Abstract
Purpose
To present a novel surgical technique for the Bernese peri-acetabular osteotomy (PAO) using electromagnetic navigation (EMN) and patient-specific templates (PST), and to evaluate it against the traditional fluoroscopic technique.
Methods
We included 40 dysplastic hips. All PAOs were performed using PST and EMN. We recorded learning-related complications. For the purpose of acetabular fragment correction analysis, patients were divided into two groups. In the study group (EMN group, 30 hips), the acetabular fragment was reoriented with the help of EMN. In the control group (XR group, 10 hips), the acetabular fragment was reoriented using fluoroscopy. We compared the difference between the planned and achieved position of the acetabular fragment and outcomes between both groups.
Results
Two major complications occurred in four PAOs in the XR group only (first ten PAOs). The average absolute difference in planned and achieved lateral centre -edge angle (LCEA) and acetabular index (AI) was 1.2° ± 1.5° and 1.1° ± 2° for the EMN and 7° ± 6.1° and 6.3° ± 6.3° for the XR group (p = 0.02; p = 0.03). The average surgery duration was 183 ± 32 minutes for the EMN and 203 ± 42 minutes for the XR group (p = 0.19). At the last follow-up, the average Harris Hip Score (HHS) value was 88 ± 12 in the EMN and 86 ± 14 in the XR group (p = 0.84).
Conclusions
Our study indicates that PAO performed with EMN and PST seems to be a safe and reproducible procedure with a short learning curve. Additionally, navigated reorientation of the acetabular fragment is significantly more accurate than the fluoroscopic technique.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Shallow acetabulum with reduced femoral coverage is the hallmark of developmental dysplasia of the hip (DDH), which manifests clinically as regional pain, fast tiring, instability, and pathologically increased range of motion [1]. Residual congenital dysplasia of the acetabulum is the main indication for peri-acetabular osteotomy (PAO) [2,3,4]. The purpose of PAO is to reduce symptoms and delay osteoarthritis (OA) progression by optimizing the acetabular fragment’s position, resulting in improved coverage of the femoral head and increased joint stability [5]. The most versatile is the Bernese PAO [2, 3], with good long-term results [5, 6]. The classic PAO technique is demanding, performed only by highly skilled surgeons. Osteotomy cuts and reorientation of the acetabulum are traditionally performed freehand. However, many surgeons take the advantage of intra-operative fluoroscopy [2, 7], which delivers two-dimensional (2D) information (plain of osteotomy cuts and acetabular fragment position) about very complex three-dimensional (3D) structures and its anatomical relations [8]. Anatomical complexities are the main reason for the very steep learning curve with an increased incidence of major complications in the first 20–50 patients, as published by different authors [2, 3, 7, 9,10,11,12]. As pointed out by Trousdale and Cabanela [13], the learning curve for PAO is long.
Since nobody was performing PAO for almost two decades in our country, we were motivated to reintroduce the technique, which was abandoned in the nineties of the past century due to the demanding technique and unfavourable midterm results [14]. However, the revival was reasonable because the means to upgrade the surgical technique became available. We decided to improve the classic surgical technique by using electromagnetic navigation (EMN) system and patient-specific templates (PST), with the fundamental goal to reduce the learning-related complications and improve the accuracy, repeatability, and safety of PAO [15]. In all PAOs, we used jigs and navigation for optimal execution of the planned osteotomy cuts but we performed the first ten reorientations of the acetabulum with traditional fluoroscopic technique. Soon, we noted that intra-operative fluoroscopy is time-consuming and unreliable in the estimation of the true reoriented acetabular position. Consequently, we also developed a computer-based navigation technique for acetabular fragment positioning with the real-time visualization of its real-time position in relation to the planned position on the monitor.
The first aim of our study was to analyze the impact of EMN and PST on learning-related complications. The second goal was to compare the accuracy of the acetabular fragment position performed with EMN and traditional fluoroscopic technique.
Methods
Study design and patients selection
We conducted a retrospective data analysis of a prospective longitudinal cohort on 40 dysplastic hips in 35 patients with no or slight OA (Tönnis grade 0–1) on pre-operative x-ray (Fig. 1), having persistent pain at daily activities refractory to conservative treatment, scheduled for PAO between April 2013 and May 2019. All patients selected for the surgery underwent pre-operative computed tomography (CT) of the hip and pelvis for pre-operative planning and personalized PST design and manufacturing.
In all patients, in both groups, the four osteotomy cuts of the PAO were performed with the help of EMN and PST (Video 1). In the study group (EMN group), including 30 patients, the reorientation of the acetabular fragment was performed with the help of EMN with the intra-operative real-time 3D visualization of the fragment on the monitor (Video 2). In the control group (XR group), including the first ten operated upon patients, the reorientation of the acetabular fragment was controlled with intra-operative fluoroscopy. Two high-volume hip surgeons with limited experience with PAO (RM and RT) performed all the procedures. The preparations included numerous learning visits to experienced institutions and several cadaver surgical procedures [16].
We collected the following patient data: age, sex, side, body mass index (BMI), number of complications, surgery duration, estimated blood loss (EBL), Harris Hip Score (HHS), lateral centre-edge angle (LCEA), acetabular index (AI), and the length of follow-up.
Pre-operative planning and surgical procedure
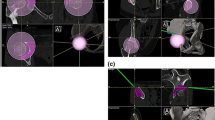
In the first phase, a DICOM format file from a pre-operative CT scan is uploaded into a medical software application (EBS, Ekliptik, d.o.o., Ljubljana, Slovenia) where a virtual 3D model of the pelvis and the hips is generated (Video 1). The surgeon and the software specialist plan the bone cuts and determine the desired acetabular fragment position and orientation on a 3D virtual pelvic model (Fig. 2, Video 1) taking into consideration also the virtual pre-operative hip range of motion (ROM) trial estimates to avoid any potential impingement. We also design the PST congruent to the exposed bones with holes for guidance of the Kirschner wires along with the planned posterior acetabular cut and a plane for the guidance of the superior iliac cut (Video 1).
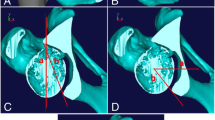
In the second phase, we manufacture the PST made of biocompatible plastic (VisiJet, 3D systems, Rock Hill, SC, USA) for guiding the supra- and retroacetabular osteotomy (Fig. 3, Video 1).
The third phase is the surgery. The patient is lying supine on the radiolucent operating table. The skin incision starts in the inguinal fold extending approximately 2 cm below the anterior superior iliac spine to the middle third of the iliac crest. The soft tissue exposure is then continued as described by Siebenrock et al. [17]. Next, the reference sensor is carefully fixed onto the iliac crest on the Steinmann pin–like holder and the acquisition of pelvic anatomy is accomplished with the pointer (Video 1). The accuracy of the acquisition is then checked and rechecked with the pointer and navigated chisel. The surgery continues with the execution of ischial osteotomy, performed through infra-articular space as described by Siebenrock et al. [17], with the help of navigated curved osteotome (Video 1). The pubic osteotomy entry point is then marked with the navigated osteotome and finished with a straight non-navigated osteotome (Video 1). Next, we position the PST on the iliac crest, and two Kirschner wires are inserted through pre-designed holes in the template into the posterior column (Video 1). The retroacetabular osteotomy is cut along K-wires, and the superior iliac cut along the predesigned plane on the PST. In the EMN group, we position the measuring sensor onto the fragment before we mobilize it and then we navigate it to the pre-operatively planned position with the help of the EMN (Video 1, Video 2). In the XR group, its preplanned final position was determined with the help of fluoroscopy. The definitive osteosynthesis of the reoriented fragment with three 4.5-mm cortical screws was the same in both groups.
Post-operative evaluation
On the follow-up visit 3 months after the surgery, major complications were recorded and standing anteroposterior radiographs of hips and pelvis were performed in all patients. With the use of EBS software (Ekliptik, d.o.o., Ljubljana, Slovenia) we also created, 2D renders of hips and pelvis from the preoperative 3D models of hips and pelvis, with the virtually reoriented position of the acetabular fragment according to the planning (Fig. 4). The 2D pelvic renders were adjusted to the real post-operative radiographs of hips and pelvis (Fig. 5). The acetabular fragment position on the 2D renders (LCEA and AI) represented the basis for comparative analysis of the difference between planned and achieved LCEA and AI for both groups.
Blind radiographic measurements were performed in mediCAD software (mediCAD Hectec GmbH, Altdorf near Landshut, Germany) with the nearest reading of 1 mm by a software specialist and confirmed by a single independent investigator. For each patient in both groups, we measured the planned and achieved LCEA and AI. HHS was recorded at the last follow-up.
Statistical analysis
Age, BMI, surgery duration, EBL, HHS, and absolute difference in planned and executed LCEA and AI between both groups were compared using Student’s t test. Statistical analyses were performed using SPSS version 25.0 (IBM SPSS Statistics, Armonk, NY, USA). p < 0.05 was considered significant.
Results
We performed the surgeries on 40 hips (26 right, 14 left) in 35 patients (29 females, 6 males). The average follow-up was 2.87 ± 1.13 years for the EMN group and 6.18 ± 0.92 years for the XR group. The patients’ characteristics (age, BMI) and pre-operative parameters (LCEA, AI, HHS) are summarized in Table 1. There were no significant differences in patients’ characteristics and pre-surgical parameters between the groups. No patient was lost to follow-up.
Of the 40 surgeries, 29 had no complications. Two major complications occurred in the XR group (1 peripheral peroneal nerve dysfunction that improved after one year follow-up leaving residual hypesthesia, and one popliteal deep venous thrombosis with no sequelae). No major complications were observed in the EMN group. One hip in each group was later (2 and 5 years after index procedure) converted to THA. There were 3 minor complications in the XR group (1 transient dysesthesia of lateral femoral cutaneous nerve, 1 minor heterotrophic ossification (Brooker 1–2), 1 symptomatic hardware removal) and six minor complications in the EMN group (1 delayed wound closure, 5 symptomatic hardware removals, 1minor heterotrophic ossification (Brooker 1–2)).
The average surgery duration was 183 ± 32 minutes for the EMN group and 203 ± 42 min for the XR group (p = 0.19). The average EBL was 652 ± 318 ml for the EMN group and 700 ± 306 ml for the XR group (p = 0.67). The average absolute difference in planned and achieved LCEA and AI was 1.2° ± 1.5° and 1.1° ± 2° for the EMN group and 7° ± 6.1° and 6.3° ± 6.3° for the XR group (p = 0.02; p = 0.03). At the last follow-up, the average HHS value was 88 ± 12 in the EMN group and 86 ± 14 in the XR group (p = 0.84). The outcomes are summarized in Table 2.
Interestingly, there was also one patient in our cohort where the reorientation of the acetabular fragment was performed with intra-operative fluoroscopy on one side (6th consecutive PAO) and with the help of EMN on the other side (12th consecutive PAO, 2nd in the EMN group). We noted a faster procedure (20 min shorter), and better accuracy of the acetabular fragment reorientation in the latter, where the difference between planned and achieved LCEA and AI was − 0.3° and − 0.2°, respectively. While on the XR side, the difference between planned and achieved LCEA and AI was − 8.1° and 3.1°, respectively.
Discussion
We present a computer-aided system for performing PAO cuts and positioning of the acetabular fragment, based on 3D planning. To the best of our knowledge, this is the first system, which enables the EMN- and PST-guided PAO and the intra-operative real-time 3D visualization of the acetabular fragment position.
PAO is a powerful procedure allowing for nearly any correction of abnormal acetabular position [8]. Its aim is to optimize the 3D position of the acetabulum to improve the coverage of the femoral head and to remove an eventual impingement. Both goals are important for a successful clinical outcome. PAO is technically demanding and requires significant experience, particularly because ischial osteotomy and distal retroacetabular osteotomy are performed without direct visualization and the final position of the acetabular fragment highly depends on the surgeon’s experience and 2D non-standard x-ray available in the OR [2, 3, 9, 18]. The avoidance of learning-related complications, including neurovascular lesions, muscle damage, inappropriate bone cuts, and/or suboptimal correction of the osteotomized fragment [2, 3, 7, 9,10,11,12], was the main motivation for the introduction of advanced technical aids, such as EMN and PST, to the surgical procedure.
The first important finding was the low incidence of major complications, as classified by the modified Dindo-Clavien grade III or IV [19, 20]. We noted them in only two (5%) hips in the XR group (1 transient peripheral peroneal nerve dysfunction, and one popliteal deep venous thrombosis). Contrary to other authors, we observed no other major complications such as intra-articular osteotomy, fragment nonunion, intra-operative fracture, or deep infection [2, 3, 7, 9, 11]. In the original description of the procedure, Ganz et al. [2] reported that major complications occurred in 13% of cases, most of them in the first 18 cases. Hussell et al. [11] in retrospective series of 508 patients reported that major complications occurred in 13% of cases, most of them in the first 50 cases. Peters et al. [9] noted that major complications occurred in 12% of the cases, most of them in the first 30 cases. In our study, the percentage of major complications was half as high as reported by other authors and we also observed a shorter learning period (less than 10 hips) compared to other studies [2, 3, 7, 9, 11]. In our opinion, the reduced number of technique-related complications is a direct consequence of better control of the surgical steps achieved with the EMN and PST. At the most recent follow-up, two hips (5%; patient no. 2 and no. 14) were converted to THA. The first was five years after PAO and the second was two years after PAO. The reasons were non-navigated (XR group) under correction of the acetabular fragment in the first patient, and overlooked cartilage degeneration (posterior part of the hip) in the second patient noticed post hoc on pre-operative CT scans while analyzing the reasons for persistent pain in the operated hip. This is also comparable to other published studies, where the conversion rate to THA is between 4 and 17% [3, 9].
Several computer navigation systems have already been developed for improving bone cuts and surgical accuracy in PAO [21,22,23,24,25,26,27,28]. However, in those, the reorientation of the osteotomized acetabulum still depended on subjective assessment of the surgeon and intra-operative fluoroscopic control, which heavily depends on patients’ position on the operating table, surgical field visualization, and surgeon’s experience. The surgeon needs repeated verification of the acetabulum position with intra-operative fluoroscopy, which increases the exposure of the patient to the x-rays and increases the duration of the procedure. In contrast, the presented system allows intra-operative real-time 3D visualization of the acetabular fragment position and its positioning according to the pre-operative plan. We confirmed this in our study by showing that the final position of the acetabular fragment in the EMN group was significantly more consistent with the pre-operative plan than it was in the XR group (for the LCEA, p = 0.02; for the AI, p = 0.03). Our corrections could not be compared to other published studies because they usually reported the differences between pre-operative and corrected LCEA and AI, without mentioning what the individual planned target LCEA and AI were [3, 9, 13, 29, 30]. Nevertheless, in a similarly designed study, Wang et al. also confirmed that the acetabular fragments’ positions were more consistent with the pre-operative plans when the procedure was performed with the help of PST, in contrast to the conventional free-hand technique [31].
We also observed that navigated reorientation of the acetabular fragment reduced the average duration of the surgery, from initial 203 to 183 minutes, but not significantly. This is contrary to other published studies, where conventional navigation systems mostly significantly extend the duration of surgery [23, 32]. These might be important since longer surgical procedures are associated with the increased risk of infection [33].
Other findings included a significant increase in the mean HHS in both groups. At the final follow-up, an average increase of HHS in the EMN group and the XR group were 38 and 31 points, respectively. This result is even slightly better than other authors’ reports, where the average increase of HHS was approximately 24 points [30, 34, 35].
The study’s main limitation is that groups regarding the acetabular fragment position and duration of surgery were not randomly selected and that the ten hips in the XR group represented the first ten PAOs. This could represent the main reason for higher complication rates and longer surgery in the XR group. However, this was not a limitation regarding the accuracy of the acetabular fragment position, since the EMN-guided reorientation of the acetabulum enables the surgeon real-time information about its position.
Conclusions
The most important benefits of performing PAO with the help of EMN and PST are increased accuracy, reproducibility, and most probably also the safety of the technique. Since the bone cuts and the fragment position are safely navigated, all surgeon’s attention can be focused on dissection and handling of sharp instruments in the vicinity of neurovascular structures. And here, the navigation is invaluable.
Our study indicates that PAO performed with EMN and PST seems to be a safe and reproducible procedure with a short learning curve. Additionally, navigated reorientation of the acetabular fragment is significantly more accurate than the fluoroscopic technique.
Data availability
Raw data (clinical, radiography) were generated at the Valdoltra Orthopaedic Hospital, Ankaran, Slovenia. Derived anonymized data supporting findings of this study are available from the corresponding author upon request.
References
Kamath AF (2016) Bernese periacetabular osteotomy for hip dysplasia: surgical technique and indications. World J Orthop 7:280. https://doi.org/10.5312/wjo.v7.i5.280
Ganz R, Klaue K, Vinh TS, Mast JW (1988) A new periacetabular osteotomy for the treatment of hip dysplasias technique and preliminary results. Clin Orthop 232:26–36
Siebenrock KA, Schöll E, Lottenbach M, Ganz R (1999) Bernese periacetabular osteotomy. Clin Orthop 363:9–20
Siebenrock KA, Schaller C, Tannast M et al (2014) Anteverting periacetabular osteotomy for symptomatic acetabular retroversion: results at ten years. J Bone Joint Surg Am 96:1785–1792. https://doi.org/10.2106/JBJS.M.00842
Steppacher SD, Tannast M, Ganz R, Siebenrock KA (2008) Mean 20-year followup of Bernese periacetabular osteotomy. Clin Orthop 466:1633–1644. https://doi.org/10.1007/s11999-008-0242-3
Lerch TD, Steppacher SD, Liechti EF et al (2017) One-third of hips after periacetabular osteotomy survive 30 years with good clinical results, no progression of arthritis, or conversion to THA. Clin Orthop 475:1154–1168. https://doi.org/10.1007/s11999-016-5169-5
Clohisy JC, Schutz AL, St. John L et al (2009) Periacetabular osteotomy: a systematic literature review. Clin Orthop 467:2041–2052. https://doi.org/10.1007/s11999-009-0842-6
Wirth SH, Rahm S, Kamath AF et al (2020) Periacetabular osteotomy using three-dimensional cutting and reposition guides: a cadaveric study. J Hip Preserv Surg. https://doi.org/10.1093/jhps/hnz051
Peters CL (2006) Early results of the Bernese periacetabular osteotomy: the learning curve at an academic medical center. J Bone Jt Surg Am 88:1920. https://doi.org/10.2106/JBJS.E.00515
Davey JP, Santore RF (1999) Complications of periacetabular osteotomy. Clin Orthop:33–37
Hussell JG, Rodriguez JA, Ganz R (1999) Technical Complications of the Bernese periacetabular osteotomy. Clin Orthop Relat Res 363:81–92
Büchler L, Beck M (2014) Periacetabular osteotomy: a review of swiss experience. Curr Rev Musculoskelet Med 7:330–336. https://doi.org/10.1007/s12178-014-9232-0
Trousdale RT, Cabanela ME (2003) Lessons learned after more than 250 periacetabular osteotomies. Acta Orthop Scand 74:119–126. https://doi.org/10.1080/00016470310013824
Kralj M, Mavčič B, Antolič V et al (2005) The Bernese periacetabular osteotomy: clinical, radiographic and mechanical 7–15-year follow-up of 26 hips. Acta Orthop 76:833–840. https://doi.org/10.1080/17453670510045453
Mihalič R, Trebše R (2014) Modern approach to periacetabular osteotomy performed with osteotomy guiding jigs and navigation. 11th EHS Congress Stockholm, Sweden
Mihalič R, Trebše R, Kreuh D (2013) Computer aided periacetabular osteotomy performed with CAD-CAM osteotomy guiding jig. Orthop Proc 95-B:Supp_34
Siebenrock KA, Steppacher SD, Tannast M, Büchler L (2015) Anteverting periacetabular osteotomy for acetabular retroversion. JBJS Essent Surg Tech 5:e1. https://doi.org/10.2106/JBJS.ST.N.00036
Zou Z, Chávez-Arreola A, Mandal P et al (2013) Optimization of the position of the acetabulum in a ganz periacetabular osteotomy by finite element analysis. J Orthop Res 31:472–479. https://doi.org/10.1002/jor.22245
Clavien PA, Sanabria JR, Strasberg SM (1992) Proposed classification of complications of surgery with examples of utility in cholecystectomy. Surgery 111:518–526
Dindo D, Demartines N, Clavien P-A (2004) Classification of surgical complications: a new proposal with evaluation in a cohort of 6336 patients and results of a survey. Ann Surg 240:205–213. https://doi.org/10.1097/01.sla.0000133083.54934.ae
Akiyama H, Goto K, So K, Nakamura T (2010) Computed tomography-based navigation for curved periacetabular osteotomy. J Orthop Sci 15:829–833. https://doi.org/10.1007/s00776-010-1520-y
Langlotz F, Bächler R, Berlemann U et al (1998) Computer assistance for pelvic osteotomies. Clin Orthop 354:92–102. https://doi.org/10.1097/00003086-199809000-00012
Langlotz F, Stucki M, Bächler R et al (1997) The first twelve cases of computer assisted periacetabular osteotomy. Comput Aided Surg Off J Int Soc Comput Aided Surg 2:317–326. https://doi.org/10.1002/(SICI)1097-0150(1997)2:6<317::AID-IGS1>3.0.CO;2-2
Mayman DJ, Rudan J, Yach J, Ellis R (2002) The Kingston periacetabular osteotomy utilizing computer enhancement: a new technique. Comput Aided Surg 7:179–186. https://doi.org/10.3109/10929080209146028
Wong KC, Kumta SM, Leung KS et al (2010) Integration of CAD/CAM planning into computer assisted orthopaedic surgery. Comput Aided Surg 15:65–74. https://doi.org/10.3109/10929088.2010.514131
Kamath AF, Mays RR (2019) Periacetabular osteotomy performed with imageless computer-assisted navigation: case report. Case Rep Orthop Res 2:33–39. https://doi.org/10.1159/000501545
Hooper JM, Mays RR, Poultsides LA et al (2019) Periacetabular osteotomy using an imageless computer-assisted navigation system: a new surgical technique. J Hip Preserv Surg 6:426–431. https://doi.org/10.1093/jhps/hnz058
Imai H, Kamada T, Miyawaki J et al (2020) Outcomes of computer-assisted peri-acetabular osteotomy compared with conventional osteotomy in hip dysplasia. Int Orthop 44:1055–1061. https://doi.org/10.1007/s00264-020-04578-x
Clohisy JC, Nunley RM, Curry MC, Schoenecker PL (2007) Periacetabular osteotomy for the treatment of acetabular dysplasia associated with major aspherical femoral head deformities. J Bone Joint Surg Am 89:1417–1423. https://doi.org/10.2106/JBJS.F.00493
Trousdale RT, Ekkernkamp A, Ganz R, Wallrichs SL (1995) Periacetabular and intertrochanteric osteotomy for the treatment of osteoarthrosis in dysplastic hips. J Bone Joint Surg Am 77:73–85. https://doi.org/10.2106/00004623-199501000-00010
Wang X, Liu S, Peng J et al (2019) Development of a novel customized cutting and rotating template for Bernese periacetabular osteotomy. J Orthop Surg 14:217. https://doi.org/10.1186/s13018-019-1267-x
Li J, Gao X, Li X (2019) Comparison of iASSIST navigation system with conventional techniques in total knee arthroplasty: a systematic review and meta-analysis of radiographic and clinical outcomes. Orthop Surg 11:985–993. https://doi.org/10.1111/os.12550
Cheng H, Chen BP-H, Soleas IM et al (2017) Prolonged operative duration increases risk of surgical site infections: a systematic review. Surg Infect 18:722–735. https://doi.org/10.1089/sur.2017.089
Trumble SJ, Mayo KA, Mast JW (1999) The periacetabular osteotomy. Minimum 2 year followup in more than 100 hips. Clin Orthop:54–63
Mayo KA, Trumble SJ, Mast JW (1999) Results of periacetabular osteotomy in patients with previous surgery for hip dysplasia. Clin Orthop:73–80
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethics approval
The study was approved by the Ethics Committee of the Valdoltra Orthopaedic Hospital (No. 3/2019).
Informed consent
Informed consent was obtained from patients for their anonymized data to be published in this article.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
(MOV 74465 kb)
(MOV 12744 kb)
Rights and permissions
About this article
Cite this article
Mihalič, R., Brumat, P. & Trebše, R. Bernese peri-acetabular osteotomy performed with navigation and patient-specific templates is a reproducible and safe procedure. International Orthopaedics (SICOT) 45, 883–889 (2021). https://doi.org/10.1007/s00264-020-04897-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00264-020-04897-z