Abstract
Purpose
The use of adjuvant radiation in the treatment of soft-tissue sarcoma (STS) is equivocal in selected cases. Our objective was to compare the short-term outcomes in patients operated on for a local recurrence who had radiation for the primary tumour to those who were spared radiation.
Methods
This was a retrospective study of 103 patients treated for a local recurrence: 48 (47%) with previous radiation and 55 (53%) without. Our primary outcome criterion was to identify the differences in the local treatment provided. Secondary outcomes were the cumulative incidence of a surgical site infection/wound complication (SSI/WC), variables associated with SSI/WC, and local recurrence.
Results
Amputation and the incidence of re-operation were significantly more frequent in patients who received previous radiation compared to patients without previous radiation (27% vs 9%, p = 0.02, for amputation; 26% vs 36% at 2 years for SSI/WC, p = 0.049). Multivariable regression models found previous radiation (p = 0.049), arteriopathy (p = 0.012), location at lower limb (p = 0.09), and use of a flap (0.0048) associated with the risk of SSI/WC.
Conclusions
Previous radiation is associated with an increased risk of amputation and reoperation for SSI/WC when treating a local recurrence. This information should be accounted for when deciding for the use of radiation.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Soft tissue sarcoma (STS) represents 1% of all cancers [1]. The incidence in France is about 2.8 to 3.3 new cases for 100,000 [2, 3]. The local management of STS involves a wide surgical resection with sometimes the addition of external beam radiation therapy (EBRT).
The rate of wound complication after limb-salvage surgery ranges between 18 and 48% [4,5,6,7,8], and increases in case of recurrence surgery. EBRT is often used as an adjuvant treatment, before or after the resection. The ESMO guidelines stipulates that adjuvant radiation should be used “in selected cases in the case of low- or high-grade, superficial, > 5 cm and low-grade, deep, < 5 cm STSs” [9]. According to the national cancer data base, 30 to 60% of the patients with negative margins receive adjuvant radiotherapy [10]. The use of EBRT varies mainly according to providers’ experience, surgical margins, tumour grade, size, depth, and histology. Numerous studies have well shown the benefit of EBRT on the risk of local recurrence [11, 12]. However, despite reducing the risk of local recurrence, radiation does not prevent it entirely. In fact, 80% of local recurrences in patients who had adjuvant radiation occur in the irradiated field [13,14,15]. Unfortunately, EBRT is also associated with morbid complications. According to different series, wound complications after sarcoma resection surgery are largely increased by radiation [16, 17]. Half these complications will need repeat surgery [6].
When opting for adjuvant radiation in equivocal cases, surgeons face the risk of having to deal with a local recurrence in an irradiated field. This is most true when dealing with patients who had the resection of a STS outside a reference center for instance. The absence of pre-operative imaging, little information on the surgical procedure, and a pathology report from a non-expert in the field make it difficult to appreciate the risk of local recurrence. Consequently, practitioners may find it difficult to decide for or against the use of radiation, whether it is associated with or without surgical revision. Some may choose to use adjuvant radiation with the risk of dealing with a local recurrence on an irradiated field should it occur. Others may discard radiation altogether, with an increased risk of local recurrence, but knowing that if the tumour recurs, it will occur in non-radiated tissues. To the best of our knowledge, there is no study comparing the short-term outcomes, and most importantly the occurrence of wound complications, in patients operated on for a local recurrence who had radiation for the primary tumor to those who were spared radiation. We aimed at providing useful and new information to care providers dealing with soft tissue sarcoma.
Therefore, we retrospectively analyzed a series of patients operated on for a local recurrence of an extremity STS with or without previous radiation. Our primary question was to look for differences in the treatments used to treat the local recurrence (type of surgery including the ability to preserve the limb, use of radiotherapy, use of chemotherapy). Our secondary questions were to estimate the proportion of patients developing a surgical site infection/wound complication (SSI/WC), the variables associated with this latter complication, and the local control rate between the two groups.
Patients and methods
This retrospective study was performed between 2000 and 2015 at tertiary university care centers specialized in the treatment of bone and soft tissue sarcomas. All care providers were experienced in the treatment of sarcomas. Patients were included in the present study if they were 16 years or older, presented with a histologically proven recurrence of an extremity STS. Patients were excluded if no information was available regarding the treatment of the primary occurrence; if the recurrence occurred within four months of the primary surgery, if the resection was macroscopically contaminated, or if the tumor was a well-differentiated liposarcoma. Patients were split into two groups based on the use of adjuvant radiation at the time of the primary occurrence: patients who had previous radiation and patients who had no previous radiation. This retrospective study was conducted in accordance with the International Ethical Guidelines and Declaration of Helsinki [18].
One hundred thirty-six patients with a recurrence of an extremity STS were eligible. Thirty-three patients were excluded because the recurrence occurred within less than four months (n = 11), the resection was macroscopically contaminated (n = 7), or the tumour was a well-differentiated liposarcoma (n = 15). Overall, 103 locally recurrent patients were included, 48 (47%) with previous radiation and 55 (53%) without previous radiation (Table 1). The median follow-up was 22 months. There were 56 females (54%) and 47 males (46%), with a mean age of 64 years. Potential healing detractors did not differ between groups, including ASA score, diabetes (n = 10, 10%), history of tobacco use (n = 26, 26%), history of peripheral vascular arteriopathy (n = 3, 3%), and corticosteroid use (n = 2, 2%). Main tumors were myxofibrosarcomas (n = 24, 23%), liposarcomas (n = 18, 17%), and leiomyosarcomas (n = 14, 14%). Initial surgery consisted of a limb-sparing surgery for all patients. Initial margins were similar between these two groups.
Differences were identified between these two groups. Patients who had previous radiation were more likely to have a high-grade (n = 34, 71%) and deeply seated tumour (n = 47, 98%) at the time of the primary occurrence of the sarcoma compared to those without previous radiation (n = 25, 46% high-grade, p = 0.004; n = 43, 78% deep tumours p = 0.002).
All treatments were decided at weekly multidisciplinary team (MDT) meetings with surgeons, radiation and medical oncologists, pathologists, and radiologists. Surgery of the local recurrence aimed for a wide resection circumferentially with about a 2-cm margin of muscle or fat, or an anatomical barrier whenever possible [19]. Close margins, and possibly planned microscopic positive margins to preserve important fixed structures such as bone, vessels, and nerves, were permitted [20]. The previous surgical site was resected together with the recurrence only in some cases. In case of limb salvage was not deemed possible, an amputation was performed. Radiotherapy of the recurrence was decided based on the surgical margins. Although the use of radiation for the primary tumour was not a strict contraindication for using radiation to treat the recurrence [21], it participated greatly in the decision for not giving it. When given, the standard radiation protocol was external beam fractionated radiotherapy, either 66 Gy post-operatively or 50 Gy pre-operatively.
Our primary objective was to look for differences in the treatments used to treat the local recurrence (type of surgery, use of radiotherapy, use of chemotherapy). Our secondary objectives were to know the proportions of patients developing a surgical site infection/wound complication (SSI/WC), the variables associated with, and the local control rate between the two groups.
Patients were considered to have surgical site infection when they presented with the following clinical criteria of infection such as fever, or pus oozing from the wound, and/or biological criteria of inflammatory syndrome and/or positivity of bacteriological cultures during the revision. Patients were considered to have a wound complication if they presented any wound complication requiring debridement and/or negative pressure therapy applied on an outpatient basis, or a surgical intervention for wound repair, such as debridement, drainage of seroma or haematoma, or secondary wound closure without evidence of infection as defined above.
Point estimates with 95% exact confidence intervals are reported. Univariable and multivariable logistic regression models were computed to look for variables associated with the primary outcome. Variables of interest tested were related to the patient: age (continuous), sex (woman or man), diabetes (yes or no), arteriopathy (yes or no), corticosteroid use (yes or no), smoking (yes or no), ASA score (continuous), body mass index (continuous), previous radiation of the surgical site (yes or no), chemotherapy (yes or no), and presence of metastasis (yes or no); to the tumor: size (continuous), grade (low, intermediate, or high), depth (superficial or deep), location (upper extremity, or lower extremity); to the surgery: type (limb-sparing surgery or amputation), resection margins (R0, or R1/R2) and soft-tissue reconstruction (yes or no). Variables with a relevant effect (p < 0.2) on the primary outcome in univariable models were fitted into a multivariable Cox regression model. Variable selection was then performed using a stepwise, backward, and forward selection procedure based on Akaike index criterion. All tests were bilateral at the 0.05 significance level. All computations were performed using R software (R foundations for statistical computing, Vienna, Austria).
Results
We found significant differences in the treatment of patients with and without previous radiotherapy (Table 2). Amputation was significantly more frequent for patients who received previous radiation (n = 13, 27%) compared to patients who did not have previous radiation (n = 5, 9%); p = 0.02. Adjuvant radiation to treat the recurrence was less used for patients who had previous radiation (n = 6, 13%) than for patients who did not have previous radiation (n = 27, 49%); p < 0.001. Adjuvant chemotherapy was more used for patients in the previous radiation group (n = 12, 25%) than for the group of patients who did not have previous radiation (n = 2, 4%); p = 0.003. There was, however, no difference in resection margins and in the use of a flap.
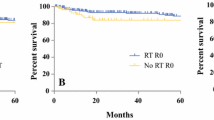
Eighteen (38%) patients underwent an operation to treat SSI/WC in the previous radiation group (nine (19%) for wound complication and nine (19%) for infection), and 12 (22%) in the group of patients with no previous radiation (eight (15%) for wound complications and four (7%) for infection). This difference was statistically significant (p = 0.049). The overall cumulative incidence of re-operation for SSI/WC was 27% (19–37%), 31% (21–41%), and 36% (25–48%) for all patients at 12, 24, and 60 months respectively. The cumulative incidence of re-operation for SSI/WC was 36% (22–50%), 36% (22–50%), and 48% (27–67%) for patients with previous radiation and 20% (10–32%), 26% (14–39%) and 26% (14–39%) for patients without previous radiation and at the same times (Fig. 1). This difference was statistically significant (p = 0.049).
Univariable regression Cox models found previous radiation (HR = 1.8 (0.88–3.8); p = 0.1), arteriopathy (HR = 7.6 (1.7–34); p = 0.0073), location at lower limb (HR = 2.6 (1.0–6.8); p = 0.048), and the use of a flap (HR = 4.1 (1.6–11); p = 0.0042) associated with the higher risk of being operated on for an SSI/WC. Variable selection in the multivariable regression Cox models retained previous radiation (HR = 2.1 (1.0–4.6); p = 0.049), arteriopathy (HR = 13 (2.6–68); p = 0.012), location at the lower limb (HR 2.3 (0.88–6.2); p = 0.09) and the use of a flap (HR = 4.3 (1.6–12); p = 0.0048) as important predictors significantly associated with a higher risk of being operated on for an SSI/WC (Table 3).
However, we found no difference with regard to local recurrence between both groups. The overall cumulative incidence of local recurrence was 6% (3–12%), 13% (8–21%), 27% (18–37%), and 41% (30–51%) for all patients at three, six, 12, and 24 months respectively; p = 0.93.
Discussion
Adjuvant radiotherapy is frequently used for treating patients with a STS [22] because it is associated with a significant reduction in risk of local recurrence [11, 12]. However, it is also associated with significant morbid complications at the time of the index surgery and potentially also at the time of treating a local recurrence. In some cases, when surgeons decided on adjuvant radiation, they face the risk of having to deal, later, with a local recurrence in an irradiated field. We therefore compared the treatments and wound complications in two groups of locally recurrent patients: one who had been treated with EBRT at the time of the previous surgery and one who never had EBRT.
Our work found a strong association, with a 40% increase at two years, between the use of EBRT and the occurrence of a SSI/WC, should a local recurrence occur and be operated on. EBRT is a known risk factor of SSI/WC at the time of index surgery [4,5,6,7,8,9,10,11,12,13,14,15,16]. The literature is, however, poor regarding the effect of previous radiation on the outcome of surgically treated local recurrence. Trovic et al. [5], in a retrospective study of 205 local recurrences based on the Scandinavian Sarcoma Group Register between 1987 and 1995, did not find an association between radiation and SSI/WC after recurrence surgery. However, only 18% of patients had previous radiation to treat the initial tumour, compared to 47% in our series, which could explain the limited power of their study. Numerous reasons can be proposed to explain the deleterious effect of previous EBRT on the occurrence of a SSI/WC. First, EBRT increases local tissue inflammation [6]. It impairs division of endothelial cells, fibroblasts, and keratinocytes, leading to a delay in wound healing. Secondly, EBRT causes ineffective angiogenesis and decreases tissue oxygenation [23]. Finally, radiation therapy, associated with limb-sparing surgery, disturbs the lymphatic network, especially for lower limb, increases the risk of developing a seroma, and a subsequent infection [4]. These effects persist indefinitely over time [24]. Plastic surgeons found that the rate of flap failure on irradiated fields is more important if radiation occurred more than 1 year before the flap, than if radiation occurred more recently [24]. A histological exam of these flaps confirmed this result by showing a significant reduction of vascularization and a significant decrease of the mean capillary lumen. Marre et al. have confirmed this result [6]. This is probably due to the long-term toxic impact of EBRT during many years.
We also found that amputation was significantly associated with previous use of EBRT. There was three times more amputation for treating the local recurrence in an irradiated field. Overall, the total amputation rate in the present series (17%) is, however, comparable with the literature [5,6,7,8]. Some reasons can be offered to explain this aggressive surgical choice. First and foremost, care providers may be reluctant to offer complex conservative surgery in a previous irradiated field if they think it is bound to fail, which experience and the present findings suggest (see paragraph above). Second, surgical resection in an irradiated field is more likely to require a soft tissue reconstruction (twice as many in our series), for which all surgeons or centres do not have the necessary experience. Therefore, for equivocal cases, surgeons may opt for an amputation rather than organizing a complex salvage limb operation. Last patients with previous radiation may have had tumours which were initially more frequently close to a fixed structure (bone, vessels, nerve). Therefore, recurrences in the radiation group could also be closer to fixed structures and therefore more likely to require an amputation [15].
Regression models found numerous variables, apart from previous radiation, associated with the occurrence of SSI/WC: arteriopathy (HR = 13), location at lower limb (HR = 2.3), and the use of a flap (HR = 4.3) associated with the risk of SSI/WC. O’Sullivan [16] as well as Morre [4] reported that lower limb sarcoma were more likely to be associated with SSI/WC. The disruption of a less redundant lymphatic network and of more voluminous tumours could be an explanation [4]. Arteriopathy seems to increase considerably the rate of SSI/WC. Also, Mouthon et al. suggest that irradiation transforms resting endothelial cells to a proadhesive surface for platelets, which could ultimately lead to thrombosis [25]. However, because of the small number of patients with an arteriopathy in our series, readers should extrapolate this result with caution. The use of a flap was significantly associated with the risk of SSI/WC in our study and should be interpreted as a confounding factor, with patients at high risk of SSI/WC more likely to benefit from soft tissue reconstruction [6].
Our study presents some limitations. Patient selection could explain some of the differences observed. Indeed, patients initially selected for EBRT probably differ from those being denied this adjuvant. However, the effects of radiation on surrounding tissues (which cause surgical site infections and wound complications) are the same for all patients, and heterogeneity would have a limited role in explaining the present results. Second, given the number of patients included, we cannot rule out that some significant predictors were missed. However, significant findings are not affected by the number of patients, if only to emphasize the strength of these associations. Last, this study is comparative but not randomized. Although ideally this would eliminate selection biases, it is not feasible given the timing between radiation and the local recurrence.
Conclusion
Previous radiation is associated with an increased risk of amputation and reoperation for SSI/WC when treating a local recurrence. Patients that are more at risk are those with known arteriopathy. This information should be accounted for when deciding for the use of radiation when its oncologic effects are thought to be marginal.
References
Clark MA, Fisher C, Judson I, Thomas JM (2005) Soft-tissue sarcomas in adults. N Engl J Med 353:701–711
Mathoulin-Pélissier S, Chevreau C, Bellera C et al (2014) Adherence to consensus-based diagnosis and treatment guidelines in adult soft-tissue sarcoma patients: a French prospective population-based study. Ann Oncol 25:225–231
Ducimetière F, Lurkin A, Ranchère-Vince D et al (2011) Incidence of sarcoma histotypes and molecular subtypes in a prospective epidemiological study with central pathology review and molecular testing. PloS One 6(8):e.20294
Moore J, Isler M, Barry J, Mottard S (2014) Major wound complication risk factors following soft tissue sarcoma resection. Eur J Surg Oncol 40(12):1671–1676
Trovik CS, Gustafson P, Bauer HC et al (2000) Consequences of local recurrence of soft tissue sarcoma: 205 patients from the Scandinavian Sarcoma Group Register. Acta Orthop Scand 71(5):488–495
Marré D, Buendía J, Hontanilla B (2012) Complications following reconstruction of soft-tissue sarcoma: importance of early participation of the plastic surgeon. Ann Plast Surg 69(1):73–78
Geller DS, Hornicek FJ, Mankin HJ, Raskin KA (2007) Soft tissue sarcoma resection volume associated with wound-healing complications. Clin Orthop Relat Res 459:182–185
Lohman RF, Nabawi AS, Reece GP et al (2002) Soft tissue sarcoma of the upper extremity: a 5-year experience at two institutions emphasizing the role of soft tissue flap reconstruction. Cancer 94:2256–2264
Soft tissue and visceral sarcomas: ESMO Clinical Practice Guidelines, (2014) Ann Oncol, 25: p. 102–112
Sherman KL, Wayne JD, Chunq J et al (2014) Assessment of multimodality therapy use for extremity sarcoma in the United States. J Surg Oncol 109(5):395–404
Pisters PW, Harrison LB, Leung DH et al (1996) Long-term results of a prospective randomized trial of adjuvant brachytherapy in soft tissue sarcoma. J Clin Oncol 14(3):859–868
Yang JC, Chang AE, Baker AR et al (1998) Randomized prospective study of the benefit of adjuvant radiation therapy in the treatment of soft tissue sarcomas of the extremity. J Clin Oncol 16:197–203
Dickie C, Griffin AM, Parent AL et al (2012) The relationship between local recurrence and radiotherapy treatment volume for soft tissue sarcomas treated with external beam radiotherapy and function preservation surgery. Int J Radiat Oncol Biol Phys 82(4):1528–1534
Sampo MM, Tuomikoski L, Tarkkanen M et al (2014) Marginal miss or radioresistance? The pattern of local recurrence after operation and 3D planned radiation treatment in soft tissue sarcoma of the extremities and the limb girdles; an analysis based on image fusion. Acta Oncol 53(4):557–562
D’Andrea FP, Safwat A, Burns JS et al (2012) Tumor microenvironment and radiation response in sarcomas originating from tumorigenic human mesenchymal stem cells. Int J Radiat Biol 88(6):457–465
O’Sullivan B, Davis AM, Turcotte R et al (2002) Preoperative versus postoperative radiotherapy in soft-tissue sarcoma of the limbs: a randomized trial. Lancet 359(9325):2235–2241
Davis AM, O’Sullivan B, Turcotte R et al (2005) Late radiation morbidity following randomization to preoperative versus postoperative radiotherapy in extremity soft tissue sarcoma. Radiother Oncol 75(1):48–53
The International Ethical Guidelines and Declaration of Helsinki. http://www.who.int/bulletin/archives/79%284%29373.pdf (date last accessed 9 Jan 2018)
Enneking WF, Spanier SS, Malawer MM (1981) The effect of the anatomic setting on the results of surgical procedures for soft parts sarcoma of the thigh. Cancer 47(5):1005–1022
Gerrand CH, Wunder JS, Kandel RA et al (2001) Classification of positive margins after resection of soft-tissue sarcoma of the limb predicts the risk of local recurrence. J Bone Joint Surg Br 83(8):1149–1155
Riad S, Biau D, Holt GE et al (2012) The clinical and functional outcome for patients with radiation-induced soft tissue sarcoma. Cancer 118(10):2682–2692
Biau DJ, Fergusson PC, Chung P et al (2012) Local recurrence of localized soft tissue sarcoma: a new look at old predictors. Cancer 118(23):5867–5877
Tsai JH, Makonnen S, Feldman M et al (2005) Ionizing radiation inhibits tumor neovascularization by inducing ineffective angiogenesis. Cancer Biol Ther 4(12):1395–1400
Schultze-Mosgau S, Grabenbauer GG, Radelspiel-Tröger M et al (2002) Vascularization in the transition area between free grafted soft tissue and pre-irradiated graft bed tissue following preoperative radiotherapy in the head and neck region. Head Neck 24:42–51
Mouthon MA, Vereycken-Holler V, Van der Meeren A et al (2003) Irradiation increases the interactions of platelets with the endothelium in vivo: analysis by eintravital microscopy. Radiat Res 160:193–199
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
This retrospective study was conducted in accordance with the International Ethical Guidelines and Declaration of Helsinki
Conflicts of interest statement
The authors declare that they have no conflict of interest.
Rights and permissions
About this article
Cite this article
Eloy, G., Daveau, C., Kreps, S. et al. Higher complications after previous external beam radiation for extremity soft-tissue sarcoma in the surgical treatment of a local recurrence: a comparative retrospective study of one hundred and three patients. International Orthopaedics (SICOT) 43, 727–733 (2019). https://doi.org/10.1007/s00264-018-4064-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00264-018-4064-y