Abstract
An individuals’ experience with conspecific signaling during development can lead to variation in their mating signals and behavior later in life. It is unclear whether experience with sexual signals also alters receivers’ fitness through changes in investment in offspring. Male field crickets attract mates using a long-distance calling song. To determine how developmental experience with calling song quality and quantity alters mating responsiveness and fitness, we raised juvenile female Teleogryllus oceanicus in five acoustic environments. These environments mimicked two mate quantities (high and low) crossed with two mate qualities (high and low), and a silent control. At adulthood, we measured females’ responsiveness in phonotaxis trials. Following phonotaxis, females were offered opportunities to mate and lay eggs. We measured egg number and proportion hatching as components of fitness and reproductive investment. Corroborating previous research in this system, female crickets raised in silence approached a broadcast calling song nearly 45% faster than their counterparts reared hearing a high-quantity/high-quality combination of calling song. Additionally, females adjusted other aspects of phonotaxis behavior in response to the quantity, but not quality of song. We found no evidence that females adjusted mating rates or investment in offspring. Regardless of acoustic experience, females laid equivalent numbers of eggs that had equivalent hatching success. Our results show that female mating behavior responds to juvenile experience mimicking a lack of mating opportunities, but is less responsive to variation in mate quality. Furthermore, reproductive investment may be less plastic than mating behavior.
Significance statement
Social experience can inform organisms about current environmental conditions and lead to changes in behavior. Existing work suggests that field crickets in the genus Teleogryllus change their mating behavior in response to acoustic environments that mimic varied mate availabilities and qualities. However, we do not know if females alter their investment in offspring under similar circumstances. Variable investment could speed evolutionary change when combined with behavioral plasticity. We found that although female mating behavior is sensitive to variation in acoustic experience, reproductive investment is not. The number of eggs laid and the percent hatching did not depend on acoustic experience. We discuss evolutionary explanations for these patterns, including the possibility that investment is less plastic than mating behavior.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
An organism’s experience can inform it about current environmental conditions and can have a considerable impact on its behavior. Evidence for behavioral plasticity in response to social experience comes from a variety of taxa. Examples include insect mating behavior responding to variation in mate availability and quality (e.g., Bailey and Zuk 2008; Fowler-Finn and Rodriguez 2012), male geckos adjusting territorial behavior depending on previous experience with females (e.g., Sakata et al. 2002), and mammals altering boldness in response to variation in conspecific behavior and maternal care (e.g., Griffin and Evans 2003; Champagne and Meaney 2007). Recently, there has been considerable interest in behavioral plasticity because it can dramatically change evolutionary trajectories (Snell-Rood 2012). For instance, if reproductive behavior is altered in response to social experience, individuals may choose mates with different traits in one social environment than they would in another social environment. That choice could then lead to future generations with different genetic compositions.
Plastic changes in reproductive behavior have been found in a variety of organisms, with social experience leading to variation in signaling, male competition, mate location behavior, and mating decisions. For example, stickleback fish experiencing alternative adult sex ratios adopt different reproductive behaviors late in life that depend on the competitive environment they experienced previously (Tinghitella et al. 2013). Acoustic cues experienced by juvenile crickets during development influence the expression of adult male sexual signals and the responses of females to those traits (Wagner et al. 2000; Bailey and Zuk 2008; Kasumovic et al. 2011; Kasumovic et al. 2012; Atwell and Wagner 2014), as well as sperm competition phenotypes (Gray and Simmons 2013). And, while the strength of male competition increased with rising densities when sex ratios were male-biased in Bicyclus anynana, females rejected fewer male mating attempts under the same conditions (Holveck et al. 2015). Such plasticity in reproductive behavior could influence the strength and/or direction of selection on extravagant male traits, leading to divergence if environments differ among populations. Plastic mating behavior may also rescue populations from mate choice Allee effects if females are more relaxed in their mating requirements when available mates are rare or less preferred (Tinghitella et al. 2013; Fowler-Finn and Rodriguez 2012). While variation in the strength of selection on sexual signals is unlikely to result in divergence in male traits that leads to reproductive isolation, the strength of selection is important for the speed of divergence (slower when weaker; Rodriguez et al. 2013). Reductions in the strength of selection on sexual signals may be key for population persistence when environmental and evolutionary change are rapid (Fowler-Finn and Rodriguez 2012).
Importantly, evolutionary change following from plastic mating behavior would be amplified if females also invested heavily in matings with chosen males (Weigel et al. 2015). However, few studies have addressed the post-copulatory effects of social experience with conspecifics (but see Heubel et al. 2008; Weigel et al. 2015). This is despite the idea that life histories are shaped by trade-offs between the quantity and the quality of current and future reproductive opportunities (Stearns 1992) and that these trade-offs can select for plasticity in female reproductive investment. Plasticity in mating behavior and reproductive investment may interact with one another in ways that dramatically alter sexual selection (Weigel et al. 2015). For instance, if females relax their mating requirements, but either do not alter reproductive investment or increase current investment because future mating opportunities are uncertain (Stearns 1992), sexual selection should be weak. If, however, females relax mating decisions and simultaneously reduce investment, sexual selection remains relatively strong. Investing heavily in matings when mates are rare could also increase the likelihood of population persistence.
In this study, we varied female experience with the quantity and quality of available mates during development and tested the hypotheses that (1) social experience with mating signals alters choosiness (the effort or energy that an individual invests in mate assessment; Jennions and Petrie 1997; Gray and Cade 1999; Brooks and Endler 2001) and investment in offspring and that (2) those changes occur in parallel. We focus on mate quantity and quality because sexual selection is largely driven by mate availability (Andersson 1994; Emlen and Oring 1977) and information about both can be gleaned from the social environment. Little research examines these two variables together (but see Kasumovic et al. 2011, 2012), and varying both quantity and quality of mates allowed us to assess their relative importance. Additionally, existing literature suggests that variation in mate quality and quantity affects reproductive investment decisions in addition to preference and choosiness.
We place our questions in a realistic context by addressing them in a system with a recent history of colonization and rapid evolutionary change in male signaling. The Pacific field cricket, Teleogryllus oceanicus, is native to the continent of Australia, and has island-hopped throughout the Pacific, perhaps as Polynesian settlers moved among these islands (Tinghitella et al. 2011). Natural variation in population density (mate availability) exists among island populations (unpublished results, R. Tinghitella and G. Kitchell). Additionally, the recent sweep of a morphological mutation (flatwing) that renders males silent in some Hawaiian populations leads to dramatic differences in perceived mate availability across populations (Zuk et al. 2006). More than 90% of males on Kauai are now obligately silent. Kauai females are less stringent in their mating decisions than females from other locations, likely due to a combination of plastic responses to the changed acoustic environment (Bailey and Zuk 2008) and evolved differences among islands (Tinghitella et al. 2009).
How might experience with sexual signals alter mating behavior? Because T. oceanicus is characterized by recent colonization of novel habitats and variation in the abundance and types of available mates, we hypothesized generally that selection would favor plasticity that ensures that mating happens when preferred mates are rare or absent (Palokangas et al. 1992; Borg et al. 2006; Kokko and Rankin 2006; Fowler-Finn and Rodriguez 2012; Tinghitella 2014; Tinghitella et al. 2015). Ensuring mating when mates are rare or absent could involve changes in choosiness like increased responsiveness (the speed or likelihood that a female will respond to a mate; Bailey 2008) or decreased discrimination (the degree to which females respond differentially to mates with different signal values; Bailey 2008). In addition to attracting mates, sexual signals often indicate aspects of signaler quality including health, dominance, size, and immune system function (e.g., Johnstone 1995; Tregenza et.al. 2006; Rendall et al. 2009). If selection favors not mating with non-preferred, low-quality mates when they are present, we predict females will be less discriminating when experiencing non-preferred signals (Fowler-Finn and Rodriguez 2012).
How might experience with sexual signals shape female life history strategies? If few mates are available, females may increase investment in current reproduction as future mating opportunities are uncertain (Real 1990; Heubel et al. 2008). If future mating opportunities are likely, however, investment in the first mating may be relatively lower. The differential allocation (Burley 1986, 1988; Johnson et al. 2005; Harris and Uller 2009) and reproductive compensation (Stearns 1992; Gowaty 2008) hypotheses attempt to explain how we might expect organisms to alter their reproductive investments depending on mate attractiveness (Sheldon 2000). The two are often (though not always) regarded as making opposite directional predictions, with differential allocation predicting that parents increase investment with preferred mates, and reproductive compensation predicting increased investment when paired with non-preferred or low-quality mates (Ratikainen and Kokko 2010). There is a rich literature in support of both hypotheses (Williams 1966; Burley 1988; Stearns 1992; Bluhm and Gowaty 2004; Johnson et al. 2005; Gowaty 2008; Harris and Uller 2009; Goncalves et al. 2010). In many systems, females can alter their reproductive investments by adjusting the number of eggs laid (Bretman et al. 2006), provisioning eggs differentially (Sinervo 1989; Eising et al. 2001), or manipulating the sex ratio of offspring (Austad and Sunquist 1986).
Acoustically signaling organisms are excellent study systems in which to address our questions. Male crickets use two songs in mate choice: a long-distance, conspicuous song to draw the female to the male from a distance (calling song), and a short-distance, less conspicuous song to induce her to mate with him (courtship song) (Balakrishnan and Pollack 1996). Recorded songs can be manipulated digitally and played back to simulate different qualities and quantities of available mates (e.g., Bailey 2008; Bailey and Zuk 2008; Gray and Simmons 2013; Kasumovic et al. 2011). Crickets from the genus Teleogryllus are known to change their mating behavior in response to acoustic environments that mimic different mate availabilities and mate qualities. For example, female T. oceanicus reared in silence are more responsive than females exposed to abundant male song during rearing (Bailey and Zuk 2008). Additionally, female field crickets are capable of adjusting the number of eggs laid in response to characteristics of mates (Simmons 1987; Bretman et al. 2006), so it is reasonable to hypothesize that reproductive investment may vary with acoustic experience in this system as well.
We exposed juvenile females to one of the five acoustic treatments that varied in quantity and quality of calling song, and then measured willingness to respond to a calling male and response effort (Bailey 2008) and two components of reproductive investment (number of eggs and hatching success). We made the following specific predictions. We first predicted that mate quantity would exert a greater effect than quality because (1) a complete lack of mating should more negatively affect fitness than the available mates being of low quality (Stearns 1992), and (2) this system is characterized by recent changes in actual and perceived mate availability. We predicted that females would vary from least to most responsive to male calling song in the following order: no song, low quantity of low-quality song, low quantity of high-quality song, high quantity of low-quality song, and high quantity of high-quality song. We also predicted that females’ responses to different song models (discrimination) would depend on acoustic experience, with females who experienced high mate availability and/or quality discriminating more strongly against less preferred song variants (0 and/or 100% long chirp; Bailey 2008). With respect to female investment, we again predicted a greater influence of mate availability than quality. First, we anticipated that, consistent with life history theory, females would invest heavily at the first mating event when mate availability is low. If mate availability is high, females ought to invest less at the first mating event as they are likely to have more mating opportunities in the future. Second, we predicted that females would invest most in matings with high-quality males as there is evidence for differential allocation in field crickets (Bretman et al. 2006). Thus, we expected responsiveness and reproductive investment to be modified in parallel by acoustic experience.
Methods
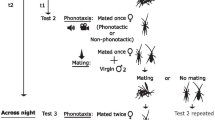
To assess the impact of acoustic rearing environment on mating behavior and reproductive investment, we raised females in five different acoustic environments, conducted phonotaxis trials to test the nature and strength of their mating responses, and then mated the females with novel, untreated males to assess female reproductive investment. We collected females on the island of Kauai (University of Hawaii Agricultural Station (21.966108-159.573791)) in April of 2014 and returned eggs of field-caught females to the University of Denver to establish laboratory populations. Laboratory populations regularly consist of 100–300 crickets and are raised in Percival incubators set to 27 °C on a 12:12 light/dark cycle. Populations are supplemented roughly yearly with offspring of field caught females to avoid inbreeding. Laboratory populations are raised in 35.5 cm × 23.5 cm × 16.5 cm boxes, and provided with Purina rabbit chow and water ad libitum, as well as egg carton for shelter. Experiments were conducted with second-generation lab-reared crickets. A single researcher (VFL) carried out all measurements and conducted all trials after a period of training and remeasurement by RMT. To minimize observer bias, we assigned females alphanumeric IDs and songs letter IDs (A-F). However, it was not feasible to record phonotaxis data blind because differences in song structure are detectable to the trained ear.
Acoustic treatments
To determine how developmental experience with song quality and quantity of available mates affects responsiveness and reproductive investment of adult female T. oceanicus, we assigned females from the larger lab populations randomly to one of the five acoustic environments (treatments). Treatment assignments were made as soon as females’ ovipositors were visible (i.e., when their sex became distinguishable). Crickets assigned to treatments were placed into boxes (35.5 cm × 23.5 cm × 16.5 cm) at a density of 20 females per box. We prepared five replicate boxes per treatment. All replicates were treated identically, and females were reared in treatment boxes until their final molt. We regularly rotated the position of replicate boxes within the incubators to control for possible placement effects and rotated the acoustic treatments between incubators every 2 weeks to avoid incubator effects.
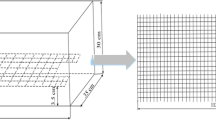
To produce the five acoustic environment treatments, we used digitally produced calling songs made from field-recorded chirps that were previously used (Bailey 2008; Bailey and Zuk 2008; 2009). Songs were used with permission from N. Bailey. These digital songs were either high or low quality and were broadcast for different amounts of time per day to alter females’ perception of mate availability. Each T. oceanicus calling song consists of a trill-like long chirp followed by a series of paired pulses (often called the short chirp component; Fig. 1). Temporal aspects of male songs vary among individuals, including long and short chirp lengths and numbers, as well as interchirp lengths (the silence between chirps). Females from Kauai most prefer songs with a ratio of 60% long chirp to 40% short chirp (Bailey 2008; Simmons et al. 2001). The “high-quality” song thus contained 60% long chip/40% short chirp, and the “low-quality” song contained 0% long chirp (the least preferred variant; Bailey 2008). We crossed these two song qualities (high and low) with two quantities of song (high and low). In Hawaiian populations of T. oceanicus, the diel distribution of calling is more truncated than it is in Pacific and Australian populations (Zuk et al. 1993). Calling begins at sunset and ceases at sunrise, though we do not have precise measurements of how much time per night an average male calls. Male Gryllus pennsylvanicus and Gryllus veletis sing for an average of 4.8 and 2.1 h per day, respectively (Bertram et al. 2013). Similarly, the most prolific Gryllus assimilis males sing for just over 6 h a day (Thomson et al. 2014). We designed our high-quantity and low-quantity treatments to fall outside of this range, such that a female experiencing a high quantity of song was exposed to more song than is reasonable for a single male to produce in a given night. This minimized the possibility that the high-quantity treatment was interpreted by females as a single high-condition caller. In the high-quantity treatments, song played on a 9-min on/1-min off cycle (10.8 h of calling per 12 h dark cycle). In the low-quantity treatments, song played on a 1-min on/9-min off cycle (1.2 h of calling per 12 h dark cycle). During the “off cycle,” we broadcast a looped background noise clipped from the quiet interval that occurs between singing bouts in a recording of cricket song. The songs were only played during the dark portion of the light/dark cycle (12 h daily) to mimic the crickets’ natural nocturnal activity and were broadcast from iPod Nanos attached to Bluetooth EcoGear ECOXBT speakers that were placed inside the incubators near the floor. Songs were projected at 72 dB at 1 m away as measured on a Radio Shack 33–2055 sound level meter. We combined these qualities and quantities of song to create four treatments: low song/low quality (LSLQ), low song/high quality (LSHQ), high song/low quality (HSLQ), and high song/high quality (HSHQ). Additionally, we conducted a treatment in which no song was played (NS).
Stylized sonogram of Teleogryllus oceanicus calling song. The calling song is a complex two part song with both long chirp (LC) and short chirp (SC) components. Song models used in phonotaxis were 0, 20, 40, 60, 80, or 100% LC (after Bailey 2008). In previous work with this population, females preferred a calling song containing 60% LC and 40% SC (Bailey and Zuk 2008)
Phonotaxis and mating behavior
We first assessed variation in responsiveness and mating rate. When females reached between 14 and 16 days of age post-eclosion, phonotaxis behavior was tested in a rectangular arena and mating behavior in mating trials with randomly assigned, untreated males. We conducted no-choice phonotaxis trials during the dark cycle in a 230-cm-long arena. The arena was inside a 2.4 m × 2.0 m room in which the walls are lined with acoustic foam. At the beginning of each phonotaxis trial, a female was carefully placed under a deli cup at the opposite end of the arena from the speaker and given 2 min to adjust to the arena. Following this, the deli cup was removed without disturbing the female and song playback began. To avoid habituation, we randomly assigned females to hear one of the six possible calling songs that varied in perceived quality (0, 20, 40, 60, 80, or 100% long chirp) during phonotaxis (following previous work in this study system; Bailey and Zuk 2008. 2009). Using all of the song variants allowed us to avoid the possibility that females would respond slower or faster to the specific song variant they heard during development. Songs were broadcast at 72 dB at 1 m away. Again, songs were those from Bailey (2008) and were used with permission from N. Bailey. We measured elapsed time before first movement and two components of female responsiveness, whether the female contacted the speaker or not and elapsed time to contact the speaker (willingness to respond and response effort, sensu Bailey 2008). The combination of willingness to respond and response effort contribute to the likelihood that a female eventually mates with a male who has a given sexual signal (Bailey 2008). Time to contact a speaker broadcasting a song is a particularly well-established measure of mate choice in Teleogryllus crickets and in other systems (Ryan 1980; Costello and Symes 2014; Davis and Leary 2015). A female was considered to have contacted the speaker if she crossed over a line 2 cm in front of the speaker or physically contacted the speaker. The trial ended 5 min after song projection began or when the female contacted the speaker.
For the remainder of the experiment, females were returned to their treatment incubators and individually housed in deli cups (area 24.4 cm2, height 7.6 cm) with an egg carton shelter, ad libitum rabbit chow, and moist cheesecloth. To test for differences in mating rate across acoustic treatments, for the 3 days following the phonotaxis trial, females were offered one opportunity per day to mate with a novel, untreated male chosen at random from the general laboratory colony. The 1st mating opportunity occurred following the phonotaxis test. Each mating trial lasted 2 h, and if a female did not mate with the male within 2 h, we returned her to her treatment incubator for another attempt the following day. If a female did not mate within the 3-day window following phonotaxis, she was returned to the general colony and we did not use her for the reproductive investment portion of the study. We recorded the number of mating trials required before a female successfully mated as a measure of interest in mating.
Reproductive investment
Finally, we assessed number of eggs laid and proportion hatching as measures of female investment. We gave each mated female 14 days from the date of mating to lay eggs in moist cheesecloth, while still in her treatment incubator. On day 14 post-mating, the adult female was removed from the deli cup and returned to the general lab population, and we counted the number of eggs laid in the cheesecloth under a dissecting scope. We then returned the cheesecloth to the deli cup container and allowed the eggs 14 days to hatch (from the date the mother was removed) before counting the hatchlings and calculating the hatching rate. Similar to other field crickets (Gershman 2010), T. oceanicus eggs generally take 10–15 days to hatch (personal obs.).
Statistical methods
All statistics were conducted in R (R Core Team, www.r-project.org). To assess the effects of acoustic experience on continuous outcome variables (time to first movement, time to contact, egg number, and hatching rate), we used linear mixed models (LMMs) in the lme4 package (Bates et al. 2013). The fixed effects in the phonotaxis-related models were acoustic treatment, song model used in phonotaxis, and the interaction of acoustic treatment and song model. Replicate nested within treatment was the random effect. We followed a model comparison method in which the full models were compared to reduced models using χ 2 tests to assess whether the fit of the model decreased significantly when the effects of interest were systematically removed (Winter 2013). When appropriate, we used post-hoc Tukey’s contrasts in the multcomp package (Hothorn et al. 2008) to assess pairwise differences between treatments or song models. If acoustic experience alters discrimination in responding females, we expected a significant acoustic treatment × song model interaction effect—in other words, females from the five acoustic treatments should respond differentially to more preferred vs less preferred songs. We expected all females to respond similarly to the 60% song, but for those who experienced high mate availability and/or quality to discriminate more strongly against the less preferred songs (0 and 100% long chirp, for instance). If willingness to respond or response effort depends on acoustic experience, we predict a significant acoustic treatment effect. A significant song model effect would indicate non-random preferences. We assessed the effect of acoustic treatment on egg number and hatchability in LMMs that included treatment as the fixed effect and replicate nested within treatment as the random effect, using the same model comparison method as above.
Finally, we used generalized linear mixed models (GLMMs) in the R package lme4 (Bates et al. 2013) to assess the effects of acoustic treatment on categorical responses. GLMMs with a binomial distribution assessed whether or not females contacted the speaker and whether or not they mated. A GLMM with a Poisson distribution and log link function assessed effects on the number of mates females rejected before mating, with females who mated on their first try assigned a value of 0 (no rejected mates), and females who never mated assigned a value of 3 (the maximum number of rejected mates in the three opportunities provided). Model comparison was conducted as described above and when appropriate, we performed post-hoc Tukey contrasts in the multcomp package (Holthorn et al. 2008).
Results
A total of 383 females were tested in phonotaxis trials. There were 78 females in the HSHQ treatment, 75 females in the HSLQ treatment, 77 females in the LSHQ treatment, 80 females in the LSLQ treatment, and 73 females in the NS treatment. Females who never moved during phonotaxis tests (N = 3, one each from the NS, LSHQ, and HSHQ treatments) were not considered in the phonotaxis-related analyses.
We found no significant effects of acoustic experience treatment, song model, or their interaction on time to first movement (Table 1). Females moved within 14.3 ± 3.7, 9.3 ± 1.0, 16.5 ± 4.2, 12.5 ± 1.6, and 10.8 ± 3.8 s for the high song/high quality, high song/low quality, low song/high quality, low song/low quality, and no song treatments, respectively.
There was no interaction between acoustic treatment and song model on whether or not females contacted the speaker (willingness to respond; Table 1). There was, however, a marginally non-significant effect of acoustic treatment on whether or not females contacted the speaker broadcasting calling song, and willingness to respond depended strongly on song model heard during phonotaxis (Table 1). We used post-hoc Tukey’s tests (α = 0.05) to determine the pairwise sources of variation among treatments and song models. In the case of acoustic treatments, only the behavior of females who experienced HSHQ and females who experienced LSHQ differed; LSHQ females contacted the speaker more often than their HSHQ counterparts (Fig. 2). Finally, females discriminated against the 0% long chirp song, contacting the speaker less often when that song was played; no pairwise comparisons between the remaining five song models differed (Fig. 2). The pairwise difference between the 0% long chirp song and the 80% long chirp song was only marginally significant (p = 0.073).
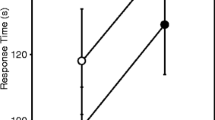
We found no interaction between acoustic treatment and song model on how quickly females contacted the speaker broadcasting calling song (response effort; Table 1). However, time to contact the speaker did depend on acoustic treatment and on the song model heard during phonotaxis (Table 1). Again, post-hoc Tukey’s tests (α = 0.05) revealed sources of variation. Consistent with the willingness to respond results described above, females discriminated against the 0% long chirp song (Fig. 3). Females reared in treatments hearing no song contacted the speaker 44.8% more quickly than females reared in the HSHQ treatment (Fig. 3). This effect size is based on the estimated treatment means from the LMM. No other pairwise comparisons differed significantly from one another.
Time to contact the speaker (response effort) depends on acoustic experience and calling song model. Shown are the estimated acoustic treatment means and standard errors from the LMM. Standard error bars show one standard error from the estimated means. Again, females discriminated against the 0% long chirp song
All 383 tested females were considered in analyses related to mating and reproductive investment. As expected, acoustic rearing environment did not influence whether or not females mated when offered the opportunity to do so (χ 2 = 0.508, df = 4, p = 0.973), nor did the number of males rejected as mates before a successful mating (χ 2 = 0.376, df = 4, p = 0.984). Across all treatments, 289 of 383 females mated. The percent of females mating in each treatment (across all three opportunities) was as follows: NS 78.9% ± 0.3, LDLQ 71.3% ± 0.3, LDHQ 75.3% ± 0.3, HDLQ 77.3% ± 0.3, and HDHQ 74.0% ± 0.2.
Finally, we found no evidence that acoustic experience alters reproductive investment (Fig. 4). There were no significant differences among treatments in number of eggs laid (χ 2 = 4.1869, df = 4, p = 0.3813) or the proportion of eggs hatching (hatchability) (χ 2 = 4.0264, df = 4, p = 0.4024).
Discussion
In this study, we varied female field crickets’ experience with the quantity and quality of available mates to assess effects of social experience on responsiveness, mating rate, and reproductive investment. We found that social experience with mating signals did alter multiple aspects of phonotaxis behavior, but it did not change likelihood of mating or female investment in offspring.
Our phonotaxis results reinforce existing literature in that female field crickets alter their response to sexual signals when they have previous experience with varied acoustic environments that alter the perception of mate availability (Bailey and Zuk 2008; Kasumovic et al. 2011). We assessed willingness to respond (whether or not females contacted the speaker broadcasting a calling song) and response effort (elapsed time to contact the broadcasting speaker) as measures of responsiveness. We found overall variation among acoustic treatments in both variables (Figs. 2 and 3). Specifically, LSHQ females contacted the speaker more often than their counterparts in the HSHQ treatment. This difference is in the predicted direction; females experiencing less calling song during development were more responsive in a simulated mate search. It is noteworthy that the adjustment in phonotaxis behavior occurs between acoustic treatments that differed in quantity of song heard, rather than quality, suggesting that phonotaxis behavior of females from Kauai is more responsive to mate quantity than quality. Given that reasoning, we might have expected a similar response (enhanced phonotaxis) by females raised in silence (NS), however. We additionally found that response effort differed between female crickets who were raised in silence and those who were raised with much high-quality song. Females raised in silence (NS) adjusted their phonotaxis behavior as predicted by approaching a novel song nearly 45% faster than their counterparts in the HSHQ treatment (Fig. 3). We interpret this to mean that females raised hearing no song are more responsive (Bailey 2008) than females raised hearing much high-quality song. This result is similar to Bailey and Zuk (2008) in which female T. oceanicus reared in silence were more responsive in phonotaxis tests than those who were reared hearing the same six songs we tested (ranging in quality from 0 to 100% long chirp) all playing at once. Interestingly, the two measures of responsiveness we assessed were not modified in parallel by experience with mate quality and quantity. This is not entirely unexpected; different measures and components of mate choice are sometimes uncorrelated (Bailey 2008).
Taken together, these results suggest that there is subtlety in the manner in which females adjust phonotaxis behavior. Selection for mate quality may be particularly relaxed in this population. The T. oceanicus population from Kauai is currently >90% silent (flatwing; Zuk et al. 2006), so selection may favor relaxed mate location responses overall to accommodate environments that mimic very low mate availability (Tinghitella and Zuk 2009). Using Kauai crickets, then, provides a conservative test of the hypothesis that mating behavior is sensitive to variation in the quantity and quality of mates available. Nonetheless, we find that females do adjust their response speed behavior in response to varied experience with mate availability.
In the reproductive investment phase of the study, we found that females who were raised hearing different quantities and qualities of calling song, and altered their phonotaxis responses accordingly, did not differ in likelihood to mate, number of eggs laid, or proportion eggs hatching. We measured mating rates (over the three opportunities) as a measure of motivation to mate, but were not surprised to find no variation across acoustic treatments because the costs of mating with a male when given a one-on-one mating opportunity in a small container are extremely low; females incur no search costs under these conditions (Real 1990). Additionally, we found no variation in the number of eggs laid by females or the proportion of eggs hatching. Our results demonstrate that changes in mating behavior and reproductive investment based on social experience do not occur in parallel in this population. It is possible that female reproductive investment is less plastic than phonotaxis behavior and that constraint is reflected in our data. It is clear from evidence in other crickets that adjustment of egg laying behavior is possible, at least in response to mate quality (Bretman et al. 2006), although in that study, the quality (dominance) of the actual mate (rather than the perceived available mates) was the variable altered.
Our results mirror those of Weigel et al. (2015) who found that female sticklebacks did not adjust maternal investments to reflect mate availability. In a previous study, the very same set of fish had been shown to adjust their mating decisions when mate availability was low late in the breeding season (Tinghitella et al. 2013). However, both Weigel et al. (2015) and our study assessed only the females’ first mating event (or first clutch in the stickleback study) and studied well-fed lab-raised animals. To gain a more complete picture of adjustment in female reproductive investment, we suggest examining multiple mating events, as in Heubel et al. (2008), to assess both current and future reproductive efforts. Huebel et al. (2008) found that females increased current reproductive effort when their future access to mates was uncertain. The difference in reproductive effort occurred at the first clutch, relative to later clutches. Further, evolutionary trade-offs follow from responses to limited resources, and are thus often most detectable when animals are resource-limited (Zera and Harshman 2001). We recommend future work on post-copulatory effects of social experience that incorporates resource limitation. Finally, future studies may benefit from more extensive investigation into reproductive investment by both females and males, as male investment into ejaculates can also affect a female’s reproductive success (Wedell 1993; Bailey et al. 2010). Our reproductive data do not extend beyond eggs and hatchlings, and further information regarding female investment (such as offspring sex ratio) and offspring quality (health, attractiveness to mates) may be enlightening.
References
Andersson MB (1994) Sexual selection. Princeton University Press, Princeton
Atwell A, Wagner WE (2014) Female mate choice plasticity is affected by the interaction between male density and female age in a field cricket. Anim Behav 98:117–183. doi:10.1016/j.anbehav.2014.10.007
Austad SN, Sunquist ME (1986) Sex-ratio manipulation in the common opossum. Nature 324(6092):58–60. doi:10.1038/32405a0
Bailey, N (2008) Love will tear you apart: different components of female choice exert contrasting pressures on male field crickets. Behav Ecol. 19:960–966. doi: 10.1093/beheco/arn054
Bailey N, Gray B, Zuk M (2010) Acoustic experience shapes alternative mating tactics and reproductive investment in male field crickets. Curr Biol 20:845–849. doi:10.1016/j.cub.2012.02.063
Bailey N, Zuk M (2008) Acoustic experience shapes female mate choice in field crickets. Proc R Soc B 275:2645–2650. doi:10.1098/rspb.2008.0859
Bailey N, Zuk M (2009) Field crickets change mating preferences using remembered social information. Biol Lett 5(4):449–451. doi:10.1098/rsbi.2009.0112
Balakrishnan R, Pollock GS (1996) Recognition of courtship song in the field cricket, Teleogryllus oceanicus. Anim Behav 51:353–366. doi:10.1006/anbe.1996.0034
Bates, D, Maechler, M, Bolker, B, Walker, S (2013) Package “lme4.” Linear mixed-effects models using Eigen and S4. Available at: https:// github.com/lme4/lme4/. Last accessed 15 September 2016
Bertram SM, Harrison SJ, Thomson IR, Fitzsimmons LP (2013) Adaptive plasticity in wild field cricket’s acoustic signaling. PLoS One 8(7):e69247. doi:10.1371/journal.pone.0069247
Bluhm CK, Gowaty PA (2004) Reproductive compensation for offspring viability deficits by female mallards, Anas platyrhynchos. Anim Behav 68:985–992. doi:10.1016/j.anbehav.2004.01.012
Borg AA, Forsgren E, Amundsen T (2006) Seasonal change in female choice for male size in the two-spotted goby. Anim Behav 72(4):763–771. doi:10.1016/j.anbehav.2005.11.025
Bretman A, Rodríguez-Muñoz R, Tregenza T (2006) Male dominance determines female egg laying rate in crickets. Biol Lett 2:409–411. doi:10.1098/rsbl.2006.0493
Brooks R, Endler JA (2001) Female guppies agree to differ: phenotypic and genetic variation in mate-choice behavior and the consequences for sexual selection. Evolution 55(8):1644–1655. doi:10.1111/j.0014-3820.2001. tb00684.x
Burley N (1986) Sexual selection for aesthetic traits in species with biparental care. Am Nat 127:415–445. doi:10.1086/284493
Burley N (1988) The differential-allocation hypothesis: an experimental test. Am Nat 132:611–628. doi:10.1086/284877
Champagne FA, Meaney MJ (2007) Transgenerational effects of social environment on variations in maternal care and behavioral response to novelty. Behav Neurosci 121(6):1353–1363. doi:10.1037/0735-7044.121.6.1353
Costello RA, Symes LB (2014) Effects of anthropogenic noise on male signaling behavior and female phonotaxis in Oecanthus tree crickets. Anim Behav 95:15–22. doi:10.1016/j.anbehav.2014.05.009
Davis AG, Leary CJ (2015) Elevated stress hormone diminishes the strength of female preferences for acoustic signals in the green tree frog. Horm Behav 69:119–122. doi:10.1016/j/yhbeh.2015.01.005
Eising CM, Eikenaar C, Schwabl H, Groothuis TGG (2001) Maternal androgens in black-headed gull (Larus ridibundus) eggs: consequences for chick development. Proc R Soc B 268(1469):839–846. doi:10.1098/rspb.2001.1594
Emlen ST, Oring LW (1977) Ecology, sexual selection, and the evolution of mating systems. Science 197:215–223. doi:10.1126/science.327542
Fowler-Finn KD, Rodriguez RL (2012) Experience-mediated plasticity in mate preferences: mating assurance in a variable environment. Evolution 66(2):459–468. doi:10.1111/j.1558-5646.2011.01446.x
Gershman S (2010) Large numbers of matings give female field crickets a direct benefit but not a genetic benefit. J Insect Behav 23:59–68. doi:10.1007/s10905-009-91950y
Goncalves IB, Mobley KB, Ahnesjö I, Sagebakken G, Jones AG, Kvarnemo C (2010) Reproductive compensation in broad-nosed pipefish females. Proc R Soc B 277:1581–1587. doi:10.1098/rspb.2009.2290
Gowaty PA (2008) Reproductive compensation. J Evol Biol 21(5):1189–1200. doi:10.1111/j.1420-9101.2008.01559.x
Gray B, Simmons LW (2013) Acoustic cues alter perceived sperm competition risk in the field cricket Teleogryllus oceanicus. Behav Ecol 24(4):982–986
Gray DA, Cade WH (1999) Quantitative genetics of sexual selection in the field cricket, Gryllus integer. Evolution 53(3):848–854. doi:10.2307/2640724
Griffin AS, Evans CS (2003) Social learning of antipredator behavior in a marsupial. Anim Behav 66(3):485–492. doi:10.1006/anbe.2003.2207
Harris WE, Uller T (2009) Reproductive investment when mate quality varies: differential allocation versus reproductive compensation. Philos Trans R Soc Lond Ser B Biol Sci 364:1039–1048. doi:10.1098/rstb.2008.0299
Heubel KU, Lindström K, Kokko K (2008) Females increase current reproductive effort when future access to males is uncertain. Biol Lett 4:224–227
Holveck MJ, Gauthier AL, Nieberding CM (2015) Dense, small and male-biased cages exacerbate male competition and reduce female choosiness in Bicyclus anynana. Anim Behav 104:229–245. doi:10.1016/j.anbehav.2015.03.025
Hothorn T, Bretz F, Westfall P (2008) Simultaneous inference in general parametric models. Biom J 50:346–363. doi:10.1002/bimj.200810425
Jennions MD, Petrie M (1997) Variation in mate choices and mating preferences: a review of causes and consequences. Biol Rev 72:283–327. doi:10.1111/j.1459-185X.1997.tb00015.x
Johnson, AK, Delhey, E, Schlicht, A, Peters, Kempanears, B (2005) Male sexual attractiveness and parental effort in blue tits: a test of the differential allocation hypothesis. Anim Behav 70:877–888. doi: 10.1016/j.anbehav.2005.01.005
Johnstone RA (1995) Honest advertisement of multiple qualities using multiple signals. J Theor Biol 177:87–94. doi:10.1016/S0022-5193(05)80006-2
Kasumovic MM, Hall MD, Try H, Brooks RC (2011) The importance of listening: juvenile allocation shifts in response to acoustic cues of the social environment. J of Evol Biol 24:1325–1334. doi:10.1111/j.1420-9101.2011.02267.x
Kasumovic M, Hall MD, Brooks RC (2012) The juvenile social environment introduces variation in the choice and expression of sexually selected traits. Ecol Evol 2(5):1036–1047. doi:10.1002/ece3.230
Kokko H, Rankin DJ (2006) Lonely hearts or sex in the city? Density-dependent effects in mating systems. Philos Trans R Soc Lond Ser B Biol Sci 361:319–334. doi:10.1098/rstb.2005.1784
Palokangas P, Alatalo RV, Korpimaki E (1992) Female choice in the kestrel under different availability of mating options. Anim Behav 43(4):659–665. doi:10.1016/S0003-3472(05)81024-3
Ratikainen II, Kokko H (2010) Differential allocation and compensation: who deserves the silver spoon? Behav Ecol 21:195–200. doi:10.1093/beheco/arp168
Real L (1990) Search theory and mate choice. I. Models of single-sex discrimination. Am Nat 136(3):376–405. doi:10.1086/285103
Rendall D, Owren MJ, Ryan MJ (2009) What do animal signals mean? Anim Behav 78:233–240. doi:10.1016/j.anbehav.2009.06.007
Rodriguez RL, Rebar D, Fowler-Finn KD (2013) The evolution and evolutionary consequences of social plasticity in mate preferences. Anim Behav 85(5):1041–1047. doi:10.1016/j.anbehav.2013.01.006
Ryan MJ (1980) Female mate choice in a neotropical frog. Science 209(445):523–525. doi:10.1126/science.209.4455.523
Sakata JT, Gupta A, Chuang CP, Crews D (2002) Social experience affects territorial and reproductive behaviours in male leopard geckos, Eublepharis macularius. Anim Behav 63:487–493. doi:10.1006/anbe.2001.1952
Sheldon B (2000) Differential allocation: tests, mechanisms, and implications. TREE 15:397–402. doi:10.1016/S016905347(00)01953-4
Simmons LW (1987) Female choice contributes to offspring fitness in the field cricket, Gryllus bimaculatus. Behav Ecol Sociobiol 21(5):313–321. doi:10.1007/BF00299969
Simmons LW, Zuk M, Rotenberry JT (2001) Geographic variation in female preference functions and male songs of the field cricket Teleogryllus oceanicus. Evolution 55(7):1386–1394. doi:10.1111/j.0014-3820.2001.tb00660.x
Sinervo B (1989) The evolution of maternal investment in lizards: an experimental and comparative analysis of egg size and its effects on offspring performance. Evolution 44(2):279–294. doi:10.2307/2409407
Snell-Rood E (2012) An overview of the evolutionary causes and consequences of behavioral plasticity. Anim Behav 85(5):1004–1011. doi:10.1016/j.anbehav. 2012.12.031
Stearns SC (1992) The evolution of life histories, vol 249. Oxford University Press, Oxford
Thomson, IR, Darveau, CA, Bertram, SM (2014) Body morphology, energy stores, and muscle enzyme activity explain cricket acoustic mate attraction signaling variation. PLoS One. 9:3:e90409. doi: 10.1371/journal.pone. 0090409
Tinghitella RM (2014) Male and female crickets modulate their courtship behaviour depending on female experience with mate availability. Anim Behav 91:9–15. doi:10.1016/j.anbehav.2014.02.022
Tinghitella RM, Stehle C, Boughman JW (2015) Females sample more males at high nesting densities, but ultimately obtain less attractive mates. BMC Evol Biol 15(1):1–14. doi:10.1186/s12862-015-0481-3
Tinghitella RM, Weigel EG, Head M, Boughman JW (2013) Flexible mate choice when mates are rare and time is short. Ecol Evol 3(9):2820–2831. doi:10.1002/ece3.666
Tinghitella RM, Wang JM, Zuk M (2009) Preexisting behavior renders a mutation adaptive: flexibility in male phonotaxis behavior and the loss of singing ability in the field cricket Teleogryllus oceanicus. Behav Ecol 20(4):722–728. doi:10.1093/beheco/arp052
Tinghitella RM, Zuk M, Beveridge M, Simmons LW (2011) Island hopping introduces Polynesian field crickets to novel environments, genetic bottlenecks, and rapid evolution. J Evol Biol 24(6):1199–1211. doi:10.1111/j.1420-9101.2011.02255.x
Tregenza T, Simmons LW, Wedell N, Zuk M (2006) Female preference for male courtship song and its role as a signal of immune function and condition. Anim Behav 72:809–818. doi:10.1016/j.anbehav.2006.01.019
Wagner WE, Reiser MG (2000) The importance of calling song and courtship song in female mate choice in the variable field cricket. AnimBehav 59:1219–1226. doi:10.1006/anbe.1999.1428
Weigel EG, Tinghitella RM, Boughman JW (2015) No evidence for adjustment of maternal investment under alternative mate availability regimes. J Fish Biol 88:508–522. doi:10.1111/jfb.12793
Wedell N (1993) Spermatophore size in bush-crickets: comparative evidence for nuptial gifts as a sperm protection device. Evolution 47(4):1202–1212. doi:10.2307/2409986
Williams GC (1966) Natural selection, the costs of reproduction, and a refinement of Lack’s principle. Am Nat 100(916):687–690. doi:10.1086/282461
Winter, B (2013) Linear models and linear mixed effects models in R with linguistic applications. arXiv:1308.5499. Available at http://arxiv.org/pdf/1308.5499.pdf/ Last accessed 15 September 2016
Zera AJ, Harshman LG (2001) The physiology of life history trade-offs in animals. Annu Rev Ecol Syst 32:95–126. doi:10.1146/annurev.ecolsys.32.081501114006
Zuk M, Rotenberry JT, Tinghitella RM (2006) Silent night: adaptive disappearance of a sexual signal in a parasitized population of field crickets. Biol Lett 2:521–524. doi:10.1098/rsbl.2006.0539
Zuk M, Simmons LW, Cupp L (1993) Calling characteristics of parasitized and unparasitized populations of the field cricket Teleogryllus oceanicus. Behav Ecol Sociobiol 33:339–343. doi:10.1007/BF00172933
Acknowledgements
We thank Brian Ketterman, Gus Kitchell, and Aimee Molloy for assistance maintaining cricket stocks and Nathan Bailey for generously providing the calling songs. Daniel Linseman and Schuyler van Engelenburg provided helpful comments on a previous version of the manuscript. Catherine Durso consulted on statistical methods in R. Funding was provided by the University of Denver’s Partners in Scholarship (PinS) and Honors Programs. The University of Hawaii’s Kauai Agricultural Research Center generously allowed us to collect crickets on their grounds.
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by S. Sakaluk
Rights and permissions
About this article
Cite this article
Lierheimer, V.F., Tinghitella, R.M. Quantity and quality of available mates alters female responsiveness but not investment in the Pacific field cricket, Teleogryllus oceanicus . Behav Ecol Sociobiol 71, 80 (2017). https://doi.org/10.1007/s00265-017-2298-0
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00265-017-2298-0