Abstract
Cooperative breeding has been studied intensively in many species of birds and mammals but remain less well studied in fish. We report a remarkable new example of a cooperatively breeding cichlid from Lake Tanganyika, Neolamprologus obscurus. Using field observations and microsatellite DNA analyses, we studied group structure, helping behavior, relatedness, and dispersal of this species. We present four major observations. First, large territorial breeding males mated with one to eight breeding females, each of which was territorial and unrelated to another. Second, one to ten smaller fish (“subordinates”) of both sexes were allowed to stay inside the breeding females’ territories. Subordinates were often highly related to both the respective breeding male and female and performed territory defense and shelter maintenance, which is regarded as helping behaviors. Third, one to three subordinate males, similar in size to breeding females, were allowed to stay inside a breeding male’s territory but were not tolerated in the breeding females’ territories. Pairwise relatedness suggests these individuals are usually sons of the respective breeding male. Fourth, pairwise relatedness estimates suggest that juveniles delay dispersal and assist their mothers in raising offspring. As female subordinates grow up, they leave the father’s territory and disperse into other groups. In contrast, male subordinates leave their mother’s territory but remain within the territory of their father. The described social system makes N. obscurus a promising new model species to study the evolution of cooperative breeding.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
A large number of bird and mammal species have been reported to engage in cooperative breeding (Lukas and Clutton-Brock 2012; Feeney et al. 2013). Cooperative breeding is defined as a breeding system in which individuals other than parents remain in the breeder’s territory and assist in raising young (Koenig and Dickinson 2004; Cockburn 2006; Lukas and Clutton-Brock 2013). In contrast to birds and mammals, only a few fish species are known to breed cooperatively, despite a wide variety of mating and parental care systems (e.g., Taborsky 1994, 2001; Kohler 1998; Wisenden 1999; Awata et al. 2005; Heg et al. 2005; Heg and Bachar 2006). Those that have been described as cooperative breeders are almost all cichlids of the tribe Lamprologini endemic to Lake Tanganyika (Heg and Bachar 2006). Helpers of cooperative breeding cichlids participate in territory defense (attacking predators and territory competitors), territory maintenance (digging and removing debris from shelters), and fry care (cleaning and fanning eggs and defending young) (Taborsky and Limberger 1981; Kohler 1998; Awata et al. 2005; Heg et al. 2005). Cichlids represent an excellent model system to study the evolution and behavioral ecology of cooperative breeding using both field data and laboratory experiments (Wong and Balshine 2010). However, detailed data on cooperative breeding cichlids have been gathered from only a few species (see Heg and Bachar 2006 for a complete list), notably Neolamprologus pulcher/brichardi (N. pulcher and N. brichardi are synonymous species according to molecular data, see Duftner et al. 2007). Other Lamprologines, such as Neolamprologus multifasciatus (Rossiter 1993; Sato and Gashagaza 1997; Kohler 1998; Schradin and Lamprecht 2000, 2002), Neolamprologus savoryi (Heg et al. 2005), Julidochromis ornatus (Awata and Kohda 2004; Awata et al. 2005; Heg and Bachar 2006) Julidochromis marlieri (Yamagishi and Kohda 1996), and Chalinochromis brichardi (MK et al., unpublished data) have been less well studied.
These species show a broad array of mating systems, ranging from monogamy and polygyny (e.g., N. pulcher: Desjardins et al. 2008; N. multifasciatus: Kohler 1998; N. savoryi: Heg et al. 2005) to classical polyandry (e.g., J. marlieri: Yamagishi and Kohda 1996; J. ornatus: Awata et al. 2005) and cooperative polyandry (J. ornatus: Awata et al. 2005, 2006; C. brichardi: MK et al., unpublished data). While the subordinates of cooperatively polyandrous species are mostly unrelated to the dominants of both breeders (Awata et al. 2005, 2006 showed more than 80 percent of the helpers were unrelated to the dominant breeders in J. ornatus), monogamous and polygynous species show age dependent relatedness between dominants and subordinates, and relatedness declines with age of the subordinates (N. pulcher: Dierkes et al. 2005; N. savoryi: DH et al., unpublished data).
The dispersal patterns of cooperative breeders are relatively well documented in birds and mammals—e.g., in Florida scrub jays (Woolfenden and Fitzpatrick 1990), Seychelles warblers (Eikenaar et al. 2007, 2008), and dwarf mongoose (Creel 1994). Usually, the dispersal patterns of birds are female biased, while they are male biased in mammals (Greenwood 1980, 1983; Greenwood and Harvey 1982; Clarke et al. 1997; Clutton-Brock and Lukas 2012). Sex-dependent differences in delayed natal dispersal directly affect group composition, the opportunity to avoid inbreeding and competition with relatives for resources and/or mates (Pusey and Wolf 1996; West et al. 2002). It also affects the probability of assisting kin, or non-kin, in raising their offspring (e.g., Griffin and West 2003; Koenig and Dickinson 2004). Whereas dispersal in birds and mammals can be studied directly using individually marked group members, information in fish is less detailed and more difficult to obtain in nature due to the challenges of marking fish (most cooperatively breeding species are <10 cm in total length, and subordinates are even smaller). Nevertheless, in N. pulcher short-term observations (e.g., Bergmüller et al. 2005), individual genotyping across years (e.g., Stiver et al. 2004), and evidence from pairwise relatedness analyses (e.g., Dierkes et al. 2005; Stiver et al. 2005, 2007) together suggest that males disperse farther and more often than females, and that subordinates are more related to breeding females than to breeding males. This further indicates higher turnover rates among males due to breeder dispersal or death. However, to our knowledge, natural dispersal has not yet been studied in any other species of cooperatively breeding cichlid.
The purpose of this study is to advance our knowledge of cooperative breeding cichlids by adding information on group structure, helping behavior, within group relatedness and dispersal patterns of the previously unstudied Lake Tanganyika cichlid N. obscurus. Based on a literature search and personal communications, Heg and Bachar (2006) concluded that at least 19 Lamprologini species are cooperative breeders, while the status of N. obscurus was inconclusive. As the cooperative breeding system of N. obscurus has not yet been described, we start with a comprehensive description of group composition, body sizes, territoriality, behavior, and reproductive maturity using gonad sizes. Next, we use within- and between-group pairwise relatedness analyses, based on microsatellite DNA, to estimate opportunities for kin selection and sex-dependent dispersal.
Methods
Study species
Neolamprologus obscurus is a small cichlid (8 cm total length in maximum) endemic to Lake Tanganyika, where it lives under stones in sediment-rich intermediate substrates near shorelines, typically at depths of 5–35 m (Poll 1978; Konings 1998; HT personal observation). N. obscurus occupy territories in which they dig out shelters under stones, which they use for breeding, foraging, and protection from predators and conspecifics. The species’ diet consists mainly of benthic animals, such as insect larvae and shrimp, but may also include zooplankton (HT, unpublished data).
Field observations
The study was conducted at the southern tip of Lake Tanganyika, at Nkumbula Island near Mpulungu (8° 45.2′ S, 31° 05.2′ W), Zambia. Data were collected by SCUBA diving from September to November 2010. The study site was located at a depth of 6.5–8 m along a steep sandy slope with many partially exposed stones (typical diameter, 10–30 cm). It measured approximately 20 × 7 m. This area was subdivided into a 0.5 × 0.5-m grids using a rope to more easily map the home range of each individual. We used the resulting detailed topographic map of all stones to trace the swimming tracks of every individual observed during the study (i.e., breeding males, independent males, breeding females, single males, and helpers; see “Definition of social rank”). Fish were individually identified by their size and distinct natural markings, which consisted of a series of unique gray lines on the head and body (Appendix 1).
Definition of social rank
We recorded two-dimensional swimming tracks of the fish as a continuous line on the topographic map and used the maximum extent of each individual’s swimming tracks to determine its home range.
We observed several large males in the study area. These males had the largest home ranges and sometimes showed aggressive behaviors against each other at the boundaries of their respective territories. Their home ranges overlapped with sexually mature females and other individuals that typically showed submissive and/or social behaviors (see “Behavioral observation” for the definition of submissive behavior and social behavior). These males had mature testes (see results of the gonad analysis), and we therefore defined them as breeding males (Fig. 1a–d).
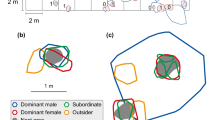
a Home range distribution of N. obscurus in the study area (20 × 7 m): home ranges of breeding males (shaded) and independent males (open). The bar represents 2 m in (a). b–d Schematic representations of home ranges of breeding males (Bm; solid line), breeding females (Bf; dotted line), and single males (Sim; broken line) of three different groups (the number of helpers is listed per breeding female). b A typical group; c the group with the most breeding females; d the group with the most single males. Shelters frequently used by the breeding male are in black, whereas all other shelters and stones are shown in gray. The bar represents 1 m in (b)–(d)
We also found males whose territory did not overlap with any breeding females but remained inside the home range of a breeding male. These males showed aggressive behavior toward their neighboring breeding females and defended their own shelters, but showed submissive and social behaviors (especially by swimming side by side; HT, unpublished data) toward, and were tolerated by, the cohabitant breeding male. We defined these as single males (Fig. 1b, c). Finally, we found that some males fought against neighboring breeding males, but their territories did not overlap with any other individuals and they remained alone. We defined these as independent males (Fig. 1a).
We found several females inside the home ranges of breeding males. These females’ home ranges rarely overlapped, and they showed aggressive behaviors against each other. Almost all had mature ovaries (see results of the gonad analysis), and we therefore defined them as breeding females (Fig. 1b–d).
We also found smaller individuals within the home ranges of breeding females. These small individuals were tolerated by, and typically used the same shelters as, the breeding female but were attacked if they stayed near neighboring females’ home ranges (Fig. 1b–d). They showed aggressive behavior toward both con- and heterospecific individuals, including other breeding females, but showed submissive behavior toward their own breeding female, and removed sand from her shelter. Similar behaviors have been described as costly helping behaviors in other cichlid species (Taborsky 1984, 1985; Grantner and Taborsky 1998; Heg et al. 2005; Heg and Bachar 2006). Thus, we consider them likely to be costly in N. obscurus as well and defined these individuals as helpers.
Finally, we observed small fish inside the shelter of some breeding females. These individuals were small (below 19 mm), rarely overlapped in size with helpers (Table 1), and never emerged from the shelter during observations. They neither removed sand nor were aggressive toward intruders, and we therefore defined them as juveniles.
We defined all individuals that lived within a breeding male’s home range as group members (i.e., the breeding male and all associated breeding females, single males, helpers, and juveniles) and individuals that overlapped only with breeding females as subgroup members (i.e., the breeding female, helpers, and juveniles).
Behavioral observation
We randomly selected 14 groups and four independent males for focal behavioral observations in the study area (N = 14 breeding males, 38 breeding females, 11 single males, 54 helpers, and 4 independent males). Each individual was observed three times within 1 day. Each observation lasted 10 min, summing up to a total of 30 min per individual. Observations were made during three time periods: between 9:00 and 10:30, 10:30 and 12:00, and 14:00 and 15:30. Juveniles were not observed because they did not emerge during the observation period. We recorded pecking frequency as a proxy of feeding behavior both in the water column and on the substrate, the frequency of sand digging from the shelter and sand removal from the home range, the frequency of aggressive behaviors (including overt aggression such as bites, chases, fast approaches, mouth-fights, and restrained aggression such as opercula spreading, also called puffed throat, S-shaped body posture, and fin spreading), the frequency of submissive behaviors (tail-quivering), and the frequency of social behaviors (soft body contact, also called bumping) toward con- and heterospecific individuals. These behaviors are similar to behaviors in N. pulcher/brichardi (described in Taborsky 1984, 1985) and N. savoryi (Heg et al. 2005). We further found that some individuals swam side by side with other individuals that approached the focal individual’s home range. This behavior has also been reported in Neolamprologus meeli as “swim together” behavior (Sunobe and Munehara 2003), and we included it as social behavior because it was only observed among group members (HT, unpublished data).
Fish sampling
After behavioral observations, all N. obscurus inside the study area were captured using gillnets and hand-nets with the help of 30 % clove oil diluted in ethanol and brought to the laboratory. Here, we measured standard length (SL; to the nearest 0.05 mm) and wet body weight (BW; to the nearest 0.001 g). Sex of fish >18 mm was determined by inspection of the genital papilla; sexing is unreliable in individuals <18 mm. After measurement, the fish were anesthetized and euthanized using an overdose of the anesthetic FA100 (10 % solution of eugenol; Tanabe Seiyaku Inc.). The right pelvic fin was preserved in 99.9 % ethanol, and the remaining body was fixed in 10 % formalin solution. After fixation, all N. obscurus bodies were dissected for gonad measurement (GW; mg).
Microsatellite analysis
Genomic DNA was extracted from all of the ethanol-preserved fin tissue samples using the AquaPure Genomic DNA Purification Kit (Bio-Rad). We used seven microsatellite loci for genotyping: 758/773 (Schliewen et al. 2001), Chb1 (Munehara et al. 2001), Pzeb1 and Pzeb3 (van Oppen et al. 1997), TmoM11 (Zardoya et al. 1996), and UME002 and UME003 (Parker and Kornfield 1996). Each forward primer was labeled with a fluorescent dye (FAM, HEX, NED, and VIC). DNA was amplified using the Qiagen Type-it Multiplex PCR Kit, arranging loci with non-overlapping size ranges in each dye, to thus allow co-amplification of all microsatellite loci in a single polymerase chain reaction (PCR). PCR was conducted in a 5-μl volume containing 1 μl genomic DNA and 2× Qiagen Type-it Multiplex PCR Master Mix and microsatellite primer pairs with varying concentrations from 0.03 to 0.09 μM, according to the intensity of the respective amplification products. Amplification was performed using a GeneAmp® PCR System 9700 (Applied Biosystems), with the following program: one cycle at 94 °C for 5 min; 35 cycles at 94 °C for 30 s, 52 °C for 40 s, and 72 °C for 70 s; and one cycle at 72 °C for 20 min. PCR products were analyzed using an ABI 3130xl Genetic Analyzer (Applied Biosystems) and automatically analyzed using GeneMapper® (Applied Biosystems). Characteristics of the seven microsatellite loci are listed in Appendix 2. One breeding male was not included in the analyses because low DNA quality yielded an unreliable microsatellite result.
Statistical analysis
We used separate linear models (LM), generalized models (GLM), or generalized linear mixed models (GLMM) for the analyses. Residuals for all models were checked for over-dispersion and heterogeneity (Bolker et al. 2009). All statistical analyses were performed in R version 2.14.0 (R Development Core Team 2011). GLMMs were performed by using the lme4 package (Bates et al. 2011).
Group composition and group structure
To investigate the difference of body size between each social rank, we used linear models with Gaussian error structure, followed by Tukey HSD post hoc tests. Using separate GLMs, we tested the effect of breeding male body size on breeding male home range size, largest female body size, group size, number of breeding females, or helpers, or juveniles within his group; respectively. In the model of home range size of the breeding male, we used a GLM with gamma error structure and log link function, and in the model of largest female body size, we used a GLM with Gaussian error structure and identity link function. In the rest of the models, we used GLMs with poisson error structure and log link function. We also tested the effects of breeding female body size on breeding female home range size, her subgroup size, number of helpers, or juveniles within her subgroup; respectively, using separate GLMMs. In each GLMM, the identity of the subgroup number was incorporated as a random factor. In the model of breeding female home range size, we used a GLMM with gamma error structure and log link function. In the rest of the model, we used GLMMs with Poisson error structure and log link function. In these LM, GLMs and GLMMs analyses, we performed likelihood ratio test to examine the significance of the explanatory variable.
Behavior
To investigate the difference in digging, aggressive, and feeding behaviors between social ranks, we used separate GLMs for each behavior, respectively. In each of the models, we used GLMs with Poisson error structure and log link function. We set breeding female as reference category to compare each behavior between social ranks in each of the model, because breeding female of other cichlids usually contribute most to territory defense and maintenance and to caring for the offspring (DH, personal observation).
Gonadal analysis
To assess differences in gonadal mass among the four different social ranks of male N. obscurus (breeding male, independent male, single male, and male helper) and two different social ranks of females (breeding female and female helper), we compared gonadal mass among social ranks for each sex by using GLMs with gamma error structure and log link function, followed by Tukey HSD post hoc test. We performed likelihood ratio test to examine the significance of the explanatory variable in GLMs. Next, to examine differences in gonadal investment among social ranks for each sex, we followed Tomkins and Simmons (2002) and used LMs in which log-transformed gonadal mass was compared among social rank, with log-transformed soma mass (log (body mass − gonadal mass)) as covariate, including the interaction between social rank and log soma mass.
Kinship structure
Dyadic estimates of KINSHIP genetic relatedness (Goodnight and Queller 1999) were calculated using KINGROUP v.2.0 software (Konovalov et al. 2004) using background allele frequencies from Konovalov and Heg (2008). We used pairwise relatedness to establish whether this fish lives in kin structured groups by comparing among non-group members, group members, and subgroup members of each pairwise social ranks. Relatedness was analyzed with Mann–Whitney U tests with Bonferroni corrected p values to avoid type I errors.
Results
Group composition and group structure
In total, we found 17 breeding males, 47 breeding females, 13 single males, and 5 independent males in the study area (Fig. 1a). The 17 breeding males had one to eight breeding females each (median, quartiles = 3, 1, 3, N = 17) and zero to three single males (median, quartiles = 0, 0, 1, N = 17) in their home ranges and occasionally visited the group members’ respective shelters. Conversely, the independent males were solitary. Body sizes differed between social ranks (GLM: χ 2 6 = 344.56, P < 0.001) and breeding males were larger than independent males (Tukey HSD test: z = −4.06, P < 0.001; Table 1). Male body size showed a significant relationship with the size of his territory and the number of group members and sizes of his group members (Tables 2 and 3 (breeding male body size (SL))). Breeding females and single males were smaller than breeding males (Tukey HSD test: z = −11.99, P < 0.001 and z = −8.04, P < 0.001, respectively; Table 1) but were of similar size to each other (Tukey HSD test: z = 1.38, P = 0.80; Table 1). Female body size also showed significant relationship with the size of her territory and the number and sizes of her subgroup members (Tables 2 and 3 (breeding female body size (SL))).
Breeding females tolerated up to 10 helpers (median, quartiles per subgroup = 1, 0, 2, N = 47) and up to four juveniles in their home ranges (median, quartiles per subgroup = 0, 0, 1, N = 47). Larger breeding males and larger breeding females had more helpers and juveniles (Table 3 (breeding male body size (SL) and breeding female body size (SL))). Of 57 helpers, 26 were males and 26 were females. For the remaining five, assessing their sex was impossible due to small gonads. Body sizes of male and female helpers did not significantly differ (Tukey HSD test: z = −2.17, P = 0.29; Table 1).
Behavior
All breeding males, independent males, breeding females, and single males had their own shelters within their home range (Fig. 1b–d). Helpers and juveniles typically used the same shelter as the breeding female of their respective subgroup. Fish often entered their shelter and spent time inside their shelters during the behavioral observation period (Table 4). Furthermore, breeding males moved freely within their home ranges and frequently entered breeding females’ shelters (median and quartiles/30 min = 3, 2, 3.5, N = 14) and the shelter of single males (0, 0, 1, N = 14). Breeding females and single males attempted to prevent breeding males from entering their home ranges by side-by-side swimming or from entering their shelters by using intense bumping to push them away from the entrance of their shelters (median and quartiles of behavior toward the breeding male/30 min: breeding female, 1, 0, 1, N = 38; single male, 1, 0.5, 1, N = 11). Breeding males and females showed no significant difference in digging/removing sand but males showed increased aggression toward intruders (Tables 4 and 5). On the other hand, single males showed less digging/removing sand from their shelters compared with breeding females and showed more aggression toward intruders (Tables 4 and 5). Helpers dug and removed sand from the breeding female’s shelters of the same subgroup and showed aggressive behavior toward con- and hetero-specifics but did so significantly less frequently than breeding females (Tables 4 and 5). Helpers also showed submissive behavior mostly toward breeders of their own group (median and quartiles/30 min = 0.5, 0, 1.75, N = 54), except for five cases in which it was directed toward breeders of another group or subgroup. Feeding rates of fish in each social rank differed significantly (Tables 4 and 5).
Reproductive potential of each social rank
Gonads of independent males, single males, and helpers of both sexes appeared very thin and underdeveloped, and accordingly, we found significant difference in gonad masses between social ranks in each sex (GLM: χ 2 3 = 185.83, P < 0.001 in males, χ 2 1 = 99.49, P < 0.001 in females). Gonad masses of independent males, single males, and male and female helpers weighed significantly less than those of both breeding males and females (Tukey HSD test: z = −7.36, P < 0.001, z = −10.36, P < 0.001, z = −11.19, P < 0.001, respectively, in males; z = −11.13, P < 0.001 in females; Table 6). The analysis of testis investment in males showed a significant interaction of social rank and log soma mass, while the same was true of ovary investment in females (LM: t = −7.14, P < 0.001 in males, t = −2.86, P = 0.006 in females; Fig. 2; Table 7).
Relatedness
We calculated pairwise mean relatedness among breeding males, breeding females, single males, and helpers/juveniles and compared among same subgroup members, same group but not the same subgroup members, and different group members of each social rank (Fig. 3). The mean relatedness of breeding males vs. helpers/juveniles from the same group was significantly higher than that of individuals from different groups (mean relatedness ± SE, within group: 0.33 ± 0.03, N = 90; between group: 0.03 ± 0.01, N = 1430; Mann–Whitney U test, z = 9.40, P < 0.001; Fig. 3a); we found the same trend for breeding females vs. helpers/juveniles from the same subgroup, and between subgroups within the same group (mean relatedness ± SE, within subgroup, 0.42 ± 0.02, N = 93; between subgroup from the same group, 0.15 ± 0.01, N = 301; Mann–Whitney U test, z = 8.63, P < 0.001; Fig. 3b). These results suggest that helpers and juveniles are related to breeding males of the same group and to females of the same subgroup. Furthermore, mean relatedness among helpers/juveniles also declined from subgroup to non-subgroup, to non-group members (mean relatedness ± SE, within subgroup, 0.39 ± 0.02, N = 181; between subgroups from the same group, 0.20 ± 0.01, N = 346; and between groups, 0.04 ± 0.00, N = 3938; Mann–Whitney U test, within subgroup vs. between subgroup, z = 8.63, P < 0.001; between subgroup vs. between group, z = 10.98, P < 0.001; Fig. 3b), suggesting that helpers and juveniles are full siblings in the same subgroup and are half siblings in the same group.
Mean relatedness (±SE) of a breeding males (Bm), b breeding females (Bf), c single males (Sim), and d helpers/juveniles to members of the same subgroup (white circle), different subgroups within the same group (gray circle), and between non-group members (black circle). A group is defined by the home range of the breeding male and a subgroup is defined by the home range of the breeding female (see results and examples in Fig. 1b–d). NS not significant; **P < 0.01; ***P < 0.001 from Mann–Whitney U tests after Bonferroni correction
The relatedness of breeding males to single males was highly variable within the same group (range of relatedness = −0.30 to 0.67), and the mean relatedness among breeding males and single males was much higher than that between different groups (mean relatedness ± SE, within group, 0.32 ± 0.12, N = 8; between group, 0.07 ± 0.02, N = 136; Mann–Whitney U test, z = 2.17, P = 0.03; Fig. 3a). This result indicates that some single males were offspring, or full or half siblings of the breeding male. The within-group relatedness of single males to helpers/juveniles was also much higher than that between groups (mean relatedness ± SE, within group, 0.21 ± 0.04, N = 49; between group, 0.02 ± 0.01, N = 806; Mann–Whitney U test, z = 4.97, P < 0.001; Fig. 3c), suggesting that single males are half siblings of helpers and juveniles of the same group.
Finally, the mean relatedness of breeding males vs. breeding females within the same group was similar to the mean relatedness between groups (mean relatedness ± SE, within group, 0.05 ± 0.05, N = 41; between group, 0.03 ± 0.01, N = 647; Mann–Whitney U test, z = 0.07, P = 0.95; Fig. 3a), suggesting that breeding females are not related to breeding males of the same group. Furthermore, mean relatedness among breeding females within the same group was not significantly different compared with the mean relatedness between breeding females with other groups (mean relatedness ± SE, within group: 0.13 ± 0.03, N = 59; between group, 0.06 ± 0.01, N = 844; Mann–Whitney U test, z = 1.93, P = 0.10; Fig. 3b), indicating that breeding females of the same group are unrelated.
Discussion
We provided the first comprehensive description of the cooperative breeding system of N. obscurus, a Lake Tanganyika cichlid previously unknown to show such behavior. We also obtained novel results on group structure, reproductive potential, and relatedness.
Cooperative breeding
Helpers of N. obscurus were allowed to remain inside breeders’ home ranges, and assisted the breeding pair with territory maintenance (digging/removing sand from shelters) and defense (aggressive behavior toward intruders). Furthermore, helpers showed submissive behaviors mostly toward the breeding male and female of their own group and subgroup, which has also been reported in other cooperatively breeding cichlids, e.g., N. pulcher/brichardi (Taborsky 1984, 1985; Wong and Balshine 2010) and N. savoryi (Heg et al. 2005). However, we did not observe direct brood care behavior, such as cleaning and fanning of eggs or caring for fry, maybe because eggs were laid inside the shelter and juvenile spent all the time inside the shelter. Still, as most helpers remained in or near the shelters in which juveniles spend most of their time and chased intruders away from the breeding female’s home range, they are likely to provide survival benefits to juveniles. Further observations and experimental verifications will help to better understand the effects of helping behavior on survival (e.g., Brouwer et al. 2005).
Most of the N. obscurus helpers were closely related to the breeding pair of the same group or subgroup. In many cases, helpers were genetically related to both breeders, which would facilitate kin selected benefits in this species (Dierkes et al. 2005). However, high variability in pairwise helper vs. breeding pair relatedness of the same group and/or subgroup (as in other cichlid species, e.g., N. pulcher: Dierkes et al. 2005; N. multifasciatus: Kohler 1998) suggests that some of the helpers were not related to the breeding pair of their own group and/or subgroup. Thus, opportunities for kin selected benefits could exist for many but not all helpers in N. obscurus.
Group structure, mating systems, and reproductive potential
The mating system of N. obscurus is harem polygyny, wherein larger males retain more females and offspring. This pattern of mating appears to be common in cooperatively breeding cichlids: N. multifasciatus and N. savoryi also show a haremic mating system (Kohler 1998; Heg et al. 2005), and the majority of females were also part of a polygynous group in a detailed study of N. pulcher/brichardi (Desjardins et al. 2008). The relationship between soma mass and gonads of both sexes indicates that males and females follow a clear ontogenetic trajectory. They increase their soma (but not their gonads, which remain regressed) up to ca. 0.0 log soma mass in male and ca. −0.5 log soma mass in female (Fig. 2). At that time point, the probability of males and females to acquire a territory or breeding position starts to rapidly increase and at the same time their gonads are also developed. Interestingly, there is no indication of a flattering-off of this effect in breeding males and females, which may be due to older and larger breeding males having more females to fertilize, and older and larger breeding females laying relatively more eggs compared with younger and smaller breeding females.
In N. obscurus, breeding females and single males were significantly smaller than breeding males. The ovaries of breeding females were significantly larger than those of female helpers, and testis size of single males were similar to those of male helpers but smaller than those of breeding males. The growth rates of breeding females and single males did not differ in the field (HT, unpublished data), indicating that females can start breeding earlier and at a smaller size than males. In contrast, single males cannot easily compete for territorial vacancies or mates because in doing so, they must compete with other males (see, e.g., Heg et al. 2011). The testis mass of single and helper males suggest reproductive suppression by the breeding male, which was also found in N. pulcher, or investment in growth at the expense of gonads (Fitzpatrick et al. 2005, 2008). If the benefit of staying inside the natal group exceeds the costs of dispersal for single males (e.g., due to predation risk, competitive costs, and energetic costs for digging a new shelter), delayed dispersal from the natal group will be the better choice (Heg et al. 2004).
Single males
Notably, single males which were highly related to breeding males but did not help them were observed in approximately 45 % of breeding male home ranges. Pairwise relatedness suggests that single males were often offspring, or full or half siblings of the breeding male of the same group. Such males were only rarely reported in N. savoryi and N. pulcher (“independents” in Heg et al. 2005; Wong and Balshine 2010). Single males removed sand from their shelters and showed aggressive behavior toward intruders of their home ranges, while their shelters were often entered by the breeding male. Why single males were tolerated by breeding males remains unknown, but we propose two non-mutually exclusive explanations. First, the diet of N. obscurus consists largely of benthic animals, including shrimp, mostly found between and under the shelter rocks. Thus for N. obscurus, the shelter is potentially also an important feeding resource, and breeding males might exploit the shelters of single males accordingly. Single males maintain their shelters not to be buried by the sand, and thus breeding males were able to access to the feeding resource in his territory. Second, single males provide benefits to their breeding male due to shared territory defense; i.e., the likelihood of an intruder leaving the breeding male’s home range is increased by the presence of single males. In both cases, the likelihood of toleration might be enhanced because single males do not impose significant costs on the fitness of breeding males (as they are prevented from participating in reproduction by breeders, or they are strategically suppressed to invest their gonads; Fitzpatrick et al. 2005). As most single males are highly related to the breeding male of their own group, parental nepotism should also allow single males to stay (e.g., Ekman et al. 1999).
From the perspective of single males, there also might be several possible reasons to stay. First, living in a group might be beneficial for single males, e.g., due to group augmentation (Kokko et al. 2001). Second, single males may be in the process of “budding-off” their own territory, while waiting to grow sufficiently to recruit their own females (e.g., Komdeur and Edelaar 2001). Indeed, some of the single males extended their home ranges and overlapped with small females outside of the breeding male’s home range, which support this idea (HT, unpublished data). Third, a chance of territory inheritance exists for single males if the breeding male disperses or dies (Balshine-Earn et al. 1998). All of the points are not mutually exclusive, and future work will resolve the factors affecting single males to stay inside the breeding male’s home range.
Dispersal patterns inferred from pairwise relatedness estimates
We used pairwise relatedness estimates to infer likely patterns of dispersal in N. obscurus, as direct dispersal observations could not be obtained. We expected that individual N. obscurus typically disperse to obtain an immediate breeding position or join a new group as a helper (Stiver et al. 2004; Wong and Balshine 2010). Many helpers were highly related to the breeding female and breeding males, suggesting that juveniles are mostly the retained offspring of these breeding pairs, which became helpers in their natal group. Furthermore, the relatedness of single and breeding males of the same group were high, while that of breeding females and males were low. As single males and breeding females were of similar size, dispersal patterns are most likely sex-dependent in N. obscurus. While female helpers will disperse from their natal group and become breeding females in other groups, male helpers will become single males in their natal group or remain independent.
The low relatedness among breeding females both within and among groups adds more support to the notion that breeding females may immigrate into breeding male’s territory from other groups. The distance between the nearest groups in our study area was 0.38 m ± 0.35 SD (N = 17 groups; Fig. 1a). Thus, movement between neighboring groups should be relatively easy for N. obscurus. Alternatively, females may not disperse, and a high turnover occurs among breeding males (i.e., death or emigration). This explanation is less likely, however, because if the breeding male is replaced, the relatedness of not only breeding females and males but also of single and breeding males should drop (except if a male full sibling of the current breeding male takes the breeder position). The discrepancy between these two pairwise estimates corroborates our prediction that females will show natal dispersal, whereas males are more likely to stay, at least until they are sufficiently grown. In a previous study of N. pulcher dispersal, males dispersed farther and more often than females (Stiver et al. 2004, 2007). In birds, males may benefit most from philopatry, as a male’s territory quality can influence both mate attraction and the survival of young (Pusey 1987). We conclude that female N. obscurus might disperse from the natal group earlier than males, and that this difference will reflect intersexual differences in the timing of reproductive onset associated with harem mating systems. Additional work is needed to determine precisely how dispersal varies with individual sex and size to fully explore the relationship between rank change and dispersal, and to shed more light on the factors driving dispersal patterns in cooperative breeding cichlids.
Conclusion
In summary, we introduced a remarkable new example of cooperative breeding system in the Lake Tanganyika cichlid N. obscurus and provided evidence for sex biased dispersal in this species. The fact that a recent phylogeny places N. obscurus in a different lineage than all other cooperatively breeding cichlids (Sturmbauer et al. 2010) underlines the potential of this species helping us to understand the evolution of cooperative systems in fish.
References
Awata S, Kohda M (2004) Parental roles and the amount of care in a bi-parental substrate brooding cichlid: the effect of size differences within pairs. Behaviour 141:1135–1149
Awata S, Munehara H, Kohda M (2005) Social system and reproduction of helpers in the cooperatively breeding cichlid fish Julidochromis ornatus in Lake Tanganyika: field observations and parentage analyses. Behav Ecol Sociobiol 58:506–516
Awata S, Heg D, Kohda M, Munehara H, Kohda M (2006) Testis size depends on social status and the presence of male helpers in the cooperatively breeding cichlid Julidochromis ornatus. Behav Ecol 17:372–379
Balshine-Earn S, Neat F, Reid H, Taborsky M (1998) Paying to stay or paying to breed? Field evidence for direct benefits of helping behavior in a cooperatively breeding fish. Behav Ecol 9:432–438
Bates D, Maechler M, Bolker B (2011) lme4: linear mixed-effects models using S4 classes. R package version 0.999375-39. http://CRAN.R-project.org/package=lme4
Bergmüller R, Heg D, Taborsky M (2005) Helpers in a cooperatively breeding cichlid stay and pay or disperse and breed, depending on ecological constraints. Proc R Soc Lond B 272:325–331
Bolker BM, Brooks ME, Clark CI, Geange SW, Poulsen JR, Stevens MH, White JS (2009) Generalized linear mixed models: a practical guide for ecology and evolution. Trend Ecol Evol 24:127–135
Brouwer L, Heg D, Taborsky M (2005) Experimental evidence for helper effects in a cooperatively breeding cichlid. Behav Ecol 16:667–673
Clarke A, Saether B, Roskaft E (1997) Sex biases in avian dispersal: a reappraisal. Oikos 79:429–438
Clutton-Brock TH, Lukas D (2012) The evolution of social philopatry and dispersal in female mammals. Mol Ecol 21:472–492
Cockburn A (2006) Prevalence of different modes of parental care in birds. Proc R Soc Lond B 273:1375–1383
Creel SR (1994) Inclusive fitness and reproductive strategies in dwarf mongooses. Behav Ecol 5:339–348
Desjardins JK, Fitzpatrick JL, Stiver KA, van der Kraak GJ, Balshine S (2008) Costs and benefits of polygyny in the cichlid Neolamprologus pulcher. Anim Behav 75:1771–1779
Dierkes P, Heg D, Taborsky M, Skubic E, Achmann R (2005) Genetic relatedness in groups is sex-specific and declines with age of helpers in a cooperatively breeding cichlid. Ecol Lett 8:968–975
Duftner N, Sefc K, Koblmüller S, Salzburger W, Taborsky M, Sturmbauer C (2007) Parallel evolution of facial stripe patterns in the Neolamprologus brichardi/pulcher species complex endemic to Lake Tanganyika. Mol Phylogenet Evol 45:706–715
Eikenaar C, Richardson DS, Brouwer L, Komdeur J (2007) Parent presence, delayed dispersal, and territory acquisition in the Seychelles warbler. Behav Ecol 18:874–879
Eikenaar C, Richardson DS, Brouwer L, Komdeur J (2008) Sex biased natal dispersal in a closed, saturated population of Seychelles warblers Acrocephalus sechellensis. J Avian Biol 39:73–80
Ekman J, Bylin A, Tegelström H (1999) Parental nepotism enhances survival of retained offspring in the Siberian jay. Behav Ecol 11:416–420
Feeney W, Medina I, Somveille M, Heinsohn R, Hall ML, Mulder RA, Stain JA, Kilner RM, Langmore NE (2013) Brood parasitism and the evolution of cooperative breeding in birds. Science 342:1506–1508
Fitzpatrick JL, Desjardins JK, Stiver KA, Montgomerie R, Balshine S (2005) Male reproductive suppression in the cooperatively breeding fish Neolamprologus pulcher. Behav Ecol 17:25–33
Fitzpatrick JL, Desjardins JK, Milligan N, Stiver KA, Montgomerie R, Balshine S (2008) Female-mediated causes and consequences of social status change in a social fish. Proc R Soc Lond B 275:929–936
Goodnight KF, Queller DC (1999) Computer software for performing likelihood tests of pedigree relationship using genetic markers. Mol Ecol 8:1231–1234
Grantner A, Taborsky M (1998) The metabolic rates associated with resting, and with the performance of agonistic, submissive and digging behaviours in the cichlid fish Neolamprologus pulcher (Pisces: Cichlidae). J Comp Physiol B 168:427–433
Greenwood PJ (1980) Mating systems, philopatry and dispersal in birds and mammals. Anim Behav 28:1140–1162
Greenwood PJ (1983) Mating systems and the evolutionary consequences of dispersal. In: Swingland IR, Greenwood PJ (eds) The ecology of animal movement. Oxford University Press, Oxford, pp 116–131
Greenwood PJ, Harvey PH (1982) The natal and breeding dispersal of birds. Annu Rev Ecol Syst 13:1–21
Griffin AS, West SA (2003) Kin discrimination and the benefit of helping in cooperatively breeding vertebrates. Science 302:634–636
Heg D, Bachar Z (2006) Cooperative breeding in the Lake Tanganyika cichlid Julidochromis ornatus. Environ Biol Fish 76:265–281
Heg D, Bachar Z, Brouwer L, Taborsky M (2004) Predation risk is an ecological constraint for helper dispersal in a cooperatively breeding cichlid. Proc R Soc Lond B 271:2367–2374
Heg D, Bachar Z, Taborsky M (2005) Cooperative breeding and group structure in the Lake Tanganyika cichlids Neolamprologus savory. Ethology 111:1017–1043
Heg D, Rothenberger S, Schürch R (2011) Habitat saturation, benefits of philopatry, relatedness, and the extent of co-operative breeding in a cichlid. Behav Ecol 22:82–92
Koenig WD, Dickinson JL (2004) Ecology and evolution of cooperative breeding in birds. Cambridge University Press, Cambridge
Kohler U (1998) Zur Struktur und Evolution des Sozialsystems von Neolamprologus multifasciatus (Cichlidae, Pisces), dem kleinsten Schneckenbuntbarsch des Tanganjikasees. Shaker Verlag, Aachen
Kokko H, Johnstone RA, Clutton-Brock TH (2001) The evolution of cooperative breeding through group augmentation. Proc R Soc Lond B 268:187–196
Komdeur J, Edelaar P (2001) Male Seychelles warblers use territory budding to maximize lifetime fitness in a saturated environment. Behav Ecol 12:706–715
Konings A (1998) Tanganyika cichlids in their natural habitat. Cichlid Press, El Paso
Konovalov DA, Heg D (2008) TECHNICAL ADVANCES: a maximum-likelihood relatedness estimator allowing for negative relatedness values. Mol Ecol Resour 8:256–263
Konovalov DA, Manning C, Henshaw MT (2004) KINGROUP: a program for pedigree relationship reconstruction and kin group assignments using genetic markers. Mol Ecol Notes 4:779–782
Lukas D, Clutton-Brock T (2012) Cooperative breeding and monogamy in mammalian societies. Proc R Soc Lond B 279:2151–2156
Lukas D, Clutton-Brock TH (2013) The evolution of social monogamy in mammals. Science 341:526–530
Munehara H, Awata S, Katoh R, Kohda M, Sunobe T (2001) Primer sequences and cross-species amplification for parentage discrimination of Tanganyikan cichlid fishes. Bull Fac Fish, Hokkaido Univ 52:131–133
Parker A, Kornfield I (1996) Polygynandry in Pseudotropheus zebra, a cichlid fish from Lake Malawi. Environ Biol Fish 47:345–352
Poll M (1978) A new cichlid fish genus-Lamprologus Schth. Description of four new species from Lake Tanganyika [in Central Africa]. B Cl Sci Ac Roy Belg 64:725–758
Pusey AE (1987) Sex-biased dispersal and inbreeding avoidance in birds and mammals. Trend Ecol Evol 2:295–299
Pusey AE, Wolf M (1996) Inbreeding avoidance in animals. Trend Ecol Evol 11:201–206
R development core team (2011) R: a language and environment for statistical computing. R Foundation for Statistical Computing, Vienna
Rossiter A (1993) Studies on the biology of Neolamprologus multifasciatus. In: Nagoshi M, Yanagisawa Y, Kawanabe H (eds) Ecological and limnological study on Lake Tanganyika and its adjacent regions VIII. Kyoto University Press, Kyoto, pp 32
Sato T, Gashagaza MM (1997) Shell-brooding cichlid fishes of Lake Tanganyika: their habitats and mating systems. In: Kawanabe H, Hori M, Nagoshi M (eds) Fish communities in Lake Tanganyika. Kyoto University Press, Kyoto, pp 221–240
Schliewen U, Rassmann K, Markmann M, Markert J, Kocker T, Tautz D (2001) Genetic and ecological divergence of a monophyletic cichlid species pair under fully sympatric conditions in the Lake Ejagham, Cameroon. Mol Ecol 10:1471–1488
Schradin C, Lamprecht J (2000) Female-biased immigration and male peace-keeping in groups of the shelldwelling cichlid fish Neolamprologus multifasciatus. Behav Ecol Sociobiol 48:236–242
Schradin C, Lamprecht J (2002) Causes of female emigration in the group-living cichlid fish Neolamprologus multifasciatus. Ethology 108:237–248
Stiver KA, Dierkes P, Taborsky M, Balshine S (2004) Dispersal patterns and status change in a co-operatively breeding cichlids Neolamprologus pulcher: evidence from microsatellite analyses and behavioural observations. J Fish Biol 65:91–105
Stiver KA, Dierkes P, Taborsky M, Gibbs HL, Balshine S (2005) Relatedness and helping in fish: examining the theoretical predictions. Proc R Soc Lond B 272:1593–1599
Stiver KA, Desjardins JK, Fitzpatrick JL, Neff B, Quinn JS, Balshine S (2007) Evidence for size and sex-specific dispersal in a cooperatively breeding cichlid fish. Mol Ecol 16:2974–2984
Sturmbauer C, Salzburger W, Duftner N, Schelly R, Koblmuller S (2010) Evolutionary history of the Lake Tanganyika cichlid tribe Lamprologini (Teleostei: Perciformes) derived from mitochondrial and nuclear DNA data. Mol Phylogenet Evol 57:266–284
Sunobe T, Munehara H (2003) Mating system and kin relationship between adults and young in the shell-brooding cichlid fish Neolamprologus meeli in Lake Tanganyika. J Ethol 21:87–92
Taborsky M (1984) Broodcare helpers in the cichlid fish Lamprologus brichardi: their costs and benefits. Anim Behav 32:1236–1252
Taborsky M (1985) Breeder-helper conflict in a cichlid fish with broodcare helpers: an experimental analysis. Behaviour 95:45–75
Taborsky M (1994) Sneakers, satellites, and helpers: parasitic and cooperative behavior in fish reproduction. Adv Stud Behav 23:1–100
Taborsky M (2001) The evolution of parasitic and cooperative reproductive behaviors in fishes. J Hered 92:100–110
Taborsky M, Limberger D (1981) Helpers in fish. Behav Ecol Sociobiol 8:143–145
Tommkins JL, Simmons LW (2002) Measuring relative investment: a case study of testes investment in species with alternative male reproductive tactics. Anim Behav 63:1009–1016
van Oppen MJH, Rico C, Deutsch JC, Turner GF, Hewitt GM (1997) Isolation and characterization of microsatellite loci in the cichlid fish Pseudotropheus zebra. Mol Ecol 6:387–388
West SA, Pen I, Griffin AS (2002) Cooperation and competition between relatives. Science 296:72–75
Wisenden BD (1999) Alloparental care in fishes. Rev Fish Biol Fish 9:45–70
Wong M, Balshine S (2010) The evolution of cooperative breeding in the African cichlid fish, Neolamprologus pulcher. Biol Rev 86:511–530
Woolfenden GE, Fitzpatrick JW (1990) Florida Scrub Jays: a synopsis after 18 years of study. In: Stacey PB, Koenig WD (eds) Cooperative breeding in birds: long-term studies of ecology and behavior. Cambridge University Press, New York, pp 241–266
Yamagishi S, Kohda M (1996) Is the cichlid fish Julidochromis marlieri polyandrous? Ichthyol Res 43:469–471
Zardoya R, Vollmer DM, Craddock C, Streelman JT, Karl S, Meyer A (1996) Evolutionary conservation of microsatellite flanking regions and their use in resolving the phylogeny of cichlid fishes (Pisces: Perciformis). Proc R Soc Lond B 263:1589–1598
Acknowledgments
We thank Tetsumi Takahashi, Kazutaka Ota, Haruki Ochi, Hiroki Hata, Michio Hori, and the staff of the Lake Tanganyika Research Unit, Mpulungu, Zambia, especially Harris Phiri, Danny Syniynza, Ruben Shapola, and Henry Simpembwa for supporting our studies at the field. We are grateful to Satoshi Nanami and Sho Furuichi for statistical discussions and Joachim Frommen and three anonymous referees for helpful comments on early versions of this manuscript. This work was financially supported by Grants-in-Aid from the Ministry of Education, Culture, Sports, Science and Technology, Japan, to MK.
Ethical standards
The research presented here was conducted with permission from the Zambian Ministry of Agriculture, Food and Fisheries and complies with current Zambian law. We treated fish in compliance with the guidelines of the Animal Care and Use Committee of Osaka City University, the Japan Ethological Society, and the Ichthyological Society of Japan.
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by J. Frommen
Rights and permissions
About this article
Cite this article
Tanaka, H., Heg, D., Takeshima, H. et al. Group composition, relatedness, and dispersal in the cooperatively breeding cichlid Neolamprologus obscurus . Behav Ecol Sociobiol 69, 169–181 (2015). https://doi.org/10.1007/s00265-014-1830-8
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00265-014-1830-8