Abstract
Aims
Recently, the determination of biochemical markers has been intensely explored to better understand the mechanisms underlying knee OA. In this study, we aimed to explore the expression pattern of five biochemical markers in patients with knee OA.
Methods
After IRB approval and signed informed consent, 26 patients were enrolled. Serum and synovial samples were collected prior to knee arthroscopy. Pre-operative assessment included diagnosis, Lysholm, Tegner Activity Scale, IKDC score, and radiographic Kellgren and Lawrence classification. ELISA of CTX-I, CTX-II, NTX-I, MMP3, and MMP13 were measured in serum and synovial fluid samples.
Results
Twenty-six patients were included, with a mean age of 42 ± 15 years old. Mean results and standard deviation of the biomarkers in serum were as follows: CTX-I 5.8 ± 5.5 ng/mL, CTX-II 3.8 ± 1.7 ng/mL, NTX-I 52 ± 71 (nM BCE), MMP3 1.18 ± 0.6 ng/mL, and MMP13 1243.6 ± 1422 pg/mL; synovial fluid results were as follows: CTX-I 0.74 ± 0.5 ng/mL, CTX-II 5.1 ± 2.5 ng/mL, NTX-I 254 ± 85 (nM BCE), MMP3 0.4 ± 0.4 ng/mL, and MMP13 797 ± 1391 pg/mL. We observed a differential pattern of expression in serum NTX-I in patients with chronic meniscus injuries when compared with ACL injuries or cartilage lesions.
Conclusions
In conclusion, the clinical criteria of early OA are useful to categorize patients with knee conditions. The biochemical markers explored did not yield a differential pattern that can be associated with this classification. Serum NTX-I could be a useful marker of chronic meniscal lesion in future longitudinal studies, after adjusting for age and sex.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Knee osteoarthritis (OA) is the most common joint disease and is associated with significant pain, disability, and elevated costs for its diagnosis and treatment [1]. It is a complex, multifactorial disease with many different phenotypes. Unfortunately, despite the availability of diagnostic and classification criteria for established OA, there is increasing interest in defining early OA clinically and through different biomarkers [2]. The anterior cruciate ligament (ACL) injury is associated with injuries to the articular cartilage, the menisci, and sometimes to other ligaments as well. A higher risk of OA has been shown among patients who have sustained any of the former injuries, compared with those who have never sustained knee injuries. It is estimated that up to one third of the patients will have knee OA within one decade after the injury regardless if they underwent ACL reconstruction or not [3, 4].
Injury to the menisci and the articular cartilage, lesion chronicity, age, and sex are considered risk factors for OA. In a recent cohort study, 43% of 620 patients were diagnosed with at least early OA when they underwent arthroscopy due to meniscal symptoms. A high proportion of such patients had ICRS grade III to IV lesions in at least one knee compartment [5]. From the pathophysiologic standpoint, it is understood that instability and injury events result in irreversible articular cartilage damage [6, 7]. The latter results in production of types I and II collagen and aggrecan within an inflammatory environment, along with gelatinases, programmed cell death, and, lastly, osteophyte formation [8].
Despite the frequency of these injuries, the early stages and progression of severe forms of knee OA are unclear. Furthermore, the diagnosis of OA is made with plain knee X-rays, focusing on the decrease in the joint space and osteophyte formation, both of which are late changes that occur during the irreversible stage of OA [9]. This has led to an intensive search for validated imaging and biochemical markers of early OA as an attempt to diagnose the patients at risk early on and to assess the potential short- and medium-term treatments [8,9,10]. OA biomarkers may be grouped as follows: collagen fragments; collagen synthesis markers; collagen degradation enzymes; proteoglycan and glycosaminoglycan degradation enzymes; bone formation; proinflammatory, anti-inflammatory, and anabolic cytokines; and others known as chemokines [11].
The purpose of this study was to explore the expression pattern of collagen fragments and/or bone formation and of collagen degradation enzymes in Mexican subjects scheduled for knee arthroscopy who had ACL, meniscal, or articular cartilage injuries, with or without signs of clinical or radiological early OA, to conduct a cross-sectional analysis. We hypothesized that the biomarkers could serve as surrogate marker of a specific lesion.
Methods
This study is a cross-sectional analysis of an early osteoarthritis cohort, within the OA Biomarker Initiative undertaken by the local Orthopaedics College. Once the project was reviewed and approved by the Research and Ethics Committee (Comité de Investigación Universidad Autónoma de Tamaulipas, approval number 04/17, April 5, 2016), a multicenter study was started at local hospitals from January 2016 to December 2018.
Inclusion criteria were as follows: age > 18 years, suspected ACL, meniscal or articular cartilage injury (based on the physical exam, lesion chronicity, and MRI diagnosis), ability to read and answer questions in Spanish, and provision of a serum and synovial fluid sample for ELISAs. Exclusion criteria included inflammatory disease, rheumatologic disease, gout, septic arthritis, history of arthroscopy, and history of fracture within the previous six months.
Twenty-six patients were included, all of whom signed the informed consent to undergo arthroscopic procedures. They all had sustained an injury to the anterior cruciate ligament, menisci, or knee articular cartilage during the 2016–2017 period. As this is an exploratory pilot study, no power sample size calculation was conducted.
Patient-reported outcomes
To assess symptoms of OA, patients were questioned by the treating physicians and by a research assistant just prior to surgery using the following set of questions: For how long have you had pain? (with answers specified in months); How did your symptoms begin? (response being gradually, due to a specific situation, like an accident); Does your knee get stuck or locked up? The latter question was asked to detect mechanical symptoms. We considered a lesion to be chronic lesion if it was present for more than six months before surgery.
The following questionnaires were also applied to know how patients perceived their knee: Lysholm Knee Questionnaire, Tegner Activity Scale, and Subjective International Knee Documentation Committee Knee Form (IKDC). In short, the Lysholm questionnaire was designed to measure the post-ACL reconstruction outcomes. It focuses on the patient’s perception during activities of daily living. The Tegner Scale includes 11 categories rated from 0 to 10; the higher the score, the higher the physical activity level. The IKDC-S is a questionnaire designed to evaluate function of the knee, meaning higher values of function to higher values of IKDC [12,13,14].
Structural pathology at the time of arthroscopy
The ACL status was reported during the arthroscopy as either intact or injured. Meniscal injuries were classified using the modified meniscal classification of the International Society of Arthroscopy, Knee Surgery and Orthopedic Sports Medicine (ISAKOS) [15]. The articular cartilage status was reported using the International Cartilage Repair Society Classification and classified as abnormal when ICRS classification was I–IV [16, 17]. When more than one lesion was reported, the most severe one was considered for purposes of the analysis (Fig. 1).
Presence or absence of early knee OA
The algorithm by Luyten et al. [15] was used to classify patients as having early or no OA with the purpose of clinically differentiating these entities. Early OA patients were classified based on the combination of had frequent or permanent pain of more than ten days at least two times in the last year, degenerative meniscal lesion (ISAKOS), and/or cartilage lesion (ICRS I-IV) with a Kellgren and Lawrence classification of 0, 1, or 2. Patients with no OA were defined when there had absence of frequent or permanent pain of more than ten days at least two times in the last year, Kellgren and Lawrence Classification of 0, with a condition that required surgery such as ACL, meniscus, or cartilage lesions [15,16,17,18].
Sample collection and ELISA acquisition
Human serum samples and synovial fluid were obtained in the operating room at the time of surgery. Synovial fluid was centrifuged (8 °C, 3500 g, 5 minutes) and the supernatant aliquoted and frozen at − 80 °C within two hours of collection. Serum was also aliquoted and frozen at − 80 °C until analysis. The production of Human MMP-13 (OriGene/ABCAMF ®), Human MMP-3 (Invitrogen ®), Human C-telopeptide of type I collagen (CTX-I, MyBioSource company ®), Human Cross Linked C-telopeptide of type II collagen (CTX-II, MyBioSource company ®), and Human Cross Linked N-telopeptide of type I collagen (NTX-I, MyBioSource company ®) was measured by ELISA development kit, according to the manufacturer’s instructions of each determination. All reagents were equilibrated to room temperature before use. All the microplates were precoated with an antibody specific for MMP-13, MMP-3, CTX-I, CTX-II, and NTX-I. We determined the number of microwell strips required. The reconstitution of the Human MMP-13, MMP-3, CTX-I, CTX-II, and NTX-I standards were prepared two hours prior to the experiment. One hundred microliter of the standard and samples were added into the appropriate wells, as well as 100 uL of biotinylated detection antibody of each assay with exception of MMP-3 assay. Also, streptavidin-HRP solution was prepared, and 100 uL were added to the microwell strips. TMB substrate solution was tacked on the microwell strips, and the color developed in proportion to the amount of each molecule. The stop solution changes the colour, and the intensity of the color was measured at 450 nm with an ELISA plate reader (DYNEX Technologies, model Opsys ®). The washes were done by four or five times between each step. After the last wash, the microwell strips were placed on absorbent paper towel to remove excess wash buffer. The incubation time (from 30 minutes to 2.5 hours) and the temperature (from room temperature to 37 °C) were different in each assay.
For calculation of results, the optical density (O.D.) of each well was subtracted of the optical density of zero well. The standard curve was plotted as the relative O.D. 450 of each standard solution on the y-axis and standard concentration on the x-axis. The Human MMP-13, MMP-3, CTX-I, CTX-II, and NTX-I concentration of the samples was interpolated from the standard curve. GraphPad Prism 6 software was performed.
Statistics
Descriptive statistics included means and standard deviations, and medians with inter-quartile intervals or relative numbers, as appropriate. As a method of standardizing our radiographic measurements and classification, we conducted a reliability analysis between observers and within observers of 56 knee radiographs. Comparisons between more than two groups were tested with Kruskal Wallis test. Comparisons between two groups were tested with Mann-Whitney U test; correlations were explored using Spearman’s correlation coefficient (rho). p values < 0.05 were considered as statistically significant. The SPSS, version 23, was used to perform the overall statistical analysis.
Results
Demographic and clinical results
Among the 96 eligible cases, 26 patients met the inclusion criteria; 11 males and 15 females. Mean age was 42 years ± 15 (Table 1). As expected, patients with symptoms associated with early OA were older than asymptomatic patients (p < 0.01). Female sex was a predisposing factor for associated symptoms (p < 0.0.001). No patients with ACL injury within this cohort had symptoms of early OA; their reason for visiting the doctor was instability.
No patient was diagnosed with severe radiographic OA (Kellgren and Lawrence grades III or IV). The following lesions were diagnosed in all patients: ten had ACL lesions, 11 meniscal lesions, and six cartilage lesions. Twelve out of the 26 patients were classified as having clinical signs of early OA according to Luyten et al. [15] criteria. We classified our patients as early OA clinically if they had frequent or permanent pain of more than ten days at least twice in the last year. None of the meniscal lesions encountered was amenable to repair, and they were degenerative in nature. Among the patients with grade I–IV lesions, six had disabling symptoms (p < 0.05). Patients with clinical signs of early OA had worse Tegner activity status as compared with that in patients without OA (p = 0.001). The IKDC questionnaire was statistically significant worse in patients with early OA (p = 0.002). The Lysholm scale did not detect any clinically significant differences (Table 2). For X-rays, intraclass coefficient between observers was 0.75 and 0.91 within observers.
Results of biomarkers
Mean values of OA biomarkers in serum and synovial fluid are shown in Table 1. To determine whether the values of the serum biomarkers behave as surrogates of their levels found at the knee at the time of surgery, we compared the synovial fluid and serum levels collected from the same patients. In this sense, the values for CTX-II and NTX-1 were higher in synovial fluid than in serum, and those for CTXI, MMP3, and MMP13 were higher in serum than in synovial fluid. When patients were grouped according to the presence or absence of early OA symptoms, no significant differences were found in the expression pattern of the biomarkers studied (Table 3).
We also classified patients based on their main diagnosis and grouped them as follows: ACL lesion, chronic meniscal lesion, and grade I to IV articular cartilage lesion. We found significant differences resulting from age, the Tegner activity score prior to the lesion, the IKDC score, and the serum NTX-1 expression. The worst functional values corresponded to patients with articular cartilage lesion, based on both the pre-lesion Tegner score and the IKDC score. Interestingly, NTX-1 expression was higher in patients with chronic meniscal lesion (Table 4).
Correlations
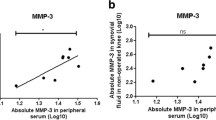
Among the patients studied, the Lysholm score was moderately correlated with the pre-operative Tegner activity score (rho = 0.6, p = 0.01); the IKDC correlated with the pre-lesion Tegner score (rho = 0.55, p = 0.002) and the pre-operative Tegner score (rho = 0.56, p = 0.01). The Lysholm function score was highly correlated with the level of CTX-I (rho = − 0.938, p < 0.001) in synovial fluid. For serum determinations, the pre-lesion Tegner score was moderately correlated with the CTX-I (rho = − 0.59, p = 0.002) and the MMP-3 (rho = − 0.59, p = 0.002) levels. Moreover, a good correlation was found between CTX-I and MMP-3 (rho = 0.9, p < 0.001).
Discussion
In this prospective cross-sectional study, we found that 46% of the patients subjected to knee arthroscopy had signs of early OA, based on the definition of knee pain, radiographic classification 0 to II of Kellgren and Lawrence, with articular cartilage lesion and/or meniscal lesions. We also found that serum NTX-I was associated with chronic meniscal lesions and that the level of CTX-I in synovial fluid was correlated with the Lysholm score.
The clinical, imaging, and biochemical characterization of the initial phases of knee OA is the subject of intense research. Given that the categorization of knee OA is based on radiographic classifications that only distinguish between advanced and early stages, different methods that might shed light on the understanding of the disease onset have been explored extensively [16,17,18,19,20,21,22].
In our study, the clinical and arthroscopic diagnosis of early OA was simple. The patients’ medical history was recorded in a simple and easy-to-apply questionnaire derived from Luyten pain criteria (pain in the knee at least two episodes for more than ten days in last year) which has not yet been validated. However, the functional differentiation by means of validated scales was not as clear as we expected. The Lysholm scale did not detect any differences between the patients who had symptoms of early OA and those who did not. The pre-lesion status was useful to predict signs of early OA when the Tegner score was used. As expected, the pre-operative activity levels were equivalent in all patients, that is, all of them had very low activity scores. Interestingly, the IKDC questionnaire significantly discriminated patients who had early OA from those who did not.
Age was a determining factor in the occurrence of early OA, together with female gender. None of the patients operated due to an ACL injury had signs of early OA. When the diagnosis of meniscal injury was compared in patients with and without early OA, no significant difference was seen. The prevalence of associated cartilage injuries was higher in patients with early OA.
The Kellgren and Lawrence radiographic classification was useful to categorize patients meeting the clinical criteria of early OA. We were able to standardize our measurements with a moderate-to-excellent concordance for grades 0 and 2. Wright et.al were also able to establish a moderate-to-very good classification of patients based on arthroscopy and X-rays in the MARS multicenter study [23]. Despite the broad use of this classification, it is still subject to criticism due to its inability to measure disease progression and its lack of sensitivity to detect change. However, the radiographic detection of symptom-associated osteophytes is useful to institute individualized treatment and place the patient within the right context [24].
In a study of patients with osteoporosis, levels of urinary NTX-I were higher compared with those without osteoporosis [25]. In an imaging study conducted by the OA Initiative, urinary CTX-II was associated with bone marrow lesions, osteophytes, and changes in bone shape; serum NTX-I was conversely associated with trabecular bone integrity in the same study [26]. A recent clinical trial assessed different markers in patients undergoing surgical ACL reconstruction and those managed conservatively and did not find any changes in urinary levels of NTX-I and CTX-II at 5 years [27]. Matsudai’s knee OA study explored the urinary levels of CTX-II and NTX-I. They found that postmenopausal women had increased uCTX-II and NTX-I levels, and those with OA grade ≥ II also had increased levels [28]. In our study, patients with meniscal lesions had a mean age of 45 years, and 65% of them were females. It is uncertain whether this contributed to the greater expression of serum NTX-I or not.
This finding is consistent with a recent meta-analysis that showed that the only biomarker that expressed a relation with early OA was NTX-I [29]. When we explored the correlation with the knee questionnaires, the synovial level of CTX-I was higher in patients with higher pre-operative Lysholm grades. The serum levels of CTX-I and MMP-3 showed a good correlation and were moderately correlated with the pre-lesion Tegner score.
Our study has the following strengths: it consists of a prospective standardized data collection of a cohort located in a well-defined geographical region. We have reliable objective measurements of the patients’ knee wellbeing perception, X-rays, arthroscopy, and ELISA tests in both synovial fluid and serum. This study adds validity to the Luyten criteria categorizing early OA in the clinical context. The weaknesses of our design include a small sample size that makes difficult to achieve statistical power in all comparisons, the cross-sectional nature of measurements, and the lack of inflammation biomarkers, such as C-reactive protein and interleukins, which are necessary to complete the biochemical profile. Thus, this study does not change clinical practice derived from laboratory findings, but the search for detection of early OA biomarkers is a real necessity across the globe.
Conclusion
The clinical criteria of early OA proposed by Luyten et al. [15] are useful to categorize patients with knee conditions. The analysis based on cartilage degradation biomarkers and matrix metalloproteinases does not yield a differential pattern that can be associated with this classification. On the other hand, serum NTX-I could be a useful marker of chronic meniscal lesion in future longitudinal studies, after adjusting for age and sex.
References
Brown TD, Johnston RC, Saltzman CL, Marsh JL, Buckwalter JA (2006) Posttraumatic osteoarthritis: a first estimate of incidence, prevalence, and burden of disease. J Orthop Trauma 20(10):739–744. https://doi.org/10.1097/01.bot.0000246468.80635.ef
Buckwalter JA, Saltzman C, Brown T (2004) The impact of osteoarthritis: implications for research. Clin Orthop Relat Res 427(Suppl):S6–S15. https://doi.org/10.1097/01.blo.0000143938.3068
Pearle AD, Warren RF, Rodeo SA (2005) Basic science of articular cartilage and osteoarthritis. Clin Sports Med 24:1–12. https://doi.org/10.1016/j.csm.2004.08.007
Lohmander LS et al (2007) The long-term consequence of anterior cruciate ligament and meniscus injuries: osteoarthritis. Am J Sports Med 35:1756–1769. https://doi.org/10.1177/0363546507307396
Pihl K, Englund M, Lohmander LS, Jørgensen U, Nissen N, Schjerning J, Thorlund JB (2017) Signs of knee osteoarthritis common in 620 patients undergoing arthroscopic surgery for meniscal tear. Acta Orthop 88(1):90–95. https://doi.org/10.1080/17453674.2016.1253329
Thorlund JB, Englund M, Christensen R, Nissen N, Pihl K, Jørgensen U, Schjerning J, Lohmander LS (2017) Patient reported outcomes in patients undergoing arthroscopic partial meniscectomy for traumatic or degenerative meniscal tears: comparative prospective cohort study. BMJ 356:j356. https://doi.org/10.1136/bmj.j356
Silverwood V, Blagojevic-Bucknall M, Jinks C, Jordan JL, Protheroe J, Jordan KP (2015) Current evidence on risk factors for knee osteoarthritis in older adults: a systematic review and meta-analysis. Osteoarthr Cartil 23(4):507–515. https://doi.org/10.1016/j.joca.2014.11.0019
Harkey MS, Luc BA, Golightly YM, Thomas AC, Driban JB, Hackney AC, Pietrosimone B (2015) Osteoarthritis-related biomarkers following anterior cruciate ligament injury and reconstruction: a systematic review. Osteoarthr Cartil 23(1):1–12. https://doi.org/10.1016/j.joca.2014.09.004
Pritzker KP, Gay S, Jimenez SA, Ostergaard K, Pelletier JP, Revell PA, Salter D, van den Berg WB (2006) Osteoarthritis cartilage histopathology: grading and staging. Osteoarthr Cartil 14(1):13–29. https://doi.org/10.1016/j.joca.2005.07.014
Kellgren JH, Lawrence JS (1957) Radiological assessment of osteoarthrosis. Ann Rheum Dis 16:494–502. https://doi.org/10.1136/ard.16.4.494
Gossec L, Jordan JM, Mazzuca SA, Lam MA, Suarez-Almazor ME, Renner JB, Lopez-Olivo MA, Hawker G, Dougados M, Maillefert JF (2008) OARSI-OMERACT task force ‘total articular replacement as outcome measure in OA.’ Comparative evaluation of three semi-quantitative radiographic grading techniques for knee osteoarthritis in terms of validity and reproducibility in 1759 X-rays: report of the OARSI-OMERACT task force. Osteoarthr Cartil 16:742–748. https://doi.org/10.1016/j.joca.2008.02.021
Lysholm J, Gillquist J (1982) Evaluation of knee ligament surgery results with special emphasis on use of a scoring scale. Am J Sports Med 10:150–154. https://doi.org/10.1177/036354658201000306
Tegner Y, Lysholm J (1985) Rating systems in the evaluation of knee ligament injuries. Clin Orthop Relat Res 198:43–49. https://doi.org/10.1097/00003086-198509000-00007
Higgins LD, Taylor MK, Park D, Ghodadra N, Marchant M, Pietrobon R, Cook C (2007) International knee documentation committee. Reliability and validity of the International Knee Documentation Committee (IKDC) Subjective Knee Form. Joint Bone Spine 74(6):594–599. https://doi.org/10.1016/j.jbspin.2007.01.036
Luyten FP, Denti M, Filardo G et al (2012) Definition and classification of early osteoarthritis of the knee. Knee Surg Sports Traumatol Arthrosc 20:401–406. https://doi.org/10.1007/s00167-011-1743-2
Anderson AF, Irrgang JJ, Dunn Wet al (2011) Interobserver reliability of the International Society of Arthroscopy, Knee Surgery and Orthopaedic Sports Medicine (ISAKOS) classification of meniscal tears. Am J Sports Med39(5):926-932. doi:https://doi.org/10.1177/0363546511400533
Brittberg M, Winalski CS (2003) Evaluation of cartilage injuries and repair. J Bone Joint Surg Am 85-A(Suppl 2):58–69. https://doi.org/10.2106/00004623-200300002-00008
Dwyer T, Martin CR, Kendra R et al (2017) Reliability and validity of the arthroscopic international cartilage repair society classification system: correlation with histological assessment of depth. Arthroscopy 33(6):1219–1224. https://doi.org/10.1016/j.arthro.2016.12.012
Catterall J, Stabler T, Flannery C, Kraus V (2010) Changes in serum and synovial fluid biomarkers after acute injury. Arthritis Res Ther 12:2–9. https://doi.org/10.1186/ar3216
Favero M, Ramonda R, Goldring MB, Goldring SR, Punzi L (2015) Early knee osteoarthritis. RMD Open 15(1(Suppl 1)):e000062. https://doi.org/10.1136/rmdopen-2015-000062
Madry H, Luyten FP, Facchini A (2012) Biological aspects of early osteoarthritis. Knee Surg Sports Traumatol Arthrosc 20:407–422. https://doi.org/10.1007/s00167-011-1705-8
Madry H, Kon E, Condello V, Peretti GM, Steinwachs M, Seil R, Berruto M, Engebretsen L, Filardo G, Angele P (2016) Early osteoarthritis of the knee. Knee Surg Sports Traumatol Arthrosc 24(6):1753–1762. https://doi.org/10.1007/s00167-016-4068-3
Wright RW, MARS Group (2004) Osteoarthritis classification scales: interobserver reliability and arthroscopic correlation. J Bone Joint Surg Am 96(14):1145–1151. https://doi.org/10.2106/JBJS.M.00929
Spector TD, Cooper C (1993) Radiographic assessment of osteoarthritis in population studies: whither Kellgren and Lawrence? Osteoarthr Cartil 1:203–206. https://doi.org/10.1016/s1063-4584(05)80325-5
Ishii Y, Noguchi H, Sato J, Takayama S, Toyabe SI (2016) Preoperative bone mineral density and bone turnover in women before primary knee arthroplasty. Open Orthop J 5(10):382–388. https://doi.org/10.2174/1874325001610010382
Deveza LA, Kraus VB, Collins JE, Guermazi A, Roemer FW, Bowes M, Nevitt MC, Ladel C, Hunter DJ (2017) Association between biochemical markers of bone turnover and bone changes on imaging: data from the osteoarthritis initiative. Arthritis Care Res 69(8):1179–1191. https://doi.org/10.1002/acr.23121
Struglics A, Larsson S, Kumahashi N, Frobell R, Lohmander LS (2015) Changes in cytokines and aggrecan ARGS neoepitope in synovial fluid and serum and in c-terminal crosslinking telopeptide of type II collagen and n-terminal crosslinking telopeptide of type I collagen in urine over five years after anterior cruciate ligament rupture: an exploratory analysis in the knee anterior cruciate ligament, nonsurgical versus surgical treatment trial. Arthritis Rheum 67(7):1816–1825. https://doi.org/10.1002/art.39146
Tanishi N, Yamagiwa H, Hayami T, Mera H, Koga Y, Omori G, Endo N (2014) Usefulness of urinary CTX-II and NTX-I in evaluating radiological knee osteoarthritis: the Matsudai knee osteoarthritis survey. J Orthop Sci 19(3):429–436. https://doi.org/10.1007/s00776-014-0535-1
Ren G, Krawetz RJ (2018) Biochemical markers for the early identification of osteoarthritis: systematic review and meta-analysis. Mol Diagn Ther 22(6):671–682. https://doi.org/10.1007/s40291-018-0362-8
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Izaguirre, A., González-Gutiérrez, G., Galindo-López, S.E. et al. Evaluation of biomarkers of joint damage in patients subjected to arthroscopy. International Orthopaedics (SICOT) 45, 1413–1420 (2021). https://doi.org/10.1007/s00264-020-04829-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00264-020-04829-x