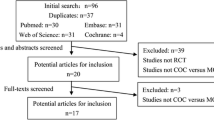
Abstract
Purpose
Ceramic coatings have been used in metal-on-polyethylene (MOP) hips to reduce the risk of wear and also infection; the clinical efficacy of this remains unclear. This retrieval study sought to better understand the performance of coated bearing surfaces.
Methods
Forty-three coated MOP components were analysed post-retrieval for evidence of coating loss and gross polyethylene wear. Coating loss was graded using a visual semi-quantitative protocol. Evidence of gross polyethylene wear was determined by radiographic analysis and visual inspection of the retrieved implants.
Results
All components with gross polyethylene wear (n = 10) were revised due to a malfunctioning acetabular component; 35 % (n = 15) of implants exhibited visible coating loss and the incidence of polyethylene wear in samples with coating loss was 54 %, significantly (p = 0.02) elevated compared to samples with intact coatings (14 %).
Conclusions
In this study we found evidence of coating loss on metal femoral heads which was associated with increased wear of the corresponding polyethylene acetabular cups.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Background
Conventional metal-on-polyethylene (MOP) total hip replacements (THRs) are susceptible to wear of the polyethylene bearing, which may be associated with osteolysis and aseptic loosening [1]. The use of titanium-nitride (TiN) or titanium-niobium-nitride (TiNbN) based coatings on the metal femoral head [2] offers theoretical advantages of minimising polyethylene wear [3, 4].
In vitro mechanical hip simulation of coated MOP bearings under standard and adverse conditions has demonstrated a reduction in wear [4–6]. However, recent retrieval studies have demonstrated that simulation does not always predict real life [3, 7–10], although these have investigated small numbers of implants.
It is reported that when coating breakthrough occurs in TiN coated components, the wear properties of the implant revert to that of a typical un-coated prostheses and the polyethylene wear rate increases [11–14]. The frequency and severity of coating removal in a larger cohort of implants is however unknown and the effect of this on risk of revision is not fully understood.
In this study we sought to: (1) characterise the location, incidence and severity of coating loss in a series of 43 retrieved, coated MOP hips, (2) identify components with gross polyethylene wear and (3) determine if there was a correlation between coating loss and polyethylene wear.
Methods
This retrieval study involved a series of 43 coated MOP THRs that were consecutively collected from a single revising institution and subsequently sent to our centre for analysis; the femoral heads had been coated with either TiN (n = 39) or TiNbN (n = 4). The hips were retrieved from 19 male and 24 female patients with a median age of 73 (27 – 88) years at primary surgery and a median time to revision of 73 (11 – 193) months; the heads were 36 mm in diameter. The reasons for revision, as reported by the revising surgeon, were loosening of cup and stem (n = 5), loosening of cup (n = 9), loosening of stem (n = 7), infection (n = 5), metallosis (n = 3), dislocation (n = 1) and gross cup damage (n = 10); the reason for revision could not be identified in three cases.
Characterising coating removal
A semi-quantitative visual grading protocol was implemented for analysing coating removal; this protocol was adapted for use in this study from previously published work describing the detailed inspection of hip implant bearing surfaces [15]. The surface of each femoral head was visually divided into eight zones (Fig. 1), and each zone was scored on a scale of 0–3 based on the extent of coating removal exhibited in that area (Table 1). The scores for each zone were totalled, providing an overall ‘coating-loss’ score for each implant. Examinations were performed independently by two examiners experienced in retrieval analysis. This methodology facilitated analysis of coating loss with regards to (1) incidence, (2) severity and (3) location.
Scanning electron microscopy
We used a JEOL JSM (Tokyo, Japan) scanning electron microscopy (SEM) with secondary electron detection at an accelerating voltage of 20 KV to examine surface differences between coated surface regions and regions where coating loss had occurred.
Assessment of polyethylene wear
Pre-revision plain radiographs were available for 35/43 hips; these were analysed by an experienced orthopaedic surgeon for evidence of gross wear of the polyethylene acetabular component. An implant head positioned asymmetrically in the acetabular component was deemed to have mechanically worn the polyethylene cup [16–18]. Gross polyethylene wear was characterised if the X-ray demonstrated implant head penetration in a supero-lateral direction [17, 19–21]. The retrieved polyethylene cups’ bearing surfaces were also macroscopically examined by a single observer for evidence of adhesive and third body abrasive wear. In all cases, the result of analysis was either (1) clear evidence of polyethylene wear or (2) no clear evidence of polyethylene wear.
Statistical analysis
The strength of agreement in the coating-loss scores reported by the two examiners was assessed by performing Cohen’s weighted Kappa (κ) statistical analysis; Kappa values were assessed using the assessment criteria of κ ≤ 0 = poor, 0.01–0.20 = slight, 0.21–0.40 = fair, 0.41–0.60 = moderate, 0.61–0.80 = substantial, 0.81–1 = almost perfect.
Non-parametric tests were performed to test the strength of correlations between coating loss and polyethylene wear.
Results
Characterising coating removal
We found that 35 % (n = 15) of femoral heads exhibited some evidence of coating removal whilst the coatings appeared to have remained intact in 65 % (n = 28) of cases. In cases with coating removal, the median (range) score for severity of loss was 4 (1–10), out of a maximum of 24. The polar regions (D zones) had significantly greater coating loss than the equatorial regions (R zones) (p < 0.01).
Scanning electron microscopy
Examination of the head surfaces under SEM revealed considerable evidence of scratching on the exposed metal surfaces, possibly due to third bodies such as coating fragments (Fig. 2). There were small localised regions of coating loss visible in the coated areas however there was notably less evidence of scratching.
Assessment of polyethylene wear
Of the 35 cases available for radiographic analysis, 37 % (n = 13) revealed evidence of gross polyethylene wear (Fig. 3). All implants that were categorised as demonstrating polyethylene wear upon radiographic analysis were revised due to a malfunctioning acetabular component, either due to cup loosening or gross cup damage.
Upon visual inspection, distinctly dull areas of apparent wear were present on the surface of these components, indicative of abrasive wear (Fig. 4). These regions were adjacent to areas of glossy wear, indicative of adhesive wear.
Statistical analysis
Weighted kappa analysis comparing the scores between the two examiners yielded a value of 0.659; this suggested substantial agreement between the two examiners in assessment of coating loss.
The incidence of polyethylene wear in samples with coating loss was 54 %, which was significantly (p = 0.02) elevated compared to samples with intact coatings (14 %) (Fig. 5).
Discussion
This study demonstrated that coating loss was visible in 35 % of samples and more common in the polar than equatorial region of the implant. Components with accompanying radiographs that exhibited gross polyethylene were revised due to a malfunctioning polyethylene component. These components exhibited adhesive and third-body abrasive wear upon visual inspection. We found a significant correlation between the incidence of coating loss and the incidence of gross polyethylene wear.
The existence of visible coating removal raises issues regarding the cause of detachment and the clinical viability of the prosthesis post-detachment in vivo. The propensity for coating failure may be related to a number of variables, such as the method of coating application, which dictates relative adhesion strength of the coating [2]. The method of adhesion used in the prostheses in this study was unknown. A possible explanation for coating removal centres on the principle that the presence of third body particles at the bearing surface may elicit a wear mechanism that leads to coating detachment. Entrapment of third body particles, such as titanium debris and PMMA cement, between the polyethylene and the femoral head component may have increased local stresses within the coating [3, 22]. This may lead to fracture propagation within the coating, thus leading to coating delamination [23, 24].
Coating removal may have led to increased polyethylene wear. The exposed metal substrate may have worn the polyethylene to a greater degree than the coated region, due to the increased surface roughness of the metal surface in comparison to the ceramic coating [25–27]. Another possible mechanism for increased polyethylene wear may be detached coating fragments manifesting as third body particles embedded in the articulating space between femoral head component and polyethylene.
The difference in the amount of coating loss in the polar and equatorial region may be due to differential loading of the regions due to geometric implant design features [28–30]. The work of Tudor et. al [31] demonstrated that relatively greater distances between articulating surfaces results in greater contact pressures on the polar region than the equatorial region. Conversely, relatively smaller distances between articulating surfaces have demonstrated greater contact pressure applied in the equatorial region of the implant than the polar region [31]. The differential contact pressures of the two areas could aid in explaining the difference in incidence of coating loss between polar and equatorial regions.
This study suggests there may be a possible association between coating loss and polyethylene wear, thus impacting the clinical performance of the prosthesis. However, this study did not fully examine all relevant surgical and patient factors. For example, surgical implantation technique data and patient related factors, such as body mass index and patient activity, were not available in the present study. Acquisition of this data in future studies may provide more information about the clinical outcome of these prostheses. Additionally, the nature of retrieval analysis is that all implants in this study had failed; it is not clear what the extent of coating loss is in well-functioning implants. It is also important to note that the extent of wear in contemporary highly cross-linked MOP hips has been favourable [32, 33]; this supports the approach of optimising the polyethylene material used rather than introducing coatings to reduce wear.
Conclusion
In this study we examined a consecutive series of retrieved, coated MOP hips and found evidence of coating loss which was associated with increased polyethylene wear. Future work will investigate which surgical, implant and patient factors increase the risk of coating loss occurring.
References
McKellop HA (2007) The lexicon of polyethylene wear in artificial joints. Biomaterials 28(34):5049–5057
Gotman I, Gutmanas E (2014) Titanium nitride-based coatings on implantable medical devices. Adv Biomater Devices Med 1:53–73
Raimondi MT, Pietrabissa R (2000) The in-vivo wear performance of prosthetic femoral heads with titanium nitride coating. Biomaterials 21(9):907–913
de Villiers D, Traynor A, Collins SN, Banfield S, Housden J, Shelton JC (2015) Chromium nitride coating for large diameter metal-on-polyethylene hip bearings under extreme adverse hip simulator conditions. Wear 328–329:363–368
Pappas MJ, Makris G, Buechel FF (1995) Titanium nitride ceramic film against polyethylene. A 48 million cycle wear test. Clin Orthop Relat Res (317):64–70
Gutmanas EY, Gotman I (2004) PIRAC Ti nitride coated Ti–6Al–4V head against UHMWPE acetabular cup–hip wear simulator study. J Mater Sci Mater Med 15(4):327–330
Harman MK, Banks SA, Hodge WA (1997) Wear analysis of a retrieved hip implant with titanium nitride coating. J Arthroplast 12(8):938–945
Prasad PSV, Takahashi T, Steele N, Richardson JB (2004) A comparative study of bone conserving hip replacements: the thrust plate and the Buechel Pappas resurfacing prosthesis. J Bone Joint Surg British 86-B(SUPP III):279
Malviya A, Lobaz S, Holland J (2007) Mechanism of failure eleven years following a Buechel Pappas hip resurfacing. Acta Orthop Belg 73(6):791
Buechel FF Sr, Buechel FF Jr, Helbig TE, D’Alessio J, Pappas MJ (2004) Two- to 12-year evaluation of cementless Buechel-Pappas total hip arthroplasty. J Arthroplast 19(8):1017–1027
Maurer AM, Brown SA, Payer JH, Merritt K, Kawalec JS (1993) Reduction of fretting corrosion of Ti-6Al-4V by various surface treatments. J Orthop Res 11(6):865–873
Komotori J, Lee BJ, Dong H, Dearnley PA (2001) Corrosion response of surface engineered titanium alloys damaged by prior abrasion. Wear 251(1–12):1239–1249
Erivan R, Villatte G, Khelif YR, Pereira B, Galvin M, Descamps S, Boisgard S (2016) The Muller self-locking cemented total hip prosthesis with polyethylene liner: After twenty years, what did they become? Int Orthop. 2016 Apr 25 [Epub ahead of print]
Yan Y, Chen H, Feng J, Chen K, Zhou K, Hong W, Wang Y, Liu Z, Zhang J, Yang Q, Guo L, He C (2016) Poor performance of Enduran polyethylene liner in total hip arthroplasty: a minimum ten-year follow up and ultra-morphological analysis of wear particles. Int Orthop. 2016 May 13. [Epub ahead of print]
Hothi HS, Berber R, Whittaker RK, Bills PJ, Skinner JA, Hart AJ (2015) Detailed inspection of metal implants. Hip Int 25(3):227–231
Weissman BN (1997) Imaging of total hip replacement. Radiology 202(3):611–623
McBride TJ, Prakash D (2011) How to read a postoperative total hip replacement radiograph. Postgrad Med J 87(1024):101–109
Paydar A, Chew FS, Manner PA (2007) Severe periprosthetic metallosis and polyethylene liner failure complicating total hip replacement: the cloud sign. Radiol Case Rep 2(4):115
Fisher J (1998) Wear of polyethylene in artificial hip joints: superolateral wear of the acetabulum. J Bone Joint Surg British 80-B(2):190–191
Murray DW, O’Connor JJ (1998) Superolateral wear of the acetabulum. J Bone Joint Surg British 80-B(2):197–200
Badhe S, Livesley P (2006) Early polyethylene wear and osteolysis with ABG acetabular cups (7- to 12-year follow-up). Int Orthop 30(1):31–34
Davidson JA, Poggie RA, Mishra AK (1994) Abrasive wear of ceramic, metal, and UHMWPE bearing surfaces from third-body bone, PMMA bone cement, and titanium debris. Bio-Med Mater Eng 4(3):213–229
Wiklund U, Gunnars J, Hogmark S (1999) Influence of residual stresses on fracture and delamination of thin hard coatings. Wear 232(2):262–269
van Hove RP, Sierevelt IN, van Royen BJ, Nolte PA (2015) Titanium-nitride coating of orthopaedic implants: a review of the literature. BioMed Res Int. Article ID 485975
Kim Y-H, Ritchie A, Hardaker C (2005) Surface Roughness of Ceramic Femoral Heads After in Vivo Transfer of Metal: Correlation to Polyethylene Wear. J Bone Joint Surg Am 87(3):577–582
Pappas MJ, Makris G, Buechel FF (1995) Titanium nitride ceramic film against polyethylene—a 48-million cycle wear test. Clin Orthop Rel Res (317):64–70
Fabry C, Zietz C, Baumann A, Bader R (2015) Wear performance of sequentially cross-linked polyethylene inserts against ion-treated CoCr, TiNbN-Coated CoCr and Al2O3 ceramic femoral heads for total hip replacement. Lubricants 3(1):14–26
Morlock M, Bishop N, Huber G (2011) Biomechanics of hip arthroplasty. In: Knahr K (ed) Tribology in total hip drthroplasty. Springer, Berlin, pp 11–24
Mattei L, Di Puccio F, Piccigallo B, Ciulli E (2011) Lubrication and wear modelling of artificial hip joints: A review. Tribol Int 44(5):532–549
Hernández-Rodríguez MAL, Mercado-Solís RD, Pérez-Unzueta AJ, Martinez-Delgado DI, Cantú-Sifuentes M (2005) Wear of cast metal–metal pairs for total replacement hip prostheses. Wear 259(7–12):958–963
Tudor A, Laurian T, Popescu VM (2013) The effect of clearance and wear on the contact pressure of metal on polyethylene hip prostheses. Tribol Int 63:158–168
Jauregui JJ, Naziri Q, Pierce TP, Elmallah RK, Cherian JJ, Delanois RE, Mont MA (2015) Is the use if thin, highly cross-linked polyethylene liners safe in total hip arthroplasty? Int Orthop 40:681–686
Scemama C, Dora C, Langlois J, Hamadouche M (2014) Minimum five-year wear rate of metal-on-highly cross-linked polyethylene in primary total hip arthroplasty. Int Orthop 39:1051–1055
Acknowledgments
We are grateful for the support of Gwynneth Lloyd, Elizabeth Ellis and Akramul Hoque for their coordination of the retrieval centre and the surgeons and patients that have contributed implants to this study.
Two authors received funding from the British Orthopaedic Association through an industry consortium of nine manufacturers: DePuy International Ltd, Zimmer GmbH, Smith & Nephew UK Ltd, Biomet UK Ltd, JRI Ltd, Finsbury Orthopaedics Ltd, Corin Group PLC, Mathys Orthopaedics Ltd, and Stryker UK Ltd.
This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Khatkar, H., Hothi, H., de Villiers, D. et al. Retrieval analysis of ceramic-coated metal-on-polyethylene total hip replacements. International Orthopaedics (SICOT) 41, 1101–1105 (2017). https://doi.org/10.1007/s00264-016-3314-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00264-016-3314-0