queens of the desert harvester ant Messor pergandei. J Exp Biol 214:3495–3506. doi:10.1242/jeb.054304
Johnston AB, Wilson EO (1985) Correlates of variation in major/minor ratio in the ant, Pheidole dentata (Hymenoptera: Formicidae). Ann Entomol Soc Am 78:8–11
Keeler KH (1993) Fifteen years of colony dynamics in Pogonomyrmex occidentalis, the western harvester ant, in western Nebraska. Southwest Nat 38:286–289. doi:10.2307/3671438
Kramer BH, Schrempf A, Scheuerlein A, Heinze J (2015) Ant colonies do not trade-off reproduction against maintenance. PLoS One 10:e0137969. doi:10.1371/journal.pone.0137969
Kwapich CL, Tschinkel WR (2013) Demography, demand, death, and the seasonal allocation of labor in the Florida harvester ant (Pogonomyrmex badius). Behav Ecol Sociobiol 67:2011–2027. doi:10.1007/s00265-013-1611-9
Kwapich CL, Tschinkel WR (2016) Limited flexibility and unusual longevity shape forager allocation in the Florida harvester ant (Pogonomyrmex badius). Behavl Ecol Sociobiol 70:221–235. doi:10.1007/s00265-015-2039-1
Lincoln FC (1930) Calculating waterfowl abundance on the basis of banding returns. USDA Circular 118:1–4
Macom TE, Porter SD (1996) Comparison of polygyne and monogyne red imported fire ants (Hymenoptera: Formicidae) population densities. Ann Entomol Soc Am 89:535–543
Mercier B, Passera L, Suzzoni JP (1985) Étude De La Polygynie Chez La Fourmi Plagiolepis pygmaea Latr. (Hym. Formicidae) Ii. La Fécondité Des Reines En Condition Expérimentale Polygyne. Insect Soc 32:349–362. doi:10.1007/BF02224013
Mersch DP, Crespi A, Keller L (2013) Tracking individuals shows spatial fidelity is a key regulator of ant social organization. Science 340:1090–1093. doi:10.1126/science.1234316
Moilanen A, Sundström L, Pedersen J (2004) Matesoft: a program for deducing parental genotypes and estimating mating system statistics in haplodiploid species. Mol Ecol Notes 4:795–797
Ode PJ, Rissing SW (2002) Resource abundance and sex allocation by queen and workers in the harvester ant, Messor pergandei. Behav Ecol Sociobiol 51:548–556. doi:10.1007/s00265-002-0462-6
Plowes N, Johnson R, Hölldobler B (2013) Foraging behavior in the ant genus Messor (Hymenoptera: Formicidae: Myrmicinae). Myrmecol News 18:33–49
Pollock GB, Rissing SW (1985) Mating season and colony foundation of the seed-harvester ant, Veromessor pergandei. Psyche 92:125–134. doi:10.1155/1985/87410
Porter SD, Jorgensen CD (1980) Recapture studies of the harvester ant, Pogonomyrmex Owyheei Cole, using a fluorescent marking technique. Ecol Entomol 5:263–269. doi:10.1111/j.1365-2311.1980.tb01149.x
Rissing SW (1987) Annual cycles in worker size of the seed-harvester ant Veromessor pergandei (Hymenoptera: Formicidae). Behav Ecol Sociobiol 20:117–124. doi:10.1007/BF00572633
Rissing SW, Pollock GB (1984) Worker size variability and foraging efficiency in Veromessor pergandei (Hymenoptera: Formicidae). Behav Ecol Sociobiol 15:121–126. doi:10.1007/bf00299379
Schmid-Hempel P (1982) Foraging ecology and colony structure of two sympatric species of desert ants, Cataglyphis bicolor and Cataglyphis albicans. Doctoral Dissertation, Universität Zürich
Schmid-Hempel P, Schmid-Hempel R (1984) Life duration and turnover of foragers in the ant Cataglyphis bicolor (Hymenoptera, Formicidae). Insect Soc 31:345–360. doi:10.1007/BF02223652
Shik JZ (2008) Ant colony size and the scaling of reproductive effort. Funct Ecol 22:674–681. doi:10.1111/j.1365-2435.2008.01428.x
Simone-Finstrom M, Walz M, Tarpy DR (2016) Genetic diversity confers colony-level benefits due to individual immunity. Biol Lett 12:20151007. doi:10.1098/rsbl.2015.1007
Smith CR, Tschinkel WR (2006) The sociometry and sociogenesis of reproduction in the Florida harvester ant, Pogonomyrmex badius. J Insect Sci 6:1–11. doi:10.1673/2006_06_32.1
Smith CR, Anderson KE, Tillberg CV, Gadau J, Suarez AV (2008) Caste determination in a polymorphic social insect: nutritional, social, and genetic factors. Am Nat 172:497–507. doi:10.1086/590961
Smith ML, Ostwald MM, Loftus JC, Seeley TD (2014) A critical number of workers in a honeybee colony triggers investment in reproduction. Sci Nat 101:783–790. doi:10.1007/s00114-014-1215-x
Tschinkel WR (1988a) Colony growth and the ontogeny of worker polymorphism in the fire ant, Solenopsis invicta. Behav Ecol Sociobiol 22:103–115. doi:10.1007/BF00303545
Tschinkel WR, Porter S (1988) Efficiency of sperm use in queens of the fire ant, Solenopsis invicta (Hymenoptera: Formicidae). Ann Entomol Soc Am 81:777–781
Tschinkel WR (1993) Sociometry and sociogenesis of colonies of the fire ant Solenopsis invicta during one annual cycle. Ecol Monogr 64:425–457
Tschinkel WR (2006) The fire ants. In. Harvard University Press, Cambridge, p 747. 716 p. of plates
Tschinkel WR (2011) The organization of foraging in the fire ant, Solenopsis invicta. J Insect Sci 11:26. doi:10.1673/031.011.0126
Tschinkel WR (2017) Lifespan, age, size-specific mortality and dispersion of colonies of the Florida harvester ant, Pogonomyrmex badius. Insect Soc 64:285–296. doi:10.1007/s00040-017-0544-0
Tschinkel WR, Adams ES, Macom T (1995) Territory area and colony size in the fire ant, Solenopsis invicta. J Anim Ecol 64:473–480
Went FW, Wheeler J, Wheeler GC (1972) Feeding and digestion in some ants (Veromessor and Manica). Bioscience 22:82–88
Wheeler J, Rissing SW (1975) Natural history of Veromessor pergandei. II. Behavior (Hymenoptera: Formicidae). Pan-Pac Entomol 51:303–314
Wheeler, Wheeler (1976) Ant larvae: review and synthesis vol 7. Memoirs of the entomological society of Washington. Entomological Society of Washington, Washington, D.C.
Wiernasz DC, Cole BJ (1995) Spatial distribution of Pogonomyrmex occidentalis: recruitment, mortality and overdispersion. J Anim Ecol 64:519–527. doi:10.2307/5654
Wiernasz DC, Hines J, Parker DG, Cole BJ (2008) Mating for variety increases foraging activity in the harvester ant, Pogonomyrmex occidentalis. Molec Ecol 17:1137–1144. doi:10.1111/j.1365-294X.2007.03646.x
William OHH, Boomsma JJ (2004) Genetic diversity and disease resistance in leaf-cutting ant societies. Evolution 58:1251–1260
Wilson EO (1983) Caste and division of labor in leaf-cutter ants (Hymenoptera: Formicidae: Atta) iii. Ergonomic resiliency in foraging by Atta cephalotes. Behav Ecol Sociobiol 14:47–54. doi:10.1007/BF00366655
Acknowledgements
We would like to thank Yocha DeChavez for her tireless and careful assistance in the laboratory and field and Scott Bingham for his support at the ASU DNA core facility. We are also grateful for the thoughtful discussions and advice from Robert A. Johnson, Walter Tschinkel, Ti Ericksson, Erick Lundgren, the Social Insect Research Group at ASU, and two anonymous reviewers. This study was made possible with permission from South Mountain Regional Park, Phoenix, AZ.
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by O. Rueppell
Electronic supplementary material
ESM 1
Example of counting procedure for each forager sample (PNG 739 kb)
ESM 2
Example of counting procedure of marked foragers recatured after 24 h, pictured under UV light (PNG 3514 kb)
ESM 3
Analysis of size free changes in allocation between months revealed three main foraging trajectories. Colonies with high variation in forager number between months, greater average annual production, larger annual foraging range size fell into cluster A (Vp29,24,199,28), or cluster B (Vp25,200), depending on timing of peak forager allocation. Cluster C was defined by low variation between months, and smaller annual foraging range (Vp23, 30). (PNG 43 kb)
Rights and permissions
About this article
Cite this article
Kwapich, C.L., Gadau, J. & Hölldobler, B. The ecological and genetic basis of annual worker production in the desert seed harvesting ant, Veromessor pergandei . Behav Ecol Sociobiol 71, 110 (2017). https://doi.org/10.1007/s00265-017-2333-1
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00265-017-2333-1