Abstract
Sex-specific senescence has been construed as a function of mating system and differential investment in parental care, with males exhibiting low parental investment predicted to have more rapid senescence due to costly reproductive behavior. In monogamous mating systems, however, where parental investment may be more evenly distributed, rates of senescence are predicted to be more equivalent between the sexes than in polygynous mating systems. While many polygynous species do appear to support this pattern, evidence from monogamous species, particularly mammals, is scarce. Wolves are an excellent system with which to test this hypothesis, as they exhibit both monogamy and cooperative breeding, where parental investment is distributed across both breeders and non-breeders of both sexes. We examined patterns of age-specific reproduction in red wolves and red wolf-coyote hybrids. We found no evidence of decline in pup production with age in male wolves; among females, the production appeared better explained by hybrid status and age at first reproduction than age per se. Remarkably, however, there was strong evidence of sex differences in pup recruitment, with males, but not females, showing a steep decline in recruitment with age. Combined with previous work on aging in captive wolves, our findings contribute not only to the current understanding of the relationship between mating system and senescence but also to the plasticity of aging and the dynamics of female mate choice in the wild.
Significance statement
In this study, we test for signs of reproductive aging in red wolves using a 20-year dataset documenting age-specific reproduction in the wild. We find evidence that reproductive aging in males is evident for number of pups recruited, though not for number of pups born; we find no evidence for reproductive aging at either stage in wild female wolves. This sex difference stands in contrast to the general prediction that patterns of aging would be more monomorphic among monogamous species. Furthermore, this is especially of interest given that red wolves are cooperative breeders, with costs of reproduction spread across family groups. In general, this study constitutes a valuable contribution to an emerging literature geared at understanding patterns of aging in the context of diverse social and ecological variables.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Evolutionary biologists have long been intrigued by the widespread diversity in rates of aging that is evident both within and among species. It has historically been difficult to understand how aging—a quintessentially maladaptive trait associated with deterioration in reproduction and survival capacity—could be shaped by natural selection. Current evolutionary theory regards aging as an inevitable consequence of increasing probability of death with advancing age due to extrinsic causes, thus reducing selection against alleles with deleterious late-life effects (Medawar 1952; Williams 1957). However, as illuminating as this body of theory and empirical study has proven to be, much remains to be understood regarding how and why rates of aging vary so dramatically in the wild, in environments that differ not only in overall rates of extrinsic mortality but also in numerous complex social and environmental factors that can have pronounced effects on how selection shapes patterns of investment in reproduction and lifespan (Lee 2003; Bonduriansky et al. 2008).
Some classic models predict sex-specific senescence based on sexual selection theory: males should “live fast, die young” when paternal investment per offspring—ranging from gamete size to offspring care—is low, and the benefits of investing in costly reproductive behavior, such as competition for mates early in life, may be worth any potential trade-off with longevity (Williams 1957; Trivers 1972; Bonduriansky et al. 2008). This prediction of rapid aging in males relative to females appears to be supported empirically in a number of species, particularly those exhibiting highly polygynous social systems and high levels of sexual dimorphism (Finch 1994; Promislow 1992; Clutton-Brock and Isvaran 2007). However, “rule proving” exceptions may occur in the case of monogamous species where parental investment may be more equivalent and favor more concordant patterns of aging between the sexes (Promislow 1992; Promislow et al. 1992; Clutton-Brock and Isvaran 2007).
While exceptions to the predicted fast-living strategy for males have begun to be documented in birds (Clutton-Brock and Isvaran 2007), similar evidence from mammals is limited—likely due at least in part to the relative rarity of monogamous mammals. Furthermore, studies of this kind have largely focused on sex differences in longevity and survival, rather than reproduction, particularly as information on paternal identity and age has historically been more difficult to obtain (but see Velando et al. 2006; Reid et al. 2010; Torres et al. 2011; Auld et al. 2013). Indeed, some of the greatest challenges in aging research in wild species are methodological, as it remains difficult to obtain the long-term, longitudinal data necessary for robust examination of age-related decline, especially in long-lived species (Nussey et al. 2008). Nevertheless, to test the generality of a theory linking aging to sexual selection theory and to further explore its complexities, it is critical to extend our understanding of patterns of aging in both survival and reproductive traits in a broader array of species.
Monogamous mammals pose an interesting case, as there are some inevitable sex differences in parental investment arising from female-only gestation and lactation. It is possible that the exclusivity of these tasks may result in males bearing proportionally more of the costs of foraging and defense, especially during early pup development. Thus, even at this most basic level, there exists the possibility of greater strength of extrinsic mortality on males, even in monogamous species, that could generate sex-specific patterns of aging. Wolves are of great interest with regard to the relationship between mating system and senescence, as they exhibit highly monogamous behavior and joint investment in parental care (Packard 2003; Phillips et al. 2003; vonHoldt et al. 2008; Sparkman et al. 2012). However, they are also cooperative breeders, and it is possible that what could otherwise be greater male-specific costs of predation and defense are spread out among group members, thus allowing mortality risks in male breeders to more closely approximate that of females (Bourke 2007). Thus, in these mammals, we might predict the most consonant patterns of male and female aging based on social system alone, regardless of any core differences in early parental investment between the sexes.
Here, we present the first study in a large, monogamous carnivore to test for evidence of both male and female reproductive senescence with regard to two indices of reproductive success: pup production and recruitment. We examine longitudinal data on pup production and recruitment from 20 years of monitoring of a reintroduced population of the red wolf, Canis rufus. Red wolves are cooperative breeders which exhibit high levels of genetic monogamy (Sparkman et al. 2011a, 2012), and reproductive success for both males and females is known based on a population-wide pedigree (Adams 2006). There has been considerably controversy over whether the red wolf lineage originated from an early hybridization between the now-extinct southeastern gray wolf and coyote or whether it is a distinct cousin of the coyote that evolved independently in North America (Kyle et al. 2006; vonHoldt et al. 2016). Whatever the case, red wolves have naturally interbred with coyotes subsequent to their reintroduction, allowing us to examine variation in age-related patterns in both “traditional” red wolves and recent coyote hybrids. Using this rich data set, we test the prediction that monogamous male wolves pose an exception to the “live fast, die young” model for male life-history and explore the ramifications of our findings (in combination with previous findings for captive red wolves) for understanding the plasticity of aging and female mate choice.
Methods
Red wolves were reintroduced into the Alligator River National Wildlife Refuge in North Carolina in 1987 after their extinction from their native distribution throughout the southeastern USA (McCarley and Carley 1979; USFWS 1984). Between 1987 and 2007, free-ranging wolves were captured primarily via foothold traps, equipped with very high-frequency radio-collars and subsequently monitored intensively to gather detailed information on life-history traits (Phillips et al. 2003). It is estimated that >95% of handled adult wolves were collared and that >90% of adults on the recovery area were “known” (A. Beyer and USFWS, unpublished data). It was not possible to record data blind because our study involved focal animals in the field.
Two levels of reproduction were analyzed: (1) the birth litter size, defined as the number of pups captured in the den site, and (2) the recruit litter size, defined as the number that survived from birth in the spring until the following autumn. Litter size estimates were obtained directly via den site visits during the first weeks after pup birth, when pups had low mobility and could be enumerated and individually marked with relative ease (Mills et al. 2008). Our analysis examined 92 birth events, involving 52 dams and 58 sires, as well as 106 recruitment events, involving 44 dams and 44 sires. Parentage was assigned using a population pedigree, the reconstruction of which has been described in detail elsewhere (Adams 2006). Briefly, genetic material was obtained for 703 individuals and genotypes were collected at 18 microsatellite loci with an average heterozygosity of 0.65 (Adams 2006). To assign parents to an individual, we used a maximum likelihood approach as implemented in the program CERVUS 2.0 (Marshall et al. 1998; Adams 2006) as well as field data on known pairings and spatial locations of individuals. When one parent was known, we could successfully assign parentage 95% of the time at the 95% confidence level and 96% of the time at the 80% confidence level. When neither parent was known, we could successfully assign parentage 88% of the time at the 95% confidence level and 99% of the time at the 80% confidence level using these 18 loci (Adams 2006).
All members of a red wolf pack, including the breeding pair and non-breeding individuals, are known to frequent dens after pups are born, and the majority of non-breeding members of packs were offspring from a previous year that delayed dispersal, which we refer to as “helpers.” Wolves were aged by PIT tagging at den sites or during pup capture in early fall.
Reintroduced red wolves naturally hybridize with the coyote (Canis latrans), although management efforts selectively remove many hybrid litters (Phillips et al. 2003). We were able to identify all pair formation and hybridization events between radio-collared red wolves and coyotes. Hybrids used in this study ranged from 50 to 97% red wolf (see Adams 2006).
Statistical analyses
Analyses were conducted using PROC GLIMMIX, with a log-link function and Poisson distribution (SAS 9.3, SAS Institute, Cary, NC, USA). We conducted generalized linear mixed model analyses of our two response variables—red wolf birth and recruit litter size. We began by testing for an effect of dam age, with dam identity as a random effect, and for an effect of sire age, with sire identity as a random effect. Random effects were included to address the presence of repeated measures of reproductive events within individuals. We also tested for a quadratic relationship between reproductive variables and age for both dams and sires. Given that we found dam age was significantly related to birth litter size, and sire age was significantly related to recruit litter size (see “Results” section), we then examined other confounding variables that could potentially explain variation in birth and recruit litter size. This allowed us to test whether the relationships we observed with age could better be explained by other factors.
For birth litter size, we constructed a series of models that contained dam age and dam identity as a random effect, and one of the following variables (summary statistics—mean ± standard deviation—are provided in parentheses): population size (103 ± 28 wolves), pack size (3 ± 2 wolves), dam adult body size (PC1 mean −0.06 ± 0.69), dam hybrid status (125 red wolves, 39 hybrids), dam presence/absence of helpers in the natal pack (75 present, 53 absent), dam age at first reproduction (AFR) (3.2 ± 1.1 years), and dam age at last reproduction (ALR) (6.7 ± 2.0 years). Pack size was defined as the number of individuals present within a pack during the spring, when pups are born. It was expressed as a categorical variable with two levels: one to two individuals in a pack (generally the dam and sire), or three or more individuals. Note that analyses were also conducted with pack size as a continuous variable, and no non-linear effects were observed. We chose to conduct the final analysis with pack size as a categorical variable to reduce the extent to which current pack size is influenced by the degree of reproductive success and offspring dispersal profile during the previous year, as packs are largely composed of parents and philopatric offspring (Sparkman et al. 2012). We included the presence/absence of helpers in sire/dam natal pack (i.e., whether or not sires and dams had helpers when they were pups themselves), as previous work has shown that helpers can have long-term effects on pup fitness (Sparkman et al. 2011b). Adult body size was defined as the first principal component from a principal component analysis (PCA) of morphological measures (ear, tail, hindfoot, shoulder, and body length) of free-ranging wolves taken at any age over 18 months, the age at which growth appears to cease in red wolves (Sparkman et al. 2011b). PC1 explains 71% of the variation in body size. AFR and ALR were also investigated, to examine whether age effects could be explained by differences in individual quality rather than age per se (Nussey et al. 2008). Age at first reproduction was defined as the first year at which an individual was known to reproduce, and age at last reproduction was defined as the last year at which an individual was known to reproduce prior to their death.
For recruit litter size, we constructed a series of models that contained sire age and sire identity as a random effect, and one of the following variables (summary statistics—mean ± standard deviation—are provided in parentheses): population size, pack size, sire adult body size (PC1 mean 1.23 ± 0.95), sire hybrid status (110 red wolves, 54 hybrids), sire presence/absence of helpers in the natal pack (73 present, 47 absent), sire age AFR (3.0 ± 1.6 years), and sire age ALR (6.2 ± 2.4 years). All variables were defined as described above.
We first screened all covariates individually, and those where P > 0.1 when fit individually were not considered further. We compared models containing variables that passed the preliminary screening tests using Akaike’s information criterion corrected for small samples (AICc) (see Burnham and Anderson 2002). There was no concern regarding multicollinearity in our analysis (r < 0.3 for all explanatory variables). In addition to each main effect, we also included interactions between dam/sire age and hybrid status, to determine whether red wolves and red wolf-coyote hybrids were exhibiting the same trends. Since some variables had data missing for some individuals, interfering with our ability to compare models using AICc, we imputed missing values as follows: we imputed a dam AFR of 3 years, the AFR average for this population, for 12/98 missing data cells; we imputed a pack size of 1–2 individuals for 4/98 packs, as all four of these packs appeared to be young transitory packs, unlikely to have had helpers from previous litters. To confirm that imputed values were not affecting conclusions, the final models arrived at through our model selection procedure were re-run with the reduced sample size (N = 83 litters) that only included observations where data was available for all covariates.
In addition to considering a simple linear and quadratic relationship between reproductive output and age, we also employed two additional methods designed to test for non-linear effects of age. First, following the approach of Hammers et al. (2012), we repeated the analyses of top models for birth and recruit litter size using generalized additive mixed models (GAMMs) with a non-parametric smoothing spline for sire/dam age using the mgcv package (version 1.8-3; Wood 2011) in R (version 3.1.2; R Core Team 2014). In addition, we conducted analyses with dam or sire age restricted to 5 years or older to test for evidence of a linear decline from mid-life to age 10 (the latest age of reproduction in our data set). To do so, we included dam age and sire age to all top models for both birth and recruit litter size using this restricted data set.
Results
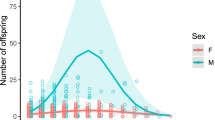
There was a significant negative relationship between birth litter size and dam, but not sire age (Table 1; Fig. 1a, b). In contrast, there was a significant relationship between recruit litter size and sire age, but not dam age. There was no significant quadratic effect of age (Table 1; Fig. 1c, d).
For birth litter size, with dam age as a covariate, population density, pack size, dam AFR, and dam hybrid status all showed P < 0.1 (Table 2). For recruit litter size, with sire age as a covariate, presence of natal helpers, pack size, and ALR all showed P < 0.1 (Table 2). All other effects showed P > 0.1 and were not considered further (Table 2), with the exception of dam ALR and sire AFR. We included both of these variables in our model selection analysis, since ideally both AFR and ALR should be tested at the same time when accounting for potential variation in individual quality.
We compared all combinations of the screened variables in models with and without age. The model with the lowest AICc for birth litter size included hybrid status, pack size, age at first reproduction, and population density, but not dam age (Table 3). Other models with similar AICc values included various combinations of these same variables; thus, all of the top models were in agreement in the variables that best explained birth litter size. No additional parameters occurred within a ΔAICc <3, with the exception of dam ALR in one model (ΔAICc = 2.1); however, ALR was highly non-significant (as in Table 2) and did not alter either the relationship between birth litter size and other variables in the model. Furthermore, all of the top models lead to the same conclusion regarding a lack of evidence for senescence. An interaction between age and hybrid status was not present in any of the top models. All four variables in the final birth litter size model were significantly related to birth litter size, with the exception of population density (Table 4). Hybrids had larger litters, dams with later AFR had smaller litters (Fig. 2a), and larger packs had larger litters (Fig. 2b).
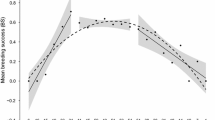
The model with the lowest AICc for recruit litter size contained both sire age and presence of natal helpers, but not pack size, AFR, or ALR (Table 3). Other models with similar AICc values (ΔAICc <3) included various combinations of sire age and natal helpers, and some also included ALR and pack size. However, while ALR and pack size were non-significant (P > 0.1), indicating the lack of a strong effect, the most critical point for the purpose of the study is that regardless of whether natal helpers, pack size, or ALR were in the model together with sire age, all models provided evidence for senescence. AFR did appear in the tenth best model (ΔAICc = 3.5), but it was highly non-significant (as in Table 2) and did not alter the relationship between recruit litter size and other variables in the model. In the top model, both sire age and natal helpers were significantly related to recruit litter size (Table 4), with older sires having fewer recruits (Fig. 4). Consistent with previous analyses of male lifetime reproductive success (discussed in Sparkman et al. 2011b), males that had helpers present in their natal packs also recruited significantly fewer pups (Fig. 3).
GAMM models fitting a non-linear smooth spline showed no significant relationship between birth litter size and either dam age (estimated degree of freedom (edf) = 1; F = 0.561; P = 0.456) or sire age (edf = 1; F = 1.161; P = 0.285). Similarly, there was no significant relationship between recruit litter size and dam age (edf = 1; F = 2.269; P = 0.134). Consistent with the generalized linear mixed model analyses, however, there was a significant relationship between recruit litter size and sire age (edf = 1; F = 11.47; P = 0.000917). The GAMM function penalizes complexity, and if there is not sufficient support for non-linearity, it will revert to a linear model, as is indicated by an estimated degree of freedom, or edf, at or near 1. In all cases, the edf was 1, the new model did not explain additional variation in the data, and the plotted relationship of the spline function was linear and matched results from the linear model. As such, there was no support for non-linear relationships with respect to age. The restricted analysis containing only sires or dams ages 5 and older revealed no hidden negative relationship with dam age for either reproductive variable (birth litter size P = 0.716, recruit litter size P = 0.159) or in sires for birth litter size (P = 0.885). Note that even a full model selection analysis on this restricted data set leads us to the same conclusions, with reproductive aging evident only for sires with respect to recruit litter size. Furthermore, even if we restrict the data set to 6+ or 7+ years, we still see no evidence for aging. That said, sample sizes begin to diminish at 8 years, leaving us little power to robustly test for presence of decline at 8+ years of age. Finally, we also confirmed that sire and dam age did not bear a significant relationship to birth litter size or recruit litter size, respectively, if included in our top models for each of these variables (Table S1).
Discussion
In this study, we report that red wolves do in fact exhibit sex differences in age-related reproductive decline. Furthermore, patterns of age-related decline vary with the stage of reproductive investment. These results suggest that a monogamous, cooperatively breeding social system per se does not result in more homogeneous patterns of reproductive senescence between the sexes, as might be predicted by demographic applications of sexual selection theory. Rather, our findings suggest that monogamous breeding pairs may differ markedly in their contribution to mutual fitness, as well as in their response to early-life social factors.
Sex-specific changes in reproduction with age
Female age appeared to bear a significant negative relation to birth litter size in our sample of red wolves (Fig. 1a). However, other competing explanatory variables—AFR and hybrid status in particular—appeared to more effectively explain variation in litter size (Fig. 2), suggesting that the low litter sizes at later ages may have been primarily an artifact of higher reproduction in hybrids (likely due to high average litter sizes in coyotes—see Rabon 2014; Gese et al. 2016) and females that started reproducing later having smaller litters (perhaps due to poor body condition, or suboptimal conditions) (Fig. 4a). This is not to say that female reproductive senescence does not occur in red wolves—on the contrary, it may occur at later ages, or even commence around 8 years of age, when our sample size becomes restrictive. Furthermore, age and AFR at later ages may to be some extent confounded, with later AFR females also beginning to experience some level of reproductive decline. However, while we had few points for ages 9 and 10, two of the largest litters, composed of 10 pups (with the more typically pattern being two to four pups per litter), occurred at ages 7 and 9. This suggests that some females may continue to be capable of high reproductive output even at late ages. Male red wolves showed a similar capacity for sustained pup production with age even at late ages, with no evidence of decline in number of pups sired by older males (Figs. 1b and 4b).
These results are not readily explained by differences in breeder quality that mask senescent decline (with only higher-quality females living to reproduce at late ages; see Nussey et al. 2008), as dam age at last reproduction did not prove to be a significant predictor of number of pups produced. Furthermore, our findings are consistent with a recent study in captive red wolves that showed no relation between birth litter size and parental age (Rabon 2014). Importantly, however, captive red wolf females did show a decline in pup survival with maternal age, commencing at 8 years of age. Since very few females reproduced at this late age in the wild, it is possible that reproductive senescence after 8 years is also accompanied by somatic senescence and a steep decline in the probability of survival and/or breeding success at older ages.
The lack of strong evidence for reproductive senescence in wild female red wolves stands in contrast to reports of female reproductive senescence in other cooperative breeders with similar lifespans, where declines in litter size and/or survival have been shown to commence as early as 4–6 years of age (e.g., Creel et al. 2004; Sharp and Clutton-Brock 2010; Stahler et al. 2013). Though some evidence suggests that red wolves represent a lineage that emerged from hybridization between gray wolves of Eurasian origin and coyotes of North American origin (vonHoldt et al. 2016), other works suggest that it may be a distinctly North American cousin of the coyote (Kyle et al. 2006). Whatever the case, in this context we found that red wolves do appear to more closely resemble the coyote, in which female pregnancy rates and breeding success have been reported to show little decrease until after 9 years of age (Windberg 1995; Green et al. 2002). Nevertheless, at least two coyote studies have shown a complete cessation in reproduction in all individuals older than 6 years (Crabtree 1988; Sacks 2005). As studies of canid senescence accumulate, it will be important to determine whether female red wolves are distinctive in showing minimal senescence up to late ages in the wild or whether the few reports that exist differ less due to phylogenetic differences, than to population differences in response to ecological challenges.
While there was no evidence of a relationship between dam age and recruit litter size (Figs. 1c and 4c), sire age did bear a strong relationship to recruitment, with sires showing a steep linear decline in recruit litter size from ages 1 to 9 (Figs. 1d and 4d). Tellingly, five or six reproductive 8- and 9-year olds had zero recruits, and the remaining individual reared only a single recruit. Thus, aging appears to have effects over the vast majority of male wolf reproductive lifespan in the wild, acting at the level of successful pup recruitment. These findings are consistent with reports of apparent somatic senescence in gray wolves, which exhibit predatory senescence commencing as early as 2–3 years of age (MacNulty et al. 2009b).
These sex-specific patterns of reproductive senescence in red wolves suggest that sex differences in parental investment and behavior may not be sufficiently diluted by cooperative living so as to decrease the strength of extrinsic mortality on males. There is in fact reason to believe that in spite of being monogamous cooperative breeders in which both sexes share parenting and foraging responsibilities, wolves do exhibit moderate sexual dimorphism in addition to characteristically mammalian differentiation in sex roles during gestation and lactation. For instance, on average male gray wolves are larger than females, and larger body size has been associated with increased predatory ability (MacNulty et al. 2009a). Furthermore, there is some evidence that competition may be stronger in males (though perhaps not as strong as in polygynous species) (Sparkman et al. 2012; Cassidy et al. 2015). Thus, it is possible that costs associated with increased effort/investment in predation and pack defense—or at least increased probability of injury—may sufficiently differentiate levels of extrinsic mortality experienced by males and females in general.
Interestingly, paternal age does not affect pup survival in captive male red wolves (Rabon 2014). Thus, it appears that age-specific declines in recruitment may be environmentally contingent. More limited resources, harsher environmental conditions, and/or increased costs associated with male-male competition, pack defense, and foraging in the wild may cause more rapid phenotypic aging in males and consequently result in decreased ability to perform these same behaviors. A similar case of environmentally contingent plasticity in aging has been reported in the black field cricket, where males suffered increased mortality rate due to predation risk under semi-natural conditions, whereas males lived longer and aged slower than females in a benign lab environment (Maklakov et al. 2009). There is also some evidence that sex differences in mortality and longevity in humans may be environmentally contingent (Graves et al. 2006; Graves 2007). While these findings suggest that male reproductive senescence is plastic, it remains to be determined whether environmental conditions simply advance the onset of senescent phenotypes, perhaps through “wear and tear,” or whether they expose evolved phenotypes that are a result of limited selection against deleterious alleles that are more readily expressed in mature wolves in the wild, even at early ages.
Ramifications for female mate choice
We have previously shown that red wolf lifetime reproductive success is strongly correlated with helper presence/absence at birth, via effects on early-life survival and reproductive lifespan (Sparkman et al. 2011b). While having helpers does increase the probability of survival to age 2 for both males and females, male red wolves that had helpers at birth had significantly lower lifetime reproductive success via reduced reproductive lifespan. In this study, we report that males that had helpers in their natal pack also recruited fewer pups (Fig. 3). Thus, it appears that for males, having had natal helpers decreases lifetime reproductive success through both a decrease in reproductive lifespan and a decrease in annual recruitment. This may be due to a trade-off between early- and late-life traits, where faster growth to larger body sizes for pups that had helpers in their natal pack may carry costs that result in reduced performance of parental or survival-related traits in adulthood (see Sparkman et al. 2011b for more thorough discussion).
The variability in reproductive quality among males with respect to age and early-life social experience combined would suggest that females should prefer to breed with (1) younger males and (2) males that did not have helpers at birth. However, with respect to (1), we have little evidence that females are actively abandoning their mates in exchange for younger males. It is true that 12% (n = 90) of breeding pairs may form due to male-male competition, with younger males replacing older breeders (Sparkman et al. 2012). We have reported three cases in which sons displaced fathers—two initially through extra-pair copulation with their mother (which is unusual, as these constituted two of only a total of four cases of paternity/maternity outside of a pairbond). The remaining eight cases of breeder replacement involved the arrival of a competitor and subsequent death or departure of the resident male. However, it is not clear (except perhaps with respect to the mother-son pair bonds) whether females are actively choosing replacement males; in fact, the vast majority of females stay with one mate until death. This extreme monogamous behavior may be male-enforced (Hosken et al. 2009), with even senescent males actively engaged in reproductive suppression and ousting of potential competitors. Alternatively, for females, the likelihood of and costs associated with finding a new, younger mate with a suitable territory may be greater than any potential benefits.
The extent to which females could preferentially breed with males that did not have helpers would likely be a complex function of both mate availability, as well as the presence of signals indicating early social history. For instance, there could be selection for preference for smaller males, since males with helpers are smaller at high density (Sparkman et al. 2011b). However, such a preference would not necessarily evolve, since small body size could trade-off with predatory and/or competitive ability (MacNulty et al. 2009a), which could also have important ramifications for pup recruitment. Unfortunately, the study of mate choice in wolves is logistically challenging, as wolf behavior is difficult to observe directly and on fine spatial and temporal scales in the wild. However, in so far as we can determine whether females select mates on different criteria associated with age and early-life factors will be of great interest, in determining whether females are responding adaptively to differences in male parenting ability.
References
Adams JR (2006) A multi-faceted molecular approach to red wolf (Canis rufus) conservation and management. PhD dissertation, University of Idaho
Auld JR, Perrins CM, Charmantier A (2013) Who wears the pants in a mute swan pair? Deciphering the effects of male and female age and identity on breeding success. J Anim Ecol 82:826–835
Bonduriansky R, Maklakov A, Zajitschek F, Brooks R (2008) Sexual selection, sexual conflict and the evolution of ageing and life span. Funct Ecol 22:443–453
Bourke AFG (2007) Kin selection and the evolutionary theory of aging. Annu Rev Ecol Syst 38:103–128
Burnham KP, Anderson DR (2002) Model selection and multimodel inference: a practical information-theoretic approach. Springer, New York
Cassidy KA, MacNulty DR, Stahler DR, Smith DW, Mech LD (2015) Group composition effects on aggressive interpack interactions of gray wolves in Yellowstone National Park. Behav Ecol 26:1352–1360
Clutton-Brock TH, Isvaran K (2007) Sex differences in ageing in natural populations of vertebrates. Proc R Soc Lond B 274:3097–3104
Crabtree RL (1988) Sociodemography of an unexploited coyote population. PhD dissertation, University of Idaho
Creel S, Mills MGL, McNutt JW (2004) African wild dogs: demography and population dynamics of African wild dogs in three critical populations. In: MacDonald DW, Sillero-Zubiri C (eds) The biology and conservation of wild canids. Oxford University Press, Oxford, pp. 337–350
Finch CE (1994) Longevity, senescence, and the genome. University of Chicago Press, Chicago
Gese EM, Roberts BM, Knowlton FF (2016) Nutritional effects on reproductive performance of captive adult female coyotes (Canis latrans). Anim Reprod Sci 165:69–75
Graves BM (2007) Sexual selection effects on the evolution of senescence. Evol Ecol 21:663–668
Graves BM, Strand M, Lindsay AR (2006) A reassessment of sexual dimorphism in human senescence: theory, evidence, and causation. Am J Hum Biol 18:161–168
Green JS, Knowlton FF, Pitt WC (2002) Reproduction in captive wild-caught coyotes (Canis latrans). J Mammal 83:501–506
Hammers M, Richardson DS, Burke T, Komdeur J (2012) Age-dependent terminal declines in reproductive output in a wild bird. PLoS One 7:e40413
Hosken DJ, Stockley P, Tregenza T, Wedell N (2009) Monogamy and the battle of the sexes. Annu Rev Entomol 54:361–378
Kyle CJ, Johnson AR, Patterson BR, Wilson PJ, Shami K, Grewal SK, White BN (2006) Genetic nature of eastern wolves: past, present and future. Cons Gen 7(2):273–287
Lee RD (2003) Rethinking the evolutionary theory of aging: transfers, not births, shape senescence in social species. P Natl Acad Sci USA 100:9637–9642
MacNulty DR, Smith DW, Mech LD, Eberly LE (2009a) Body size and predatory performance in wolves: is bigger better? J Anim Ecol 78:532–539
MacNulty DR, Smith DW, Vucetich JA, Mech LD, Stahler DR, Packer C (2009b) Predatory senescence in ageing wolves. Ecol Lett 12:1347–1356
Maklakov AA, Hall MD, Simpson SJ, Dessmann J, Clissold FJ, Zajitschek F, Lailvaux SP, Raubenheimer D, Bonduriansky R, Brooks RC (2009) Sex differences in nutrient-dependent reproductive ageing. Aging Cell 8:324–330
Marshall TC, Slate J, Kruuk LEB, Pemberton JM (1998) Statistical confidence for likelihood-based paternity inference in natural populations. Mol Ecol 7:639–655
McCarley H, Carley CJ (1979) Recent changes in distribution and status of wild red wolves (Canis rufus). Endangered Species Report 4. U.S. Fish and Wildlife Service, Albuquerque, NM
Medawar PB (1952) An unsolved problem of biology. H.K. Lewis and Co, London
Mills KJ, Brent R, Dennis L (2008) Direct estimation of early survival and movements in eastern wolf pups. J Wildl Manag 72(4):949–954
Nussey DH, Coulson T, Festa-Bianchet M, Gaillard JM (2008) Measuring senescence in wild animal populations: towards a longitudinal approach. Funct Ecol 22:393–406
Packard JM (2003) Wolf behavior: reproductive, social and intelligent. In: Mech LD, Boitani L (eds) Wolves: behavior, ecology and conservation. University of Chicago Press, Chicago, pp. 35–65
Phillips MK, Henry VG, Kelly BT (2003) Restoration of the red wolf. In: Mech LD, Boitani L (eds) Wolves: behavior, ecology and conservation. University of Chicago Press, Chicago, pp. 272–288
Promislow DEL (1992) Costs of sexual selection in natural populations of mammals. Proc R Soc Lond B 247:203–210
Promislow DEL, Montgomerie R, Martin TE (1992) Mortality costs of sexual dimorphism in birds. Proc R Soc Lond B 250:143–150
Rabon DR (2014) Effects of age and experience on reproductive performance of captive red wolves (Canis rufus). Can J Zool 92:251–258
R Core Team (2014). R: a language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria, http://www.R-project.org/
Reid JM, Bignal EM, Bignal S, McCracken DI, Bogdanova MI, Monaghan P (2010) Parent age, lifespan and offspring survival: structured variation in life history in a wild population. J Anim Ecol 79:851–862
Sacks BN (2005) Reproduction and body condition of California coyotes (Canis latrans). J Mammal 86:1036–1041
Sharp SP, Clutton-Brock TH (2010) Reproductive senescence in a cooperatively breeding mammal. J Anim Ecol 79:176–183
Sparkman AM, Adams J, Beyer A, Steury TD, Waits L, Murray DL (2011a) Helper effects on pup lifetime fitness in the cooperatively breeding red wolf (Canis rufus). Proc R Soc Lond B 278:1381–1389
Sparkman AM, Adams JR, Steury TD, Waits LP, Murray DL (2011b) Direct fitness benefits of delayed dispersal in the cooperatively breeding red wolf (Canis rufus). Behav Ecol 22:199–205
Sparkman AM, Adams JR, Steury TD, Waits LP, Murray DL (2012) Pack social dynamics and inbreeding avoidance in the cooperatively breeding red wolf. Behav Ecol 23:1186–1194
Stahler DR, MacNulty DR, Wayne RK, vonHoldt B, Smith DW (2013) The adaptive value of morphological, behavioural and life-history traits in reproductive female wolves. J Anim Ecol 82:222–234
Torres R, Drummond H, Velando A (2011) Parental age and lifespan influence offspring recruitment: a long-term study in a seabird. PLoS One 6:e27245
Trivers R (1972) Parental investment and sexual selection. In: Campbell B (ed) Sexual selection and the descent of man 1871–1971. Aldine, Chicago, pp. 136–179
USFWS (1984) Red wolf recovery plan. U.S. Fish and Wildlife Service, Atlanta, GA
Velando A, Drummond H, Torres R (2006) Senescent birds redouble reproductive effort when ill: confirmation of the terminal investment hypothesis. Proc R Soc Lond B 273:1443–1448
vonHoldt BM, Cahill JA, Fan Z, Gronau I, Robinson J, Pollinger JP, Shapiro B, Wall J, Wayne RK (2016) Whole-genome sequence analysis shows that two endemic species of North American wolf are admixtures of the coyote and gray wolf. Sci Adv 2:e1501714
vonHoldt BM, Stahler DR, Smith DW, Earl DA, Pollinger JP, Wayne RK (2008) The genealogy and genetic viability of reintroduced Yellowstone grey wolves. Mol Ecol 17:252–274
Williams GC (1957) Pleiotropy, natural selection and the evolution of senescence. Evolution 11:398–411
Windberg LA (1995) Demography of a high-density coyote population. Can J Zool 73:942–954
Wood SN (2011) Fast stable restricted maximum likelihood and marginal likelihood estimation of semiparametric generalized linear models. J Roy Stat Soc B 73:3–36
Acknowledgments
The Red Wolf Recovery Program is conducted by the US Fish and Wildlife Service (USFWS), and we are grateful to service personnel for their diligent efforts in the field and access to the data. The fieldwork was funded by the USFWS. The Natural Sciences and Engineering Research Council (Canada) supported data analysis and write-up. We also thank anonymous reviewers for their valuable comments on the manuscript. The findings and conclusions in this article are those of the authors and do not necessarily represent the views of the USFWS.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Funding
This study was funded by the US Fish and Wildlife Service
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
All applicable international, national, and/or institutional guidelines for the care and use of animals were followed. The fieldwork on red wolves was conducted solely by the US Fish and Wildlife Service, and all work and procedures conformed to national standards for wildlife handling (Gannon et al. 2011).
Informed consent
Not applicable.
Additional information
Communicated by C. Soulsbury
Electronic supplementary material
Table S1
(DOCX 15 kb)
Rights and permissions
About this article
Cite this article
Sparkman, A.M., Blois, M., Adams, J. et al. Evidence for sex-specific reproductive senescence in monogamous cooperatively breeding red wolves. Behav Ecol Sociobiol 71, 6 (2017). https://doi.org/10.1007/s00265-016-2241-9
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00265-016-2241-9