Abstract
Background
Tranexamic acid (TXA), delivered intravenously or topically, has been shown to reduce blood loss, the need for transfusion, and relevant healthcare costs when administered in primary standard total hip arthroplasty (THA). Whether the same is true of oral TXA is unclear, the purpose of this study was to determine if oral tranexamic acid is equivalent to intravenous TXA in the case of patients undergoing THA via the direct anterior approach.
Methods
In this prospective randomized controlled trial, 120 patients undergoing primary THA by the direct anterior approach were randomized to receive oral TXA (two doses of 20 mg/kg), intravenous TXA (two doses of 15 mg/kg), or no TXA. Primary outcomes were haemoglobin drop, haematocrit levels, total blood loss, intra-operative blood loss, need for transfusion, and volume transfused. Secondary outcomes included thromboembolic events, wound complications, the length of post-operative hospital stay, and 30-day readmission.
Results
Demographic characteristics were similar among the three patient groups (p > 0.05, n = 40 per group). Haemoglobin drop, haematocrit levels, total blood loss, and intra-operative blood loss were similar in the oral and intravenous groups (p > 0.05), and significantly smaller than in the control group (p < 0.05). Transfusions were given to significantly fewer patients in the oral group (3%) and intravenous group (6%) than in the control group (27%, p = 0.01). Costs of TXA and transfusions were significantly lower in the oral group than the intravenous group (p < 0.05). The three groups were similar in thromboembolic events, wound complications, the length of post-operative hospital stay, and 30-day readmission (p > 0.05).
Conclusion
Oral TXA shows similar efficacy and safety as intravenous TXA for reducing haemoglobin drop, haematocrit levels, total blood loss, and transfusion rate following THA by the direct anterior approach. Therefore, the much less-expensive oral formulation may be superior to the intravenous form.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Total hip arthroplasty (THA) is quite effective for relieving pain and promoting functional recovery of patients with hip arthrosis [1]. The number of hip replacements has risen in recent decades because of lengthening life expectancies [2, 3]. Blood loss following THA is a substantial problem and is known to interfere with patient rehabilitation. Total blood loss can range from 700 to 2000 mL, and 16–37% of patients may require transfusions [4,5,6]. Post-operative anaemia and blood transfusion have been associated with side effects as increased morbidity and cost, immunologic reactions, transmission of disease, and infection [7, 8]. Several measures are widely used to reduce blood loss and decrease the risk of transfusion, including controlled hypotensive anaesthesia, minimally invasive THA, and tranexamic acid (TXA) [8,9,10,11,12].
TXA, a synthetic amino acid derivative, is an effective antifibrinolytic agent that competitively inhibits the conversion of plasminogen to plasmin, thereby stabilizing clots [13]. The literature clearly demonstrates the ability of TXA to reduce post-operative blood loss and transfusion rates after TKA without increasing risk of thromboembolic complications [14,15,16,17], and this appears to be equally valid for TXA administered topically or intravenously [18]. Oral TXA formulations cost substantially less than topical or intravenous formulations, and oral TXA may perform as well as intravenous TXA in patients undergoing total hip or knee replacement [19, 20]. This literature suggests that oral TXA may be the most cost-effective formulation for patients undergoing standard THA.
What is unclear is whether this is true for patients undergoing THA involving a direct anterior approach, which some studies suggest can lead to less blood loss, less pain, shorter hospital stay, and faster rehabilitation than the standard procedure [21, 22]. In the muscle-sparing, internervous direct anterior approach, the prosthetic component is inserted along an intermuscular plane, resulting in less soft tissue damage and potentially improving functional recovery and post-operative quality of life [22]. On the other hand, a study from our group has shown that the direct anterior approach can prolong operating time and lead to greater blood loss than the standard procedure [23], making TXA even more necessary to reduce the need for transfusion and the associated costs [24, 25]. This highlights the need for research into whether oral TXA offers similar efficacy and safety at lower cost than intravenous TXA for patients undergoing THA by the direct anterior approach.
To address this question for the first time in a prospective randomized controlled trial, we compared the efficacy and safety of oral and intravenous TXA in patients undergoing primary THA involving the direct anterior approach as well as enhanced-recovery management. We hypothesized that the two formulations would decrease peri-operative blood loss and transfusion rates to similar extents and be associated with similar risk of post-operative complications. This would make oral TXA, because of its much lower cost, superior to intravenous TXA.
Material and methods
Study design and patients
The study protocol was approved by the Ethics Committee of West China Hospital of Sichuan University, and the study was registered in the Chinese Clinical Trial Registry (ChiCTR-INR-17013110). Written informed consent was obtained from all participants. Consecutive patients who underwent elective unilateral primary THA by the direct anterior approach between September 2016 and June 2017 were assessed for potential enrollment in this study. All patients had been diagnosed with hip osteoarthritis or femoral head necrosis (Ficat III or IV). Exclusion criteria were as follows: body weight index (BMI) > 30 kg/m2; Crowe type 3 or 4 dysplasia; previous hardware; prior hip surgery; and an inability to tolerate general anesthesia. Patients meeting the above inclusions are being operated via the direct anterior approach for THA. In addition, patients were excluded if they had bilateral arthroplasty, allergy to TXA, or history of renal failure, kidney transplant, a recent arterial thromboembolic event such as myocardial infarction or stroke, hypercoagulation, hemophilia, deep vein thrombosis, or pulmonary embolism. Patients were also excluded if they declined to participate or to receive blood products.
Patients recruited into the study were randomized into three groups using a computer-generated randomization table at an allocation ratio of 1:1:1 with a maximum number of 40 per each group. Patients and researchers who prospectively collected all clinical information were blinded to patient allocation until the final data analysis (see next section).
TXA administration
TXA in the intravenous group was administered at a dose of 15 mg/kg at ten minutes before skin incision and again at three hours after THA. In order to support the double-blind nature of the study, patients in this group also received four tablets of ascorbic acid (250 mg, so 1000 mg total) at two hours before and three hours after THA. TXA in the oral group was administered at a dose of 20 mg/kg at two hours before and three hours after THA. In order to support the double-blind nature of the study, patients in this group also received intravenous injections of saline ten minutes before skin incision and again three hours after THA. Patients in the control group received ascorbic acid tablets and intravenous injections of saline as in the other groups, but no TXA. All drugs were administered by a nurse and anesthetist who were not involved in the surgeries, care, or assessment of outcomes. All study participants, surgeons, and clinical staff participating in treatment were blinded to patient allocation throughout the study period.
Surgery and peri-operative management
All surgical procedures were performed via direct anterior approach to the hip by surgeons who had completed fellowships in reconstructive surgery on adult patients. All patients received general anesthesia, intravenous prophylactic antibiotics for 24 hours, and thromboembolic prophylaxis consisting of a half dose of low molecular weight heparin (0.2 mL 2000 IU; Clexane, Sanofi-Aventis, France) that began on post-operative day zero after the operation and continued at 24hour intervals, with a full dose (0.4 mL, 4000 IU) applied on post-operative days three. After discharge, all patients were prescribed oral rivaroxaban (Xarelto; Bayer, Cologne, Germany) at 10 mg once a day for ten days. Patients were discharged when stable surgical wounds, hip flexion of 100°, hip abduction of 40°, and adequate mobility for daily activities were achieved.
No drain was applied to any patient following THA. Patients were examined daily in the hospital for clinical symptoms of venous thromboembolism. A Doppler ultrasound examination of both lower limbs was performed on all patients by senior ultrasound physicians at two weeks after the operation. Perioperative blood transfusions were given based on guidelines of the Chinese Ministry of Health, which indicate transfusions when hemoglobin concentration < 70 g/L or when symptoms of anemia are present, such as altered mental state or palpitation (regardless of haemoglobin concentration).
Outcome measures
Data were collected on patient demographic characteristics (age, sex, weight, height, and body mass index (BMI)), American Society of Anesthesiologists (ASA) score, and pre-operative laboratory results, including haemoglobin (HB), haematocrit (HCT), prothrombin time (PT), activated partial thromboplastin time (APTT), international normalized ratio (INR), fibrinogen (FIB), and platelet count. HB and HCT levels were also measured at each time point post-operative days one, two and three.
The primary outcomes of the study were reduction in haemoglobin concentration (defined as pre-operative haemoglobin minus lowest post-operative haemoglobin), total blood loss, intra-operative blood loss, transfusion rates, and number of blood units transfused. The total blood volume was calculated according to the modification of the Gross formula, as follows: the total blood volume = (k1 × height3 (m)) + (k2 × weight (kg)) + k3, where k1 = 0.3669, k2 = 0.03219, and k3 = 0.6041 for men; k1 = 0.3561, k2 = 0.03308, and k3 = 0.1833 for women. And then total blood loss was calculated as follows: total blood loss = total blood volume × (change in haemoglobin level / mean haemoglobin) [26, 27]. If blood transfusion was performed before the lowest hemoglobin level was obtained, the total blood loss was taken to be the loss calculated from the change in the hemoglobin plus the volume of blood transfused [28]. Intr-aoperative blood loss was defined as the amount of blood collected in the suction canister and in saturated surgical sponges.
Secondary outcomes were thromboembolic events and wound complications. The oral TXA dosage cost 6.83 RMB per dose. The cost of 1 g of IV TXA was 76.30 RMB, the cost of oral form of TXA is cheaper than the intravenous form, and beside its relatively low cost, the advantage of oral TXA is simple application avoiding IV access, which is requirement for expensive nursing care for IV application. The transfusion cost per two U red blood cells was estimated to be 930 RMB at our hospital.
Statistical analysis
Statistical analysis was performed using SPSS 21.0 (SPSS Inc., Chicago, IL, USA). Quantitative data were reported as mean ± SD; qualitative data, as frequencies and percentages. Differences in continuous data between groups were assessed for significance using one-way ANOVA, while differences in categorical data were assessed using the chi-squared test. A p value of < 0.05 was considered significant.
Results
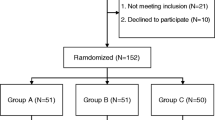
During the period of study enrollment from September 2016 to June 2017, 143 patients were scheduled for primary THA using the direct anterior approach at our hospital. Of these, 23 were excluded based on inclusion and exclusion criteria. The remaining 120 were enrolled in the study and divided randomly into groups treated with no TXA or with oral or intravenous TXA (n = 40 per group) (Fig. 1). Patients had been diagnosed either with hip osteoarthritis (oral group, 18; intravenous, 16; control, 19) or with femoral head necrosis (oral group, 22; intravenous, 24; control, 21). The three groups were similar in terms of demographic characteristics and preoperative laboratory values, including HB, HCT, PT, APTT, INR, FIB, and platelet count (Table 1). The three groups were similar in terms of operating time (p > 0.05; Table 2).
Primary outcomes
The oral and intravenous groups had significantly higher post-operative haemoglobin and haematocrit levels than the control group (Figs. 2 and 3), and the haemoglobin drop was smaller in the oral group and intravenous group than in the control group (p = 0.001); no significant differences were observed between the oral group and the intravenous group (p = 1.00; Fig. 4; Table 2). Total blood loss, intra-operative blood loss, and total units of transfused blood were significantly higher in the control group than in the two TXA groups (p < 0.05; Fig. 5; Table 2). Eight patients (27%) in the control group required blood transfusions, compared with only one patient (3%) in the oral group and two patients (6%) in the intravenous group (p = 0.01; Table 2).
Secondary outcomes
No patients in the study experienced thromboembolic events. One patient in the control group experienced wound discharge, but the wound healed fully by post-operative day ten (Table 3). In all three groups, no patients required re-operation or readmission to the orthopaedics department within 30 days of surgery because of wound complications. The length of post-operative hospital stay was similar in the oral group, intravenous group, and control group (p = 0.15; Table 3).
The cost associated with oral TXA (546 RMB total patients) was significantly lower than that of intravenous TXA (4573.2 RMB total patients; p = 0.001; Table 2). Similarly, the cost of transfusion was significantly lower in the oral group (929.65 RMB total transfusion) than in the intravenous group (1859.3 RMB total transfusion) and control group (8366.8 RMB, total transfusion; p = 0.004; Table 2).
Discussion
Patients undergoing THA are at high risk of post-operative anaemia and blood transfusions, which have been associated with complications including rigor, fever, dyspnea, morbidity, and higher healthcare costs [7, 8]. TXA, administered intravenously, topically or orally, is widely used to reduce peri-operative blood loss and allogeneic blood transfusion [29, 30]. Oral TXA appears to be associated with similar haemoglobin drop, total blood loss, and blood transfusion as intravenous TXA in patients undergoing conventional total hip or knee arthroplasty, yet the oral formulation is more convenient and much cheaper [31, 32]. Therefore, we wanted to examine whether this is true for patients undergoing THA by the direct anterior approach with enhanced recovery. Our results in this prospective, double-blind randomized controlled trial suggest that oral and intravenous TXA are associated with similar haemoglobin drop, total blood loss, transfusion rate, and adverse outcomes, while the oral formulation is associated with much lower costs.
Most blood loss in THA occurs during acetabular preparation, broach preparation of the femoral canal, and wound surface haemorrhage. The direct anterior approach to THA, although muscle sparing and preferable in many respects to the conventional posterolateral approach, can prolong operating time and lead to greater blood loss [23]. Therefore, our finding that less-expensive oral TXA can achieve similar blood-sparing efficacy as intravenous TXA in the direct anterior procedure may substantially improve outcomes and make the procedure an appropriate choice for a greater number of patients. Our findings of similar efficacy between the two formulations are consistent with previous studies involving total knee [32] or hip arthroplasty [20].
The outcomes of the study demonstrated that oral TXA was as effective as the intravenous TXA in terms of the haemoglobin drop, haematocrit levels, and total blood loss. Furthermore, our results also showed that there were no significant differences in blood transfusion and complications between oral and intravenous TXA. However, the optimal dosage time for oral TXA administration to reducing blood loss in THA remains controversial. Pilbrant et al. reported that the oral bioavailability of TXA was 34% of the dose and elimination in blood occurred within eight hours. Oral TXA achieves peak levels two or three hours after administration, and peak plasma concentration was attained immediately after the application of intravenous TXA. The half-life of equipotential doses for two forms is similar. The study also showed that a dose of 2 g of oral form produced higher plasma concentration than 1 g of intravenous form at six hours [33]. Zohar et al. compared the effective of oral TXA with the intravenous form in primary total knee arthroplasty, in his study used that a regime of 1 g of oral TXA two hours before the surgery and then used every six hours for 18 hours after surgery [34]. The dose prescribed by Zohar seems to be inadequate drug plasma concentration. From the perspective of balancing risks and benefits, the present study used a regime of 2 g of oral TXA two hours before incision and then one dose at three hours post-operatively. The dosing of the oral regimen was deemed appropriate on the basis of the above-mentioned study. In our study, all patients received 10 mg intravenous dexamethasone immediately after operation to manage post-operative nausea and vomiting. Although our study had a similar outcome of comparing oral and intravenous TXA as the previous studies [20, 32], we only focused on primary total hip arthroplasty via the direct anterior approach with enhanced recovery.
Our results suggest that oral TXA, at 6.83 RMB per dose, may be much more cost-effective than intravenous TXA, at 76.30 RMB per dose, for achieving similar efficacy and safety. This provides cost savings above what TXA already provides, regardless of administration route [35]: the increase in pharmacy costs associated with routine use of TXA is more than offset by cost savings in operating room use, blood transfusion, laboratory analyses, and room and board [36]. In our study, the cost-transfused blood was much lower with oral TXA (929.65 RMB total patients) than with intravenous TXA (1859.30 RMB total patients) or no TXA at all (8366.85 RMB total patients). Our findings are similar to a double-blind randomized trial in which the cost oral TXA treatment (1936.60 RMB) was much lower than the cost intravenous TXA treatment (6048.00 RMB) or cost topical TXA treatment (6062.40 RMB) [32]. Using oral TXA with patients undergoing total joint arthroplasty may allow hospitals to cut costs substantially without sacrificing efficacy or safety.
The results of the present study should be considered with caution in light of several limitations. First, minimum sample size was calculated based on total blood loss as the outcome, so the sample may not have been large enough to detect significant differences in thromboembolic events or wound complications. Future studies with larger populations should verify our findings of similar safety between oral and intravenous routes of TXA administration. Our hospital applies anti-coagulation therapy relatively early, which may help explain why none of the patients in our study developed deep vein thrombosis or pulmonary embolism. Our TXA-dosing regime, although based on previous work [37], may need to be optimized; further studies should systematically examine the influence of different doses and frequencies of doses on efficacy and safety. We calculated blood loss based on the lowest post-operative haemoglobin value, which means our results are vulnerable to effects from post-operative haemodilution. However, we do not believe that hemodilution significantly affected our results, since the oral and intravenous groups showed similar length of post-operative hospital stay and rates of 30-day readmission.
Conclusion
Oral TXA offers similar efficacy as intravenous TXA in reducing post-operative bleeding and transfusion rate in patients undergoing primary THA via the direct anterior approach. The much lower cost and greater convenience of the oral formulation make it an attractive alternative for patients and hospitals.
References
Laupacis A, Bourne R, Rorabeck C, Feeny D, Wong C, Tugwell P, Leslie K, Bullas R (1993) The effect of elective total hip replacement on health-related quality of life. J Bone Joint Surg Am 75:1619–1626
Kurtz SM, Ong KL, Lau E, Bozic KJ (2014) Impact of the economic downturn on total joint replacement demand in the United States: updated projections to 2021. J Bone Joint Surg Am 96:624–630. https://doi.org/10.2106/jbjs.m.00285
Kurtz S, Ong K, Lau E, Mowat F, Halpern M (2007) Projections of primary and revision hip and knee arthroplasty in the United States from 2005 to 2030. J Bone Joint Surg Am 89:780–785. https://doi.org/10.2106/jbjs.f.00222
Kim JL, Park JH, Han SB, Cho IY, Jang KM (2017) Allogeneic blood transfusion is a significant risk factor for surgical-site infection following Total hip and knee arthroplasty: a meta-analysis. J Arthroplast 32:320–325. https://doi.org/10.1016/j.arth.2016.08.026
Kim C, Park SS, Davey JR (2015) Tranexamic acid for the prevention and management of orthopedic surgical hemorrhage: current evidence. J Blood Med 6:239–244. https://doi.org/10.2147/jbm.s61915
Toy PT, Kaplan EB, McVay PA, Lee SJ, Strauss RG, Stehling LC (1992) Blood loss and replacement in total hip arthroplasty: a multicenter study. The preoperative autologous blood donation study group. Transfusion 32:63–67
Carson JL, Duff A, Poses RM, Berlin JA, Spence RK, Trout R, Noveck H, Strom BL (1996) Effect of anaemia and cardiovascular disease on surgical mortality and morbidity. Lancet (London, England) 348:1055–1060. https://doi.org/10.1016/s0140-6736(96)04330-9
Ponnusamy KE, Kim TJ, Khanuja HS (2014) Perioperative blood transfusions in orthopaedic surgery. J Bone Joint Surg Am 96:1836–1844. https://doi.org/10.2106/jbjs.n.00128
Nielsen CS, Jans O, Orsnes T, Foss NB, Troelsen A, Husted H (2016) Combined intra-articular and intravenous tranexamic acid reduces blood loss in Total knee arthroplasty: a randomized, double-blind, placebo-controlled trial. J Bone Joint Surg Am 98:835–841. https://doi.org/10.2106/jbjs.15.00810
Alshryda S, Sukeik M, Sarda P, Blenkinsopp J, Haddad FS, Mason JM (2014) A systematic review and meta-analysis of the topical administration of tranexamic acid in total hip and knee replacement. Bone Joint J 96-b:1005–1015. https://doi.org/10.1302/0301-620x.96b8.33745
Sharrock NE, Mineo R, Urquhart B, Salvati EA (1993) The effect of two levels of hypotension on intraoperative blood loss during total hip arthroplasty performed under lumbar epidural anesthesia. Anesth Analg 76:580–584
Sharrock NE, Salvati EA (1996) Hypotensive epidural anesthesia for total hip arthroplasty: a review. Acta Orthop Scand 67:91–107
Okamoto S, Hijikata-Okunomiya A, Wanaka K, Okada Y, Okamoto U (1997) Enzyme-controlling medicines: introduction. Semin Thromb Hemost 23:493–501. https://doi.org/10.1055/s-2007-996127
Camarasa Godoy MA, Serra-Prat M, Palomera Fanegas E (2008) Effectiveness of tranexamic acid in routine performance of total knee replacement surgery. Rev Esp Anestesiol Reanim 55:75–80
Jansen AJ, Andreica S, Claeys M, D'Haese J, Camu F, Jochmans K (1999) Use of tranexamic acid for an effective blood conservation strategy after total knee arthroplasty. Br J Anaesth 83:596–601
Alshryda S, Sarda P, Sukeik M, Nargol A, Blenkinsopp J, Mason JM (2011) Tranexamic acid in total knee replacement: a systematic review and meta-analysis. J Bone Joint Surg Br Vol 93:1577–1585. https://doi.org/10.1302/0301-620x.93b12.26989
Yang ZG, Chen WP, Wu LD (2012) Effectiveness and safety of tranexamic acid in reducing blood loss in total knee arthroplasty: a meta-analysis. J Bone Joint Surg Am 94:1153–1159. https://doi.org/10.2106/jbjs.k.00873
Li J, Zhang Z, Chen J (2016) Comparison of efficacy and safety of topical versus intravenous tranexamic acid in total hip arthroplasty: a meta-analysis. Medicine (Baltimore) 95:e4689. https://doi.org/10.1097/md.0000000000004689
Irwin A, Khan SK, Jameson SS, Tate RC, Copeland C, Reed MR (2013) Oral versus intravenous tranexamic acid in enhanced-recovery primary total hip and knee replacement: results of 3000 procedures. Bone Joint J 95-b:1556–1561. https://doi.org/10.1302/0301-620x.95b11.31055
Kayupov E, Fillingham YA, Okroj K, Plummer DR, Moric M, Gerlinger TL, Della Valle CJ (2017) Oral and intravenous tranexamic acid are equivalent at reducing blood loss following Total hip arthroplasty: a randomized controlled trial. J Bone Joint Surg Am 99:373–378. https://doi.org/10.2106/jbjs.16.00188
Christensen CP, Jacobs CA (2015) Comparison of patient function during the first six weeks after direct anterior or posterior Total hip arthroplasty (THA): a randomized study. J Arthroplast 30:94–97. https://doi.org/10.1016/j.arth.2014.12.038
Post ZD, Orozco F, Diaz-Ledezma C, Hozack WJ, Ong A (2014) Direct anterior approach for total hip arthroplasty: indications, technique, and results. J Am Acad Orthop Surg 22:595–603. https://doi.org/10.5435/jaaos-22-09-595
Zhao HY, Kang PD, Xia YY, Shi XJ, Nie Y, Pei FX (2017) Comparison of early functional recovery after Total hip arthroplasty using a direct anterior or posterolateral approach: a randomized controlled trial. J Arthroplast 32:3421–3428. https://doi.org/10.1016/j.arth.2017.05.056
Irisson E, Hemon Y, Pauly V, Parratte S, Argenson JN, Kerbaul F (2012) Tranexamic acid reduces blood loss and financial cost in primary total hip and knee replacement surgery. Orthop Traumatol Surg Res 98:477–483. https://doi.org/10.1016/j.otsr.2012.05.002
Lozano M, Basora M, Peidro L, Merino I, Segur JM, Pereira A, Salazar F, Cid J, Lozano L, Mazzara R, Macule F (2008) Effectiveness and safety of tranexamic acid administration during total knee arthroplasty. Vox Sang 95:39–44. https://doi.org/10.1111/j.1423-0410.2008.01045.x
Gross JB (1983) Estimating allowable blood loss: corrected for dilution. Anesthesiology 58:277–280
Nadler SB, Hidalgo JH, Bloch T (1962) Prediction of blood volume in normal human adults. Surgery 51:224–232
Liu X, Zhang X, Chen Y, Wang Q, Jiang Y, Zeng B (2011) Hidden blood loss after total hip arthroplasty. J Arthroplast 26:1100–1105.e1101. https://doi.org/10.1016/j.arth.2010.11.013
Lin ZX, Woolf SK (2016) Safety, efficacy, and cost-effectiveness of tranexamic acid in orthopedic surgery. Orthopedics 39:119–130. https://doi.org/10.3928/01477447-20160301-05
Hardy JF, Belisle S (1997) Natural and synthetic antifibrinolytics: inert, poisonous or therapeutic agents? Can J Anaesth 44:913–917. https://doi.org/10.1007/bf03011960
Zhang LK, Ma JX, Kuang MJ, Zhao J, Wang Y, Lu B, Sun L, Ma XL (2017) Comparison of oral versus intravenous application of tranexamic acid in total knee and hip arthroplasty: a systematic review and meta-analysis. Int J Surg (London, England) 45:77–84. https://doi.org/10.1016/j.ijsu.2017.07.097
Yuan X, Li B, Wang Q, Zhang X (2017) Comparison of 3 routes of administration of tranexamic acid on primary unilateral total knee arthroplasty: a prospective, randomized, controlled study. J Arthroplast 32:2738–2743. https://doi.org/10.1016/j.arth.2017.03.059
Pilbrant A, Schannong M, Vessman J (1981) Pharmacokinetics and bioavailability of tranexamic acid. Eur J Clin Pharmacol 20:65–72
Zohar E, Ellis M, Ifrach N, Stern A, Sapir O, Fredman B (2004) The postoperative blood-sparing efficacy of oral versus intravenous tranexamic acid after total knee replacement. Anesth Analg 99:1679–1683, table of contents. https://doi.org/10.1213/01.ane.0000136770.75805.19
Tuttle JR, Ritterman SA, Cassidy DB, Anazonwu WA, Froehlich JA, Rubin LE (2014) Cost benefit analysis of topical tranexamic acid in primary total hip and knee arthroplasty. J Arthroplast 29:1512–1515. https://doi.org/10.1016/j.arth.2014.01.031
Gillette BP, Maradit Kremers H, Duncan CM, Smith HM, Trousdale RT, Pagnano MW, Sierra RJ (2013) Economic impact of tranexamic acid in healthy patients undergoing primary total hip and knee arthroplasty. J Arthroplast 28:137–139. https://doi.org/10.1016/j.arth.2013.04.054
Xie J, Ma J, Yao H, Yue C, Pei F (2016) Multiple boluses of intravenous tranexamic acid to reduce hidden blood loss after primary total knee arthroplasty without tourniquet: a randomized clinical trial. J Arthroplast 31:2458–2464. https://doi.org/10.1016/j.arth.2016.04.034
Funding
This research did not receive financial support from funding agencies in the public, commercial, or not-for-profit sectors.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Zhao, H., Xiang, M., Xia, Y. et al. Efficacy of oral tranexamic acid on blood loss in primary total hip arthroplasty using a direct anterior approach: a prospective randomized controlled trial. International Orthopaedics (SICOT) 42, 2535–2542 (2018). https://doi.org/10.1007/s00264-018-3846-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00264-018-3846-6