Abstract
Purpose
To study the clinical and pathophysiologic characteristics and summarize the experience of treatment of abdominal vascular injury related to lumbar surgery.
Methods
We analyzed patients who suffered abdominal vascular injury during lumbar surgery in our hospital retrospectively and reviewed related literature in the PUBMED database from 2002 to 2017. Combined with the existing treatment options and outcomes, we investigated further and summarized our findings.
Results
With the data from our hospital, four cases of injuries were included, i.e., left common iliac artery and vein (CIA and CIV), left internal iliac artery, and inferior vena cava. Almost all of the patients (one exception) manifesting unstable haemodynamics were primarily treated by traditional vessel suture. After treatment, two patients died eventually, while the others recovered well at follow-up. With the reported data, 77 patients with the most frequently type of laceration (58.4%) were included. For vascular laceration, unstable haemodynamics was diagnosed in most of the patients (88.9%); CIA and CIV accounted for the all the most common patients (78.7%). Extracted from these data, traditional surgical method was selected to repair laceration prevalently (86.7%), while arteriovenous fistula and pseudoaneurysm were treated with an interventional procedure. Negative outcomes included two deaths, two suffered lower limb deep vein thrombosis, and two suffered graft infection.
Conclusions
Different treatment choices should be conducted depending on different injury characteristics and patients’ condition. Moreover, early recognition and prompt treatment are critical components to successful rescue. When a vascular injury is suspected, ultrasonography and positive abdominal exploration are recommended together with unified leadership in the rescue team.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Iatrogenic abdominal vascular injury related to posterior lumbar surgery with an incidence of around 0.01–0.05% is not a frequently occurring problem [1], while the mortality rate can reach up to 10–65% [2]. This is because the abdominal aorta, inferior vena cava, iliac vessels, and other major vessels can be injured during the operation. In the previous studies, some typical cases and relevant managements have been reported. Nevertheless, there is no complete rescue process summarized by medical teams based on treatment choices and outcomes. Hence, we collected the rescue experience from our hospital and some medical teams to establish a flowchart for reference.
Materials and methods
We collected the patients’ clinical characteristics, treatment decisions, and outcomes in our hospital, then analyzed four cases of abdominal vascular injury related to posterior lumbar surgery. Additionally, the relevant literature were reviewed from PUBMED database using the following key words: “lumbar disc,” “disc herniation,” “lumbar discectomy,” “vessel injury,” “spinal surgery,” “spine surgery,” “iatrogenic trauma,” “arteriovenous fistula,” and “pseudoaneurysm.” All the cases conformed to research conditions were collected from 2002 to 2017.
Results
Retrospective analysis of cases
With the same surgical method of posterior interbody fusion and fixation, the four patients’ clinical information is shown in Table 1. Three of the patients underwent abdominal ultrasonography immediately when major vascular injury was suspected and traditional vessel repair was performed as soon as free fluid was detected; one patient received angiography for confirmation, and subsequently, an immediate artery compliant balloon was applied to occlude the abdominal aorta, and then the Fluency-covered stents were implanted (Fig. 1). Due to poor haemostasis effect, abdominal exploration was conducted on the intervention-treated patient the following day. Unfortunately, two patients died in the following one and two days after the operation, respectively, while others were discharged from hospital after 14 and 30 days.
Operation procedure of the intervention (the arrow “→” points to the extravasation of contrast). a Implanting the complaint balloon to occlude the abdominal aorta; extravasation of contrast was observed from the left common iliac artery. b Implanting the 8 mm × 6 cm Fluency-covered stent, and angiography still revealed less extravasation of contrast. c Implanting the 8 mm × 6 cm balloon and a 10 mm × 4 cm balloon coating subsequently. d Angiography result displayed no extravasation of contrast
Literature review
Excluding the cases without complete information, 77 patients with iatrogenic vascular injury related to lumbar surgery were enrolled. Their clinical materials are presented in Table 2. Laceration was the most common injury type (45 cases, 58.4%) and injured various vessels (61 times in all) based on this review, though the incidence of AVF (66.0%) was reported higher than laceration (33.0%) and pseudoaneurysms (3%) [1]. Common iliac artery (CIA) and common iliac vein (CIV) injuries accounted for the majority of injuries (48 times, 78.7%), especially the left side (39 times, 63.9%) (Fig. 2).
Different intervals from operation to recognition are presented in Fig. 3. Generally, laceration was much easily recognized during (62.2%) or within the first 24 hours post-operatively (35.6%). While the diagnosis of AVF and pseudoaneurysm could take more than one year (12 cases, 37.5%), a 17-year confirmation case was reported by Sarmiento [3]. Haemodynamic instability (88.9%), intervertebral space haemorrhage (28.9%), and abdominal pain (22.2%) were usually detected in laceration; AVF could be recognized initially by abdominal pain (31.8%), while half of the pseudoaneurysm (50.0%) did not have any typical symptoms before diagnosis. In addition, some early symptoms such as swelling and lower-limb pain within the first 24 hours could help diagnose AVF [4] and pseudoaneurysm [5]. Other non-specific manifestations such as pallor, back pain, chest pain, inguinal cyanosis, ascites, and oedema could also be seen (Table 3).
Among the 77 patients, 47 (61.0%) underwent abdominal exploration as the first diagnosis and treatment method. Based on this review, laceration was primarily treated via traditional operations (39 cases, 86.7%), including vascular repair and suture, vessel resection and anastomosis, and artificial or autologous vessel transplantation. Interventional operations like endovascular stenting or embolization were mainly used for AVF and pseudoaneurysm. In order to control the continuous bleeding, four patients received the balloon occlusion of proximal AA or CIA firstly, and the covered stent was implanted in the two of them, while the others subsequently performed traditional operation [6]. Eventually, two death cases were mentioned [7, 8], one being likely attributed to diffuse intravascular coagulation (DIC) induced by massive blood transfusion. Two patients had lower limb deep vein thrombosis in seven days [9] and 12 days [10] post-operatively, and two others received patellar femoral artery bypass grafting operation secondary to graft infection in ten days and one month post-operatively, respectively [11].
Discussions
According to the literatures, the risk factors are summarized as follows [1, 10, 12, 13]: (1) lumbar operation history which may lead to adhesion between retroperitoneal vessel and vertebral body; (2) chronic disc disease which causes degeneration of the annulus fibrosus and anterior longitudinal ligament; (3) improper use of pituitary rongeur; (4) improper intra-operative position of patient; (5) pressure to the abdomen in the prone position which shortens the distance between retroperitoneal vessel and vertebral body; (6) proliferative spurs of anterior longitudinal ligament which may puncture major vessels; (7) anterior longitudinal ligament defect; (8) the abdominal radiotherapy history; (9) hernia-towards-abdomen disc; and (10) anatomical variations.
Anatomically, abdominal vessels lie in close proximity to the anterior vertebral bodies, making the vessels vulnerable to injury if the anterior longitudinal ligament is penetrated. For degenerative lumbar disease, L4–L5 and L5–S1 are the most common surgical levels [14], and 75% of abdominal vascular injuries were secondary to L4–L5 discectomy, as reported [12]. Since AA and IVC bifurcate at L4 level [14] (Fig. 4a), surgical procedures such as pedicle screwing (Fig. 4b) and pituitary rongeur probing (Fig. 4c) may cause major vascular injury here [14, 15], especially to the left CIA and CIV [10, 16]. Three cases of our hospital existed left common iliac vessel injury, which was consistent with the statistical results.
Regarding lumbar degenerative diseases, surgeons with varying experience and concept levels may select to operate via various methods. Minimally invasive spinal surgery (MISS) has achieved therapeutic successes, while iatrogenic vascular injury was also reported due to deeper insertion of pituitary rongeur in microendoscopic (MED) [17], high-energy (20 W) laser radiation in percutaneous lumbar laser discectomy (PLDD) [18] and expandable split-blade retractor in extreme lateral interbody fusion (XLIF) [19] during MISS. In open lumbar operation, discectomy, placing pedicle screw, and interbody fusion cage (Cage) are three necessary steps. Traditionally, pedicle screw is placed freehand (without computer-assistance) by the landmark of vertebra. Improper length and direction will increase the risk of vascular injury. When acute injury occurs, the tip of screw can penetrate vascular wall directly, and chronically, the perforated screw may erode the anterior longitudinal ligament and vascular wall little by little and result in bleeding after operation for several years. C-arm fluoroscopy is utilized to confirm the location of screw intra-operatively. Once the vascular injury is suspicious, removal of the offending screw automatically is recommended but should be put off without adequate vascular protection [20]. In the process of discectomy, deep bite or wide angle of pituitary rongeur should be avoided. In addition, the Shelin test (fill the wound with irrigating saline: its rapid escape through the disc space hints the disintegrity of annulus and anterior ligament) is helpful for confirmation [21]. During embedding of the Cage, selecting a suitable size based on pre-operative imaging evaluation and intra-operative try-mold is necessary, and the prohibition of inserting the Cage too deep is equally important. Cage migration is a common complication post-operatively, and its anterior displacement may hurt ligamentous and/or vascular structure. The biomechanical results from Abbushi et al. [22] showed that the postero-medial position of the Cage presented the lowest migration and highest fusion rate are worth reading as reference.
Different clinical symptoms may be detected due to different injury sites and types. Laceration usually manifested apparent intervertebral pulsatile bleeding or haemodynamic instability [12]. Other symptoms including abdominal pain [12], pallor [7], back pain [11], lower limb discomfort [7, 10], and chest pain [8] were also reported. Commonly, typical clinical signs were difficult to be recognized in AVF and pseudoaneurysm; some relevant symptoms caused by blood loss and retroperitoneal haematoma, including lower limb swelling, abdominal pain and distension, progressive dyspnea, systemic oedema, inguinal freckle, ascites, and heart failure [13, 23], can be regarded as the diagnostic clues. Sometimes, no detectable clinical performances can be recognized due to some factors: (1) young patients’ compensation [10, 15]; (2) vascular compression function caused by the prone position which may block the injury sites temporarily [15]; (3) self-sealing effect of anterior longitudinal ligament which can block flowing [24]. Additionally, patients’ medical history may mislead the judgment of surgeons and anesthesiologists. Hence, if fatness or mucosa (may be the retroperitoneal adipose tissue or vessel wall) was found in the pituitary ronguer, surgeon needs to raise vigilance of the patient immediately, even though no brisk intervertebral bleeding was observed at that moment. Anesthesiologist monitors should judge status of patient continuously; non-invasive blood pressure (NIBP), electrocardiography (ECG), pulse oximeter (SpO2), and end-tidal CO2 (EtCO2) should also be applied commonly. Beyond that, invasive monitoring (e.g., central venous pressure, radial artery pressure, intermittent arterial blood gas, and Flotrac/Vigileo system) was strongly recommended when the abovementioned risk factors or big surgical trauma existed. When vascular injury happened during the operation, progressive hypotension and tachycardia were sensitive variables that can be detected in addition to wide pulse pressure and reduction of EtCO2 which were also valuable indicators. Fluctuation of invasive monitoring and decrease in haematocrit or haemoglobin reflected blood volume change as well. Though some performances appear relatively late, paleness, decrease of skin temperature, and weak pulse had equal diagnostic values, especially after enough volume replacement and appropriate positive inotropic medication supplement.
Rapid and accurate selection of diagnostic methods is an important guarantee for prompt treatment. Generally, non-invasive diagnostic examination includes ultrasonography, computed tomography (CT), and magnetic resonance imaging (MRI). The bedside ultrasonography is convenient and effective, while CT and MRI, especially the contrast-enhanced ones, are able to confirm the bleeding site and distinguish arterial or venous injury [10, 15]. The important point should be emphasized when the following conditions exist: (1) screw penetrates lateral to the pedicle on anterior-posterior radiograph; (2) screw tip penetrates to the anterior margin of vertebral bodies on lateral radiograph; (3) or new-onset symptomatic radiculopathy appears at the level of fusion; a further CT examination is recommended positively [20]. As a kind of invasive examination, interventional angiography is a significant standard to make the definite diagnosis [10, 12] and is recommended as the first diagnostic method of choice as it has potential for therapeutic benefit [12]. For some conditions, the interventional angiography is restricted by medical technology and disease severity; therefore, timely abdominal exploration is more reasonable [5]. Once acute vascular injury is suspicious, it is critical to perform immediate balloon occlusion above the suspected site to control continuous bleeding and stabilize blood volume as soon as possible if circumstances allowed. After inflation, angiography with intravenous contrast under C-arm fluoroscopic is able to identify the exact bleeding site.
Treatment choice varies based on different types and degrees of injury, patient’s status, and medical resources. With the advantages of effective haemostasis and vessel wall repair, traditional operation is suitable for critical situation, immediate identification of injury site and repair under direct vision are contributed to arresting the continuous bleeding. But anatomical differentiation can be difficult when the defect is surrounded by a large haematoma or locates in deep pelvis, and the increased risks caused by secondary laparotomy and position change should be concerned at the same time [12]. An unsuccessful rescue case with left CIA and CIV perforation treated by vascular repair was reported [8], and a similar case appeared in our hospital as well. Primary suturing should be considered first line if conditions permit; end-to-end anastomose and bypass graft are also options once suturing fails. Materials such as Polytetrafluoroethylene, Dacron graft, or autogenous vessels will help. Endovascular technology (e.g., covered stent graft, embolization, coiling) has become increasingly utilized to repair various injury types, whereas risk of stenosis and thrombogenesis, requirement of long-term anti-thrombotic medication usage should, also be taken into consideration. Laceration requires acute settings because of rapid blood loss; open operation has been described and recommended to repair laceration. Endovascular technology is gradually popular as well [12]. Though AVF and pseudoaneurysm could also be treated with traditional operation [11], the interventional method seems more suitable [13, 15]. The advantages of interventional operation like minor operative trauma and short hospital stay are more practical in some circumstances [16]. Nevertheless, compared with abdominal exploration and traditional repair, endovascular technology has some shortcomings as well: (1) failure to recognize the coexistence of arteriovenous injury; (2) failure to cover the vascular gap tightly, which is related to vascular re-expansion after adequate supplying blood volume; (3) difficulty for the guide wire to pass through due to long-segment or transverse vascular injury. Injury of aorta and inferior caval vein has better be repaired by suturing, whereas some nonessential arteries such as the deep femoral artery, internal iliac artery and external carotid artery could be embolized selectively [13]. When endovascular technology is chosen to repair the branch vessels with irregular shape, the different characteristics of various covered stents should be considered. For instance, iliac artery is tortuous and requires a flexible stent graft; therefore, and the Viabahn stent with flexible feature seems to be more suitable [25].
Experience summary
Based on the introspection of the two death cases in our hospital, the time delay between lumbar operation and abdominal exploration might be critical in influencing patients’ outcomes. Though the prompt interventional method was implemented in one case to block vascular rupture, the ultimate failure of rescue seemingly reminded us to explore abdomen immediately when major injury was suspected.
In order to reduce the occurrence of abdominal vascular injury caused by lumbar surgery, some suggestions are summarized as follows: (1) patient’s comorbidities should be evaluated carefully so as to identify potential risk factors; (2) surgeons should avoid some improper operation manoeuvres, such as removing the intervertebral disc roughly, probing the pituitary rongeur too deep and rapid insertion of pedicle screws; (3) applying X-ray examination timely to judge the location of the pedicle screw, with further evaluation by CT examination if necessary; (4) prompt abdominal exploration and vascular repair are critical if iatrogenic vascular injury is suspected; (5) immediate balloon occlusion may help to stop the continuous bleeding. During the management of abdominal vascular injury, establishing an emergency command team is significant, ensuring that the rescue process is well-organized and efficient. The active participation of general and vascular surgeons, effective therapeutic measures by anaesthetists, positive cooperation with nurses, abundant blood supply from blood transfusion department, post-operative monitoring, and therapy in the intensive care unit (ICU) are all important components of a successful rescue. In short, time is the most precious factor in such an emergency accident; therefore, responses to major vascular injury must be rapid enough, which may help to gain better curative effect.
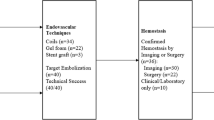
Finally, we summarized a diagnosis and treatment flowchart of the suspected abdominal vascular injury for reference (Fig. 5).
Conclusion
Iatrogenic abdominal vascular injury needs a serious complication of posterior lumbar surgery, in which early recognition, diagnosis, and treatment are critical. The choice of diagnosis and treatment depends primarily on injury type, as well as patient’s conditions. Timely abdominal exploration is appropriate for patients with haemodynamic instability under any circumstance, and interventional operation is suitable for patients with stable life signs. What is more, the effectiveness of intervention should be considered cautiously for the vascular injury confirmation during the operation and early period post-operatively. Once abdominal vascular injury occurs, prompt balloon occlusion is recommended if conditions permit.
References
Papadoulas S, Konstantinou D, Kourea HP et al (2002) Vascular injury complicating lumbar disc surgery: a systematic review. Eur J Vasc Endovasc Surg 24(3):189–195
Szolar DH, Preidler KW, Steiner H et al (1996) Vascular complications in lumbar disk surgery: report of four cases. Neuroradiology 38(6):521–525
Sarmiento JM, Wisniewski PJ, Do NT et al (2010) Bifurcated endograft repair of ilio-iliac arteriovenous fistula secondary to lumbar diskectomy. Ann Vasc Surg 24(4):551.e13–551.e17
Mulaudzi TV, Sikhosana MH (2011) Arterio-venous fistula following a lumbar disc surgery. Indian J Orthop 45(6):563–564
Wee HY, Wang CC, Kuo JR (2015) Vascular injury after lumbar discectomy mimicking appendicitis: report of a case. Asian J Neurosurg 10(3):243–245
Kwon TW, Sung KB, Cho YP et al (2003) Large vessel injury following operation for a herniated lumbar disc. Ann Vasc Surg 17(4):438–444
Keskin M, Serin KR, Genc FA et al (2013) Iatrogenic major vascular injury during lumbar discectomy: report of three cases. Turk Neurosurg 23(3):385–388
Busardò FP, Frati P, Carbone I et al (2015) Iatrogenic left common iliac artery and vein perforation during lumbar discectomy: a fatal case. Forensic Sci Int 246:7–11
Düz B, Kaplan M, Günay C et al (2008) Iliocaval arteriovenous fistula following lumbar disc surgery: endovascular treatment with a stent-graft. Turk Neurosurg 18(3):245–248
Bozok S, Ilhan G, Destan B et al (2013) Approach to the vascular complications of lumbar disc surgery. Vascular 21(2):79–82
Bingol H, Cingoz F, Yilmaz AT et al (2004) Vascular complications related to lumbar disc surgery. J Neurosurg 100(3 Suppl Spine):249–253
Jung HS, Kim DJ, Kim HS et al (2017) Vascular complications related to posterior lumbar disc surgery. Vasc Specialist Int 33(4):160–165
Rohit MK, Gupta A, Khandelwal N (2013) Endovascular transluminal stent grafting: treatment of choice for post lumbar spine surgery iliac arterio-venous fistulae. Catheter Cardiovasc Interv 88(6):203–208
Liu Y (2012) Analysis of vascular injury in lumbar spine surgery. Pakistan J Med Sci Online 28(5):791–794
Skippage P, Raja J, Mcfarland R et al (2008) Endovascular repair of iliac artery injury complicating lumbar disc surgery. Eur Spine J 17(Suppl 2):228–231
Olcay A, Keskin K, Eren F (2013) Iliac artery perforation and treatment during lumbar disc surgery by simple balloon tamponade. Eur Spine J 22(Suppl 3):350–352
Uei H, Tokuhashi Y, Oshima ML (2014) Vascular injury following microendoscopic lumbar discectomy treated with stent graft placement. J Neurosurg Spine 20(1):67–70
Jeon SH, Lee SH, Choi WC (2007) Iliac artery perforation following lumbar discectomy with microsurgical carbon dioxide laser: a report of a rare case and discussion on the treatment. Spine 32(3):124–125
Santillan A, Patsalides A, Gobin YP (2010) Endovascular embolization of iatrogenic lumbar artery pseudoaneurysm following extreme lateral interbody fusion (XLIF). Vasc Endovasc Surg 44(7):601–603
Parker SL, Amin AG, Santiagodieppa D et al (2014) Incidence and clinical significance of vascular encroachment resulting from freehand placement of pedicle screws in the thoracic and lumbar spine: analysis of 6816 consecutive screws. Spine 39(8):683–687
Shevlin WA, Luessenhop AJ, Fox JL (1973) Perforation of the anterior annulus during lumbar discectomy-case report. J Neurosurg 38(4):514–515
Abbushi A, Cabraja M, Thomale UW (2009) The influence of cage positioning and cage type on cage migration and fusion rates in patients with monosegmental posterior lumbar interbody fusion and posterior fixation. Eur Spine J 18(11):1621–1628
Luan JY, Li X (2012) A misdiagnosed iliac pseudoaneurysm complicated lumbar disc surgery performed 13 years ago. Spine 37(25):1594–1597
Leech M, Whitehouse MJ, Kontautaite R et al (2014) Abdominal aortocaval vascular injury following routine lumbar discectomy. Case Rep Anesthesiol 2014:895973
Canaud L, Hireche K, Joyeux F (2011) Endovascular repair of aorto-iliac artery injuries after lumbar-spine surgery. Eur J Vasc Endovasc Surg 42(2):167–171
Acknowledgements
We thank Xiaoyan Niu for her help in collecting the clinical materials in our hospital.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Liu, B., Ye, K., Gao, S. et al. The summary of experience of abdominal vascular injury related to posterior lumbar surgery. International Orthopaedics (SICOT) 43, 2191–2198 (2019). https://doi.org/10.1007/s00264-018-4262-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00264-018-4262-7