Abstract
Physical traits such as body size and weapon size typically reflect an individual’s resource holding potential (RHP). During male–male contests, contestants use these traits to assess their own and their opponent’s RHP. However, the advertisement of RHP does not always predict contest outcome. Here, we examined whether assessment index (body size or weapon size [major cheliped size]) and assessment tactics (self or mutual) are predictors of outcome in male–male contests of the hermit crab Pagurus minutus. In experimental contests over guarded females, intruders did not escalate the contest when their major cheliped was smaller than their opponent’s, implying that intruders use mutual assessment based on weapon size when deciding whether to escalate a contest. After escalation, intruders succeeded in taking over females within a shorter period of time with increasing major cheliped size relative to their opponent’s. Overall, males with a major cheliped that was larger than their opponent’s were more likely to win the contest, although some intruders later stopped guarding the female they had taken over. The importance of relative weapon size after escalation indicates that mutual assessment was also used in this phase of male–male contests. Together, these results suggest that males of P. minutus use mutual assessment based on weapon size throughout male–male contests, and that weapon size is an honest index of RHP.
Significance statement
We examined whether assessment index (body or weapon size) and tactics (self or mutual) are predictors of outcome in male–male contests of the hermit crab Pagurus minutus. Intruders did not escalate contests when their major cheliped was smaller than their opponent’s, implying that mutual assessment based on weapon size was used to decide whether to escalate contests. After escalation, intruders succeeded in taking over females within a shorter period of time and were more likely to win with increasing weapon size relative to their opponent’s. The importance of relative weapon size after escalation indicated that mutual assessment was also used in this phase of contests. Together, these results suggest that males of P. minutus use mutual assessment based on weapon size throughout male–male contests, and that weapon size is an honest index of actual strength.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Male–male contests over access to females or mating opportunities are a common feature of animal behavior (Andersson 1994). Resource holding potential (RHP; Parker 1974) is one of the most important factors in determining the winners of male–male contests in many species, and males with a higher RHP are more likely to win (Hardy and Briffa 2013). Because contestants expend time and energy in engaging in aggressive interactions (e.g., Marler et al. 1995; Prenter et al. 2006; Copeland et al. 2011), it is beneficial for males to be able to assess RHP when deciding whether to escalate or persist in a contest. Various traits have been shown to reflect RHP, such as budge status in several bird species (Rohwer 1982), dominant sound frequency in a frog (Riechert and Gerhardt 2013) and a fish species (Ladich 1998), and eye-span in the stalk-eyed fly (Small et al. 2009) as well as body size (Maynard Smith and Harper 2003; Briffa and Sneddon 2007), so it is likely that males use these traits as indices of RHP during decision-making. When male behavior within a contest is based entirely on their own RHP (Taylor and Elwood 2003; Fawcett and Mowles 2013), they conduct what is referred to as “self-assessment,” where the individual with the lower RHP more quickly reaches the giving-up threshold (Briffa 2013). In contrast, when males gather information on the RHP of their opponents relative to their own RHP and use this information to decide their behavior, they are said to engage in “mutual assessment” (Arnott and Elwood 2009).
Assessment indices are often based on the traits of weapons used during physical contests (Emlen 2008). Indeed, weapon size and body size are the indices most often examined experimentally in relation to male–male contests; however, the results of these studies are inconsistent (Arnott and Elwood 2009; Hardy and Briffa 2013). In some animals, male physical attributes are important not only for physical attacks but they also act as visual signals of RHP (e.g., chelicerae and forelegs of a jumping spider, Tedore and Johnsen 2012). However, in other animals, morphological traits that advertise RHP do not contribute at all to contest outcome (antlers of deer, Clutton-Brock 1982; mandible of house cricket, Briffa 2008). These results suggest that the males of some species use different assessment indices at different phases of contests, or that display traits are not an honest index of actual strength. Studies of assessment tactics (i.e., self or mutual) have shown that some species use a single assessment tactic (either self or mutual assessment) throughout a contest (cichlid fish, Enquist et al. 1990; fallow deer, Jennings et al. 2004; wasp, Tsai et al. 2014), which has also been assumed in theoretical models (e.g., Enquist and Leimar 1983). However, other species have been shown to switch assessment tactic as contests proceed (e.g., killifish, Hsu et al. 2008; reviewed in Arnott and Elwood 2009). Therefore, further investigation is needed to determine whether RHP assessment (i.e., index and tactics) is predictive of contest escalation and contest winner, especially when contests involve both prefight and combat phases.
Crustaceans are ideal for the study of RHP assessment during male–male contests. Many crustacean species possess an enlarged (major) cheliped, which they use as part of their pre-fight assessment and as a weapon during contests (Mariappan et al. 2000; Emlen 2008). Previous studies have shown that cheliped size is important in contests (i.e., for both display and as a predictor of outcome). In the shore crab Carcinus maenas (Sneddon et al. 1997) and the hermit crab Diogenes nitidimanus (Yoshino et al. 2011), cheliped size predicts the frequency of display or of contest escalation to physical combat. In both species, males with a cheliped larger than their opponent’s are more likely to win. In contrast, in the snapping shrimp Alpheus heterochaelis (Hughes 1996) and the hermit crab Pagurus bernhardus (Elwood et al. 2006; Arnott and Elwood 2010), chelipeds are displayed but their size do not predict the eventual contest winner. Therefore, cheliped display can be considered a dishonest index of actual strength in these cases (Hughes 2000; Elwood et al. 2006). Complex assessment of RHP has also been demonstrated. The fiddler crab Uca mjoebergi, for example, is known to switch assessment tactics from mutual to self as contests escalate (Morrell et al. 2005); the importance of the cheliped for display has also been shown in this genus (Backwell et al. 2000).
Male Pagurus hermit crabs show precopulatory mate guarding, where they grasp the rim of the gastropod shell occupied by a mature female with their left (minor) cheliped for several days during the reproductive season (Imafuku 1986; Goshima et al. 1998). Females are typically smaller than the males in most guarding pairs, so the females are unable to resist male guarding attempts (Yamanoi et al. 2006) and guarding itself (Okamura and Goshima 2010). Male–male contests often occur between guarding (i.e., owner) and solitary males (i.e., intruder) in which males use their right (major) cheliped, and the intruder has to grapple to take over the guarded female (Yasuda et al. 2012). Because possessing a larger body size (P. filholi, Yoshino et al. 2004; P. nigrofascia, Suzuki et al. 2012) or a larger major cheliped (P. middendorffii, Yasuda et al. 2012) relative to their opponent’s increases the probability of winning a contest, the size of both is likely correlated with male RHP. Males of P. middendorffii have been shown to use a complex assessment strategy; intruders of this species rely on self-assessment based on body size in the prefight phase but switch to mutual assessment based on major cheliped size after contest escalation. Relative cheliped size is also predictive of the eventual winner (Yasuda et al. 2012). This suggests that in P. middendorffii, unlike in other species (e.g., Hughes 2000), male major cheliped size is an honest index of RHP and is more important after escalation of the contest than during the prefight phase. However, because P. middendorffii is the only species in genus Pagurus in which assessment strategy during contests for mates has been directly examined, further study is needed to understand the assessment strategies used by other members of this genus.
Here we investigated whether RHP assessment strategy before contest escalation is predictive of the eventual winner in male–male contests of P. minutus. We recorded the progression of experimental male–male contests and examined which assessment tactics and index of RHP (i.e., body size or major cheliped size) affected an intruder’s decision to escalate a contest. We also determined which traits correlated with actual RHP by identifying predictors of an intruder taking over a guarded female and of contest outcome.
Materials and methods
We collected 112 precopulatory guarding pairs of Pagurus minutus, each male with an intact major cheliped, from the sandflat at Nunohiki, Waka-River estuary, Wakayama, Japan (34° 10′ 23″ N, 135° 10′ 49″ E), from 19 December 2014 to 9 January 2015; the mating season of this species at the study site occurs from December to April (T. Koga, unpublished data). Each precopulatory guarding pair was placed in a small vinyl pouch filled with seawater collected in the field. In the laboratory, we checked that the male was still guarding the female and then placed each guarding pair into a small plastic container (8 × 12.5 × 8 cm) with natural seawater at a depth of 2.5 cm.
Experimental design
For each contest, we placed one male (owner) and his guarded partner in an arena (11 × 19.5 × 8.5 cm) containing natural seawater at a depth of 3 cm. After confirming the guarding behavior of the owner, another male (intruder) randomly chosen from the other pairs was introduced into the arena after removing his partner. We used the video function built into digital cameras (WG-10, Pentax) to record the interactions between the two males from the time the intruder was introduced into the arena. This method helped to minimize observer bias in a manner similar to that of a blinded protocol. All experimental trials were conducted within 6 h of collection. All crabs were used only once (N = 56 contests), and no crabs were injured or lost an appendage during the contests.
The recordings of the contests were observed for up to 15 min from when the intruder initiated movement. A contest was considered escalated when the intruder initiated grappling behavior (for details of this behavior, see Yasuda et al. 2012). In the escalated contests, we recorded whether or not and at what time the intruder succeeded in taking the female from the owner. Some intruders did not continue guarding the female after takeover, so we did not record or examine total contest duration as an index of RHP assessment in this study. After the 15-min observation period, contest outcome was determined based on which male was guarding the female at that time. Contests that had not finished by the end of the observation period were recorded as a draw.
After the contests, we measured the shield length (SL, calcified anterior portion of the cephalothorax) of all of the crabs as an index of body size and the major cheliped propodus length (PL, total length of propodus) of the males to the nearest 0.01 mm under a stereomicroscope.
Statistical analyses
All statistical analyses were performed by using R version 3.0.1 (R Core Team 2013). Akaike’s information criterion was used for model selection (see below), and the model with the lowest Akaike’s information criterion value was considered the most parsimonious (Akaike 1983).
To examine the assessment strategy used by intruders when deciding whether to initiate grappling, we compared several generalized linear models (GLMs) each with a binomial error distribution, focusing on RHP index (i.e., body size or major cheliped size) and assessment tactics (i.e., self or mutual). The response variable was whether the intruder initiated grapping (Yes = 1, No = 0; N = 56). To avoid multicollinearity between SL and PL for both contestants (see Results), four measurements were used separately as an explanatory variable for each GLM: PL or SL of intruder (PLI, SLI) and difference in PL or SL between the intruder and the owner (DPLI-O, DSLI-O). The SL of females guarded by owners (SLOF) was also treated as an explanatory variable in the GLMs.
We next used Cox’s proportional hazard models (Cox 1972) to examine which trait determined whether and when the intruder took over the female after escalation (N = 47). The response variable was duration until takeover (seconds). Because the actual RHP of both males was predicted to affect the occurrence of takeover, one of six measurements was included in each model: PLI, SLI, DPLI-O, DSLI-O, PL of owner (PLO), and SL of owner (SLO). SLOF was also included in the models.
To examine which factors affected the frequency with which intruders stopped guarding the female after takeover, we compared the GLMs with a binomial error distribution. The response variable was whether intruders stopped guarding the female after takeover (Yes = 1, No = 0; N = 29). Tanikawa et al. (2012) reported this phenomenon in the hermit crab P. filholi and suggested that the size of females guarded by intruders in the field affected this behavior. We therefore used three explanatory variables of body size in the GLMs: SLI, SLOF, and SL of females guarded by intruders in the field (SLIF). SLO was not included in the models because success in taking over the female indicated that the intruder had a higher actual RHP than did the owner and so this variable was considered to have little effect on the intruder’s decision whether to guard the female.
The factor that best predicted contest outcome after escalation was determined by a comparison of the GLMs with a binomial error distribution. Outcome at the end of the observation period was treated as the response variable (intruder win = 2, draw = 1, owner win = 0; N = 47). The explanatory variables were the same as in the analysis of duration until takeover (i.e., PLI, SLI, DPLI-O, DSLI-O, PLO, and SLO). SLOF was also included in each model.
Results
Contestant size
In the male contestants (N = 112), mean shield length (SL, index of body size) was 3.76 (±0.368 SD, min = 2.70, max = 4.50, mm) in owners and 3.76 (±0.367 SD, min = 2.50, max = 4.55, mm) in intruders. Mean propodus length of the major cheliped (PL) was 6.38 (±1.113 SD, min = 3.75, max = 8.30, mm) in owners and 6.39 (±1.064 SD, min = 3.35, max = 8.20, mm) in intruders. Statistically significant correlations between SL and PL were found for both owners (t = 8.764, P < 0.001) and intruders (t = 8.389, P < 0.001; Fig. 1). There were no significant differences between contestants in terms of SL (Welch’s t test, t = 0.013, P = 0.990) or PL (t = −0.062, P = 0.951).
Process of male–male contests
In the 56 experimental male–male contests, 47 intruders initiated grappling behavior and hence escalated the contest (Fig. 2a). In the escalated fights, 18 intruders failed to take over the female from their opponents (including three contests finally settled as a draw) and 29 intruders succeeded in taking over the female during grappling. However, 14 of 29 intruders stopped guarding the newly acquired female after an assessment-like behavior by inserting their cheliped into and rolling the female’s shell (see digital video image: http://www.momo-p.com/showdetail-e.php?movieid=momo151128pm01b&embed=on). In the 14 contests where the female was relinquished by the intruder, seven intruders were considered to have won because they resumed guarding the female (Fig. 2b), and seven intruders were considered to have lost because the owner was able to resume guarding the female (Fig. 2b). Although 15 of 29 intruders continued guarding after takeover, two contests were finally settled as a draw because the original owner was challenged again for the female. Therefore, nine intruders did not win the contest even though they had taken over the female at least once during the contest. Thus, the number of contests won after escalation was 20 for the intruders and 22 for the owners, and the remaining five contests were recorded as a draw.
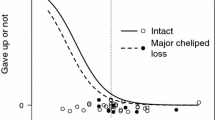
Logistic relationships estimated by using the best generalized linear model based on Akaike’s information criterion for a when the intruder escalates the contest (i.e., initiates grappling) and b the eventual outcome after escalation in the hermit crab Pagurus minutus. DPL I-O is the difference in propodus length of the major cheliped (PL) between the intruder (I) and the owner (O) in each contest. Points 0 and 1 on the y-axis in a refer to whether the intruder gave up or escalated the contest, respectively. Points 0, 1, and 2 in b refer to when the owner won, the contest was a draw, or when the intruder won, respectively. Solid triangles and open circles in b indicate intruders that stopped guarding the female after takeover and intruders that did not stop guarding the female after takeover (including males that failed to take over the female), respectively. Average shield length of females guarded by the owner (SLOF), which is an index of body size, was used in the regression curves
Consequences of intruder decision-making
Whether intruders initiated grappling behavior was best described by the DPLI-O model (N = 56, Table 1). The frequency of grappling increased with increasing intruder PL relative to that of the owner (Table 1, Fig. 2a).
In the escalated contests, the occurrence of and duration until takeover was best described by the DPLI-O model (N = 47, Table 1). When intruders possessed a larger PL relative to that of the owner, they were more likely to succeed in taking over the female in a shorter period of time (Table 1).
Whether intruders stopped guarding behavior after takeover was best described by the null model (N = 29, Table 2).
Eventual contest outcome after escalation was best described by the DPLI-O model (N = 47, Table 2). When an intruder possessed a larger PL relative to the owner, the intruder had a higher probability of winning the contest (Table 2, Fig. 2b).
Discussion
In male–male contests of the hermit crab P. minutus, relative major cheliped size (i.e., weapon size) between contestants was the best predictor of frequency of escalation, when and whether takeover occurred, and eventual contest winner. These results strongly suggest that weapon size is positively correlated with actual RHP and hence is an honest index in this context in this species. However, the advertisement of RHP does not always provide accurate information about strength (Searcy and Nowicki 2005). For example, small males of the green frog Rana clamitans produce a dishonest advertisement call in which frequency alteration does not provide honest information about fighting ability (Bee et al. 2000). Moreover, Wilson and Angilletta (2015) argue that male arthropods provide some of the best evidence of dishonest advertisement during contests. Additional examples where the actual strength of the weapon’s possessor is dishonestly advertised are the mandible in house crickets (Briffa 2008) and fig wasps (Moore et al. 2009) and the major cheliped in several crustacean species (e.g., Hughes 2000; Wilson et al. 2007; Lailvaux et al. 2009).
In males of P. minutus, one factor that may affect the honesty of weapon size as an index of RHP is a relatively higher frequency of physical combat in this species. Because physical fights allow direct assessment of the quality of an index, dishonest weapon use would be easily detected and punished by opponents once contests escalated past the display phase. In the present study, 83.9 % of contests (47 of 56) escalated to grappling, which is markedly higher than has been reported in other studies (e.g., Hughes 2000; Wilson et al. 2007). Moore et al. (2009) reported that if contests resolved without escalation, dishonest weapon use could still exist. Males of the fig wasp Philotrypesis sp. have two morphs that differ in the relative size of their weapon to their body size, where atypical males possess a relatively larger weapon than typical males (Moore et al. 2009). Although atypical males are less likely to win during contests, opponents often retreat without escalation from the atypical males because of their larger (dishonest) weapon (Moore et al. 2009). Therefore, such dishonestly in weapon size may not be favored in a system with typical escalation such as male–male contests in P. minutus. Another factor affecting the honesty of weapon size may be the type of resources contested. Unlike contests for territory or burrows, when males compete for portable resources such as females or food, intruders need to use their weapon and force to take the resource away from their opponents (Yasuda et al. 2014). Given that size often predicts the performance of a given morphology (e.g., Herrel et al. 2005), it can be hypothesized that weapon size in P. minutus is positively correlated with actual RHP. Additionally, because escalation and weapon use are well known in male–male contests of Pagurus sp. (Suzuki et al. 2012; Tanikawa et al. 2012), the honesty of weapon size in this context is common in this genus.
In the present study, relative weapon size between contestants was seen to determine both whether P. minutus intruders decided to escalate a contest as well as the eventual contest outcome. Our analysis therefore suggests that males of P. minutus use mutual assessment in both the prefight and escalation phases of contests, although it should be noted that we did not directly assess contest duration (see Material and Methods ). Similar results regarding RHP assessment have been found in both vertebrates and invertebrates (e.g., cichlid fish, Enquist et al. 1990; spider, Hack et al. 1997; fiddler crab, Pratt et al. 2003). However, this is in contrast with males of P. middendorffii, which have been shown to switch assessment tactics from self to mutual as contests escalate (Yasuda et al. 2012). Although few studies have directly compared the differences in contest behavior and assessment strategy between species in a contest context (Briffa 2013), some differences have been shown in related species. For example, Fuxjager and Marler (2010) reported variance in contest behaviors between mice that differ in social biology (i.e., degree of territoriality). Furthermore, in male–male contests of two species of fig wasp, prefight assessment allows weaker individuals to retreat from contests in Philotrypesis sp. B but not in Sycoscapter sp. A. This interspecific difference in assessment strategy is related to the number of mating opportunities (Moore et al. 2008).
This may also explain the different assessment strategies used during male–male contests in P. middendorffii and P. minutus. Pagurus middendorffii has a limited mating season (approximately 1 month) and females produce only one clutch per year (Wada et al. 1995), whereas P. minutus has a longer reproductive season (approximately 5 months) and females can produce several clutches (Wada et al. 2007). Because a longer mating season would provide more mating opportunities (Johannesson et al. 2010), males of P. minutus would have more potential reproductive opportunities compared with those of P. middendorffii. If this is true, compared with P. middendorffii, solitary males of P. minutus would encounter potential opponents (i.e., guarding pairs) more often due to the longer mating season, and the potential number of male–male contests would be higher. Therefore, to minimize the cost of fighting, intruders of P. minutus might decide whether to escalate contests based on careful assessment of their opponents’ RHP (based on weapon size) rather than rely on self-assessment (Yasuda et al. 2012).
Although actual RHP, as reflected by weapon size, is critically important in P. minutus, in the present study, some intruders eventually lost the contest despite possessing a larger weapon relative to that of the owner (Fig. 2b). Intruders often stopped guarding and relinquished females that they had succeeded in taking over, and some of them never resumed guarding the female. Given that intruders showed assessment-like behavior before relinquishing the female, they were likely assessing the quality of the female after takeover to decide whether to continue guarding. Previous reports have highlighted the importance of resource quality in contest behavior (Enquist and Leimar 1987; Arnott and Elwood 2008), and female body size, which is an index of fecundity, often affects male decision-making (dung fly, Sigurjónsdóttir and Parker 1981; spider, Hack et al. 1997). Indeed, intruders of P. filholi are also known to sometimes stop guarding females after takeover (Tanikawa et al. 2012), and because the chance of P. filholi intruders winning contests decreases relative to the size of their previous partner (i.e., females that they have guarded in the field) (Tanikawa et al. 2012), intruders must make a value choice between a newly acquired female and a previous partner.
However, in the present study, we found no effects of female body size on whether the intruder stopped guarding the female after takeover, which is consistent with the results of a study by Yasuda and Koga (2016), who also report that female body size has no significant effects on the initiation of contests or the timing of takeover by intruders in P. minutus. Thus, female body size appears not to affect male decision-making in P. minutus. Furthermore, although clutch size in P. minutus increases with female body size, about half of females molt just before copulation, independent of their prior size or shell size, and molted females produce significantly fewer eggs compared with non-molted females, especially in molted females with a relatively large body size (Wada et al. 2007). Female size is therefore unlikely to be a reliable index of fecundity in this species. Alternatively, males may be assessing female maturity sometimes rather than body size, as has been shown in other animals (e.g., hermit crab; Suzuki et al. 2012; salamander, Eddy et al. 2016). Since it remains unclear which female characteristics affect male decision-making, further investigation of the effects of resource value, especially of reproductive status, on male–male contests in P. minutus is needed.
In summary, the present results show that males of P. minutus use a strategy of mutual assessment based on weapon size in all phases of male–male contests and also that weapon size is a more crucial assessment index than body size. Differences in assessment tactics among Pagurus species emphasize the importance of interspecific comparisons when discussing animal contests (Briffa 2013). Although we did not focus on the effects of the value of the female as a resource on male decision-making, the contest process in P. minutus could not be explained solely by RHP, particularly after takeover of the female. The lack of effect of female size on male decision-making suggests that resource value in this system relates to female ovary maturity. Further studies addressing female value are needed to clarify the temporary changes in the relative importance of RHP and resource quality in P. minutus.
References
Akaike H (1983) Information measures and model selection. Bull Int Stat Inst 44:277–291
Andersson M (1994) Sexual selection. Princeton University Press, Princeton
Arnott G, Elwood RW (2008) Information gathering and decision making about resource value in animal contests. Anim Behav 76:529–542
Arnott G, Elwood RW (2009) Assessment of fighting ability in animal contests. Anim Behav 77:991–1004
Arnott G, Elwood RW (2010) Signal residuals and hermit crab displays: flaunt it if you have it! Anim Behav 79:137–143
Backwell PRY, Christy JH, Telford SR, Jennions MD, Passmore NI (2000) Dishonest signalling in a fiddler crab. Proc R Soc Lond B 267:719–724
Bee MA, Perrill SA, Owen PC (2000) Male green frogs lower the pitch of acoustic signals in defense of territories: a possible dishonest signal of size? Behav Ecol 11:169–177
Briffa M (2008) Decisions during fights in the house cricket, Acheta domesticus: mutual or self assessment of energy, weapons and size? Anim Behav 75:1053–1062
Briffa M, Sneddon LU (2007) Physiological constraints on contest behaviour. Funct Ecol 21:627–637
Briffa M (2013) Contests in crustaceans: assessments, decisions and their underlying mechanisms. In: Hardy ICW, Briffa M (eds) Animal contests. Cambridge University Press, New York, pp. 86–112
Clutton-Brock TH (1982) The functions of antlers. Behaviour 79:108–124
Copeland DL, Levay B, Sivaraman B, Beebe-Fugloni C, Earley RL (2011) Metabolic costs of fighting are driven by contest performance in male convict cichlid fish. Anim Behav 82:271–280
Cox DR (1972) Regression models and life-tables. J R Stat Soc B 34:187–220
Eddy SL, Wilburn DB, Chouinard AJ, Doty KA, Kiemnec-Tyburczy KM, Houck LD (2016) Male terrestrial salamanders demonstrate sequential mate choice based on female gravidity and size. Anim Behav 113:23–29
Elwood RW, Pothanikat RME, Briffa M (2006) Honest and dishonest displays, motivational state and subsequent decisions in hermit crab shell fights. Anim Behav 72:853–859
Emlen DJ (2008) The evolution of animal weapons. Annu Rev Ecol Evol Syst 39:387–413
Enquist M, Leimar O (1983) Evolution of fighting behaviour: decision rules and assessment of relative strength. J Theor Biol 102:387–410
Enquist M, Leimar O (1987) Evolution of fighting behaviour: the effect of variation in resource value. J Theor Biol 127:187–205
Enquist M, Leimar O, Ljungberg T, Mallner Y, Segerdahl N (1990) A test of the sequential assessment game: fighting in the cichlid fish Nannacara anomala. Anim Behav 40:1–14
Fawcett TW, Mowles SL (2013) Assessments of fighting ability need not be cognitively complex. Anim Behav 86:e1–e7
Fuxjager MJ, Marler CA (2010) How and why the winner effect forms: influences of contest environment and species differences. Behav Ecol 21:37–45
Goshima S, Kawashima T, Wada S (1998) Mate choice by males of the hermit crab Pagurus filholi: do males assess ripeness and/or fecundity of females? Ecol Res 13:151–161
Hack MA, Thompson DJ, Fernandes DM (1997) Fighting in males of the autumn spider, Metellina segmentata: effects of relative body size, prior residency and female value on contest outcome and duration. Ethology 103:488–498
Hardy ICW, Briffa M (2013) Animal contests. Cambridge University Press, New York
Herrel A, Podos J, Huber SK, Hendry AP (2005) Bite performance and morphology in a population of Darwin’s finches: implications for the evolution of beak shape. Funct Ecol 19:43–48
Hsu Y, Lee S, Chen M, Yang S, Cheng K (2008) Switching assessment strategy during a contest: fighting in killifish Kryptolebias marmoratus. Anim Behav 75:1641–1649
Hughes M (1996) Size assessment via a visual signal in snapping shrimp. Behav Ecol Sociobiol 38:51–57
Hughes M (2000) Deception with honest signals: signal residuals and signal function in snapping shrimp. Behav Ecol 11:614–623
Imafuku M (1986) Sexual discrimination in the hermit crab Pagurus geminus. J Ethol 4:39–47
Jennings DJ, Gammell MP, Carlin CM, Hayden TJ (2004) Effect of body weight, antler length, resource value and experience on fight duration and intensity in fallow deer. Anim Behav 68:213–221
Johannesson K, Saltin SH, Duranovic I, Havenhand JN, Jonsson PR (2010) Indiscriminate males: mating behaviour of a marine snail compromised by a sexual conflict? PLoS One 5:e12005
Ladich F (1998) Sound characteristics and outcome of contests in male croaking gouramis (Teleostei). Ethology 104:517–529
Lailvaux SP, Reaney LT, Backwell PRY (2009) Dishonest signalling of fighting ability and multiple performance traits in the fiddler crab Uca mjoebergi. Funct Ecol 23:359–366
Mariappan P, Balasundaram C, Schmitz B (2000) Decapod crustacean chelipeds: an overview. J Biosci 25:301–313
Marler CA, Walsberg G, White ML, Moore M (1995) Increased energy expenditure due to increased territorial defense in male lizards after phenotypic manipulation. Behav Ecol Sociobiol 37:225–231
Maynard Smith J, Harper D (2003) Animal signals. Oxford University Press, New York
Moore JC, Obbard DJ, Reuter C, West SA, Cook JM (2008) Fighting strategies in two species of fig wasp. Anim Behav 76:315–322
Moore JC, Obbard DJ, Reuter C, West SA, Cook JM (2009) Male morphology and dishonest signalling in a fig wasp. Anim Behav 78:147–153
Morrell LJ, Bakwell PRY, Metcalfe NB (2005) Fighting in fiddler crabs Uca mjoebergi: what determines duration? Anim Behav 70:653–662
Okamura S, Goshima S (2010) Indirect female choice mediated by sex pheromones in the hermit crab Pagurus filholi. J Ethol 28:323–329
Parker GA (1974) Assessment strategy and the evolution of fighting behaviour. J Theor Biol 47:223–243
Pratt AE, McLain DK, Lathrop GR (2003) The assessment game in sand fiddler crab contests for breeding burrows. Anim Behav 65:945–955
Prenter J, Elwood RW, Taylor PW (2006) Self-assessment by males during energetically costly contests over precopula females in amphipods. Anim Behav 72:861–868
R Core Team (2013) R: a language and environment for statistical computing. R Foundation for Statistical Computing, Vienna
Riechert MS, Gerhardt HC (2013) Gray tree frogs, Hyla versicolor, give lower-frequency aggressive calls in more escalated contests. Behav Ecol Sociobiol 67:795–804
Rohwer S (1982) The evolution of reliable and unreliable badges of fighting ability. Am Zool 22:531–546
Searcy WA, Nowicki S (2005) The evolution of animal communication: reliability and deception in signaling systems. Princeton University Press, Princeton
Sigurjónsdóttir H, Parker GA (1981) Dung fly struggles: evidence for assessment strategy. Behav Ecol Sociobiol 8:219–230
Small J, Cotton S, Fowler K, Pomiankowski A (2009) Male eyespan and resource ownership affect contest outcome in the stalk-eyed fly, Teleopsis dalmanni. Anim Behav 78:1213–1220
Sneddon LU, Huntingford FA, Taylor AC (1997) Weapon size versus body size as predictor of winning in fights between shore crab, Carcinus maenas (L.) Behav Ecol Sociobiol 41:237–242
Suzuki Y, Yasuda C, Takeshita F, Wada S (2012) Male mate choice and male-male competition in the hermit crab Pagurus nigrofascia: importance of female quality. Mar Biol 159:1991–1996
Tanikawa D, Yasuda C, Suzuki Y, Wada S (2012) Effects of male size and mate quality on male-male contest in the hermit crab Pagurus filholi. Jap J Benthol 67:15–19
Taylor PW, Elwood RW (2003) The mismeasure of animal contests. Anim Behav 65:1195–1202
Tedore C, Johnsen S (2012) Weaponry, color, and contest success in the jumping spider Lyssomanes viridis. Behav Process 89:203–211
Tsai YJJ, Barrows EM, Weiss MR (2014) Pure self-assessment of size during male-male contests in the parasitoid wasp Nasonia vitripennis. Ethology 120:816–824
Wada S, Goshima S, Nakao S (1995) Reproductive biology of the hermit crab Pagurus middendorffii Brandt (Decapoda: Anomura: Paguridae). Crust Res 24:23–32
Wada S, Ito A, Mima A (2007) Evolutionary significance of prenuptial molting in female Pagurus hermit crabs. Mar Biol 152:1263–1270
Wilson RS, Angilletta MJ Jr (2015) Dishonest signaling during aggressive interactions: theory and empirical evidence. In: Irschick DJ, Briffa M, Podos J (eds) Animal signaling and function: an integrative approach. Wiley Blackwell, Hoboken, pp. 205–227
Wilson RS, Angilletta MJ Jr, James RS, Navas C, Seebacher F (2007) Dishonest signals of strength in male slender crayfish (Cherax dispar) during agonistic encounters. Am Nat 170:284–291
Yamanoi T, Yoshino K, Kon K, Goshima S (2006) Delayed copulation as a means of female choice by the hermit crab Pagurus filholi. J Ethol 24:213–218
Yasuda CI, Koga T (2016) Do weaponless males of the hermit crab Pagurus minutus give up contests without escalation? Behavior of intruders that lack their major cheliped in male-male contests. J Ethol 34:249–254
Yasuda C, Takeshita F, Wada S (2012) Assessment strategy in male-male contests of the hermit crab Pagurus middendorffii. Anim Behav 84:385–390
Yasuda CI, Matsuo K, Wada S (2014) Rapid regeneration of the major cheliped in relation to its function in male-male contests in the hermit crab Pagurus middendorffii. Plankton and Benthos Res 9:122–131
Yoshino K, Ozawa M, Goshima S (2004) Effects of shell size fit on the efficacy of mate guarding behaviour in male hermit crabs. J Mar Biol Assoc UK 84:1203–1208
Yoshino K, Koga T, Oki S (2011) Chelipeds are the real weapon: cheliped size is a more effective determinant than body size in male-male competition for mates in a hermit crab. Behav Ecol Sociobiol 65:1825–1832
Acknowledgments
We are grateful to the two referees for their extensive comments and contributions, which helped to improve the manuscript. This study was financially supported by a JSPS Research Fellowship for Young Scientists (No. 25-2149 and 15J07721) to CY.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Ethical approval
All applicable international, national, and/or institutional guidelines for the care and use of animals were followed.
Conflict of interest
The authors declare that they have no competing interests.
Additional information
Communicated by D. Kemp
Rights and permissions
About this article
Cite this article
Yasuda, C.I., Koga, T. Importance of weapon size in all stages of male–male contests in the hermit crab Pagurus minutus . Behav Ecol Sociobiol 70, 2175–2183 (2016). https://doi.org/10.1007/s00265-016-2221-0
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00265-016-2221-0