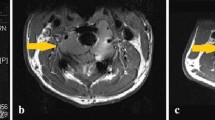
Abstract
Recent clinical studies have suggested that denosumab is associated with beneficial tumour response, surgical down-staging, and reduced surgical morbidity in patients with giant cell tumour of bone. However, these studies reported results of patients still on denosumab treatment, or patients after denosumab treatment but with a short follow-up. Other studies reported that the new osseous tumour matrix and thickened cortical bone that develop with denosumab treatment does not allow the surgeon to delineate the true extent of the tumour, and probably increases the risk for local recurrence. A study showed that cell proliferation is only diminished by denosumab; the cells continue to proliferate in vitro, albeit at a slower rate. More importantly, nine cases of malignant transformation of GCT during denosumab therapy without previous radiation exposure have been reported; inhibition of RANKL may increase the risk of new malignancies due to immunosuppression. With these concerns in mind, this article is an attempt to put essential information in one place, creating a comprehensive review that the curious reader would find interesting and informative.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Editorial
The treatment of giant cell tumour (GCT) remains controversial [1]. Surgical treatment options include intralesional surgery (curettage) using a high-speed burr or resection [2]. Curettage has a higher recurrence rate, but preserves adjacent joint function. Resection with wide margins minimises tumour recurrence; however, it is associated with worse functional results [3]. Some authors recommend the use of local adjuvants combined with curettage to reduce the risk of recurrence [4,5,6], while according to others, local adjuvants do not improve the outcome to local recurrence [7, 8]. On 13 June 2013, the Food and Drug Administration (FDA) approved denosumab (Xgeva®, subcutaneous injection; Amgen, Thousand Oaks, CA, USA), a monoclonal antibody that binds receptor activation of nuclear factor kappa-β ligand (RANKL), for the treatment of adults and skeletally mature adolescents with giant cell tumour of bone that is unresectable or where surgical resection is likely to result in severe morbidity [9,10,11].
Recent clinical studies have suggested that denosumab is associated with beneficial tumour response [9,10,11,12], surgical down-staging [11,12,13] and reduced surgical morbidity in patients with GCT [9,10,11,12,13]. However, these studies reported results of patients still on denosumab treatment, or patients after denosumab treatment but with a short follow-up (median, 13 months; range, 4–13 months) [11, 13]. Thomas et al. [10] reported the first open-label phase II study showing clinical benefits of denosumab treatment in 37 patients with GCT; however, only a small minority of the patients in that series underwent intralesional surgery after denosumab. Chawla et al. [11], in a similar open-label phase II study in 282 patients with GCT, confirmed the safety and efficacy of denosumab, including a capacity of reducing the need for morbid surgery [11]. As in the study of Thomas et al. [10], the study of Chawla et al. [11] reported results of patients still on denosumab treatment or patients that underwent surgery after denosumab treatment with a short follow-up (median, 9.2 months). Another open-label phase II study evaluated reduction of surgical morbidity after denosumab treatment in patients with resectable GCT [13]. Overall, 222 patients were evaluable for surgical down-staging. Of the 115 patients who had surgical treatment, local recurrence occurred in 17 patients (15%). The median postoperative follow-up for all patients who had surgical treatment was 13.0 months (range, 8.5–17.9 months). The median post-operative time until local recurrence was 13.6 months (range, 10.5–15.7 months). It is obvious that the median post-operative follow-up was shorter than the median post-operative time until local recurrence. Therefore, as the authors reported, because of the discrepancy and short-term follow-up, these results must be interpreted with caution [13].
Traub et al. [14] reported the results of a prospective non-randomised study of patients with GCT who received denosumab for six to 11 months pre-operatively; all patients underwent intralesional surgery. Local recurrence occurred in 3/18 patients (17%), at ten, 12 and 25 months post-operatively. The median follow-up after surgical treatment was 30 months (range, 20–45 months). The authors reported that the new osseous tumour matrix and thickened cortical bone that develop with denosumab treatment raises a new surgical challenge by not allowing the surgeon to delineate the true extent of the tumour [14]. In fact, tumour cells can “hide” within the thickened cortex and subchondral bone, which could unfavourably increase the risk of local recurrence.
Other authors confirmed these data, reporting a local recurrence rate of 8.3% in 12 patients with GCT treated by curettage after denosumab treatment, and emphasised on the same conclusions: tumour cells can remain in the newly-formed bone induced by denosumab and the stiff newly formed bone makes intralesional surgery more difficult [15]. Rekhi et al. [16] reported a local recurrence rate of 18.5% in 27 patients with GCT treated by surgery and denosumab therapy at a median follow-up of 18 months. Intralesional surgery was undertaken on 15 patients and resection on 12 patients. Unfortunately, the authors did not differentiate the two groups of patients with respect to local recurrence, and it is not possible to know the real local recurrence rate after curettage following denosumab treatment. Moreover, the follow-up was again too short for important conclusions to be drawn regarding the local recurrence rate.
Goldschlager et al. [17] reported no local recurrence in two patients with GCT of the spine treated with denosumab and en bloc vertebrectomy. Müller et al. [15] reported that five patients had resection after denosumab treatment without any local recurrence [15]. Therefore, resection following denosumab therapy seems to decrease local recurrence compared to resection only. Probably, denosumab improves subchondral and cortical bone by reconstituting a peripheral rim that allows for easier resection (Table 1) [9, 14, 15, 17, 18].
A recent in vitro study examined the viability and osteoclastogenic capabilities of neoplastic stromal cells of GCT [19]. This study showed that cell proliferation is only diminished by denosumab; the cells continue to proliferate in vitro, albeit at a slower rate. These data show that denosumab appears to be biologically active in inhibiting osteoclastogenesis. However, although the stromal cells are quiescent during denosumab treatment, the neoplastic cells remain proliferative once the microenvironment is free of denosumab [19]. Although generally considered benign, rarely GCT can metastasise despite maintaining a benign histology [2]. In this setting, nine cases of malignant transformation of GCT during denosumab therapy without previous radiation exposure have been reported (Table 2) [10, 11, 13, 20, 21]. In the study of Thomas et al. [10], two patients developed new sarcomas; one patient developed a high-grade sarcoma in the upper extremity during denosumab treatment and another patient developed a malignant GCT with lung metastases eight months after discontinuing denosumab. Similarly, in the study of Chawla et al. [11], two patients developed new sarcomas; in the first patient, the sarcoma was retrospectively suspected to be present at baseline, and in the second patient, the sarcoma was thought to be a malignant transformation [11]. In the study of 222 patients with GCT of Rutkowski et al. [13], the GCT lesions in two patients developed malignant transformation under denosumab treatment. These authors considered the diagnosis of primary malignant GCT that was missed by sampling error at the time of the initial core biopsy [13]. Aponte-Tinao et al. [20] reported a patient with a recurrent GCT who developed a bone sarcoma while receiving denosumab treatment. Broehm et al. [21] reported two patients with malignant transformation of their GCT to osteosarcoma while receiving denosumab treatment [21]. All patients in these series reported a clinical benefit to denosumab treatment until the occurrence of malignant transformation, while none of these patients had undergone previous radiation therapy. The expression of RANKL plays an important role in B- and T-cell differentiation and dendritic cell survival; its inhibition of bone destruction could eventually increase the risk of new malignancies due to immunosuppression [22,23,24].
We have a concern regarding the ability to perform a complete curettage of GCT after denosumab treatment. The rim of new bone may contain neoplastic cells that may reactivate once denosumab treatment is finished. Therefore, if curettage is feasible, we do not suggest denosumab administration for the treatment of GCT. In addition, as the present literature review has summarised, the scientific community and treating physicians should be aware of the possible association of denosumab treatment with malignant transformation of GCT or occurrence of new malignancies.
References
Klenke FM, Wenger DE, Inwards CY et al (2011) Giant cell tumor of bone: risk factors for recurrence. Clin Orthop 469:591–599. doi:10.1007/s11999-010-1501-7
Errani C, Ruggieri P, Asenzio MAN et al (2010) Giant cell tumor of the extremity: a review of 349 cases from a single institution. Cancer Treat Rev 36:1–7. doi:10.1016/j.ctrv.2009.09.002
van der Heijden L, Dijkstra PDS, van de Sande MAJ et al (2014) The clinical approach toward giant cell tumor of bone. Oncologist 19:550–561. doi:10.1634/theoncologist.2013-0432
Balke M, Schremper L, Gebert C et al (2008) Giant cell tumor of bone: treatment and outcome of 214 cases. J Cancer Res Clin Oncol 134:969–978. doi:10.1007/s00432-008-0370-x
Kivioja AH, Blomqvist C, Hietaniemi K et al (2008) Cement is recommended in intralesional surgery of giant cell tumors: a Scandinavian sarcoma group study of 294 patients followed for a median time of 5 years. Acta Orthop 79:86–93. doi:10.1080/17453670710014815
Lackman RD, Crawford EA, King JJ, Ogilvie CM (2009) Conservative treatment of Campanacci grade III proximal humerus giant cell tumors. Clin Orthop 467:1355–1359. doi:10.1007/s11999-008-0583-y
Prosser GH, Baloch KG, Tillman RM et al (2005) Does curettage without adjuvant therapy provide low recurrence rates in giant-cell tumors of bone? Clin Orthop:211–218
Turcotte RE, Wunder JS, Isler MH et al (2002) Giant cell tumor of long bone: a Canadian sarcoma group study. Clin Orthop:248–258
Gaston CL, Grimer RJ, Parry M et al (2016) Current status and unanswered questions on the use of Denosumab in giant cell tumor of bone. Clin Sarcoma Res 6:15. doi:10.1186/s13569-016-0056-0
Thomas D, Henshaw R, Skubitz K et al (2010) Denosumab in patients with giant-cell tumour of bone: an open-label, phase 2 study. Lancet Oncol 11:275–280. doi:10.1016/S1470-2045(10)70010-3
Chawla S, Henshaw R, Seeger L et al (2013) Safety and efficacy of denosumab for adults and skeletally mature adolescents with giant cell tumour of bone: interim analysis of an open-label, parallel-group, phase 2 study. Lancet Oncol 14:901–908. doi:10.1016/S1470-2045(13)70277-8
Ueda T, Morioka H, Nishida Y et al (2015) Objective tumor response to denosumab in patients with giant cell tumor of bone: a multicenter phase II trial. Ann Oncol 26:2149–2154. doi:10.1093/annonc/mdv307
Rutkowski P, Ferrari S, Grimer RJ et al (2015) Surgical downstaging in an open-label phase II trial of denosumab in patients with giant cell tumor of bone. Ann Surg Oncol 22:2860–2868. doi:10.1245/s10434-015-4634-9
Traub F, Singh J, Dickson BC et al (2016) Efficacy of denosumab in joint preservation for patients with giant cell tumour of the bone. Eur J Cancer 59:1–12. doi:10.1016/j.ejca.2016.01.006
Müller DA, Beltrami G, Scoccianti G et al (2016) Risks and benefits of combining denosumab and surgery in giant cell tumor of bone-a case series. World J Surg Oncol 14:281. doi:10.1186/s12957-016-1034-y
Rekhi B, Verma V, Gulia A et al (2016) Clinicopathological features of a series of 27 cases of post-denosumab treated giant cell tumors of bones: a single institutional experience at a tertiary cancer referral centre. Pathol Oncol Res 23:157-164. doi:10.1007/s12253-016-0123-0
Goldschlager T, Dea N, Boyd M et al (2015) Giant cell tumors of the spine: has denosumab changed the treatment paradigm? J Neurosurg Spine 22:526–533. doi:10.3171/2014.10.SPINE13937
de Carvalho Cavalcante RA, Silva Marques RA, dos Santos VG et al (2016) Spondylectomy for giant cell tumor after denosumab therapy. Spine 41:E178–E182. doi:10.1097/BRS.0000000000001191
Mak IWY, Evaniew N, Popovic S et al (2014) A translational study of the neoplastic cells of giant cell tumor of bone following neoadjuvant denosumab. J Bone Joint Surg Am 96:e127. doi:10.2106/JBJS.M.01332
Aponte-Tinao LA, Piuzzi NS, Roitman P, Farfalli GL (2015) A high-grade sarcoma arising in a patient with recurrent benign giant cell tumor of the proximal tibia while receiving treatment with denosumab. Clin Orthop 473:3050–3055. doi:10.1007/s11999-015-4249-2
Broehm CJ, Garbrecht EL, Wood J, Bocklage T (2015) Two cases of sarcoma arising in giant cell tumor of bone treated with denosumab. Case Rep Med 2015:767198. doi:10.1155/2015/767198
Criscitiello C, Viale G, Gelao L et al (2015) Crosstalk between bone niche and immune system: osteoimmunology signaling as a potential target for cancer treatment. Cancer Treat Rev 41:61–68. doi:10.1016/j.ctrv.2014.12.001
Smith MR, Egerdie B, Hernández Toriz N et al (2009) Denosumab in men receiving androgen-deprivation therapy for prostate cancer. N Engl J Med 361:745–755. doi:10.1056/NEJMoa0809003
Ellis GK, Bone HG, Chlebowski R et al (2008) Randomized trial of denosumab in patients receiving adjuvant aromatase inhibitors for nonmetastatic breast cancer. J Clin Oncol 26:4875–4882. doi:10.1200/JCO.2008.16.3832
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
No benefits have been or will be received from a commercial party related directed or indirectly to the subject matter of this article.
Funding
There is no funding source.
Ethical approval
This article does not contain any studies with human participants or animals performed by any of the authors.
Informed consent
Informed consent was obtained from all individual participants included in the study.
Rights and permissions
About this article
Cite this article
Errani, C., Tsukamoto, S. & Mavrogenis, A.F. How safe and effective is denosumab for bone giant cell tumour?. International Orthopaedics (SICOT) 41, 2397–2400 (2017). https://doi.org/10.1007/s00264-017-3536-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00264-017-3536-9