Abstract
Purpose
Quantitative evaluation of vascular ingrowth to the bone tunnel walls and tendon graft after anterior cruciate ligament reconstruction for up to two years post-surgery using magnetic resonance angiography (MRA).
Methods
The study population consists of 100 patients that underwent reconstruction with multi-stranded semitendinosus tendons. The patients were retrospectively divided into those that underwent MRA two, three, four to six, and ≥ seven months after surgery (46, 17, 16, and 21 patients, respectively). Digital imaging and communication in medicine (DICOM) MRA images were imported into image processing software (OsiriX®), and the mean signal-to-noise ratio (SNR) of the bone tunnel walls in the femur and tibia and tendon graft parenchyma in the bone tunnels were measured.
Results
On MRA, the signal intensities of the bone tunnel walls in the femur and tibia (12.6 ± 3.41 and 10.7 ± 3.04) were greater than that in the tendon graft (2.65 ± 1.94 and 2.50 ± 2.02, respectively) at two months after surgery. At three months after surgery, the intensities of the tendon grafts (6.25 ± 2.18 and 5.77 ± 1.57, respectively) were greater than those of the bone tunnel wall (2.56 ± 1.29 and 2.50 ± 1.11, respectively). At four to six months, the intensities in the bone tunnel wall were 1.76 ± 0.73 and 1.62 ± 0.72, respectively, and those in the tendon graft were 5.01 ± 2.11 and 4.01 ± 2.35, respectively. At ≥ seven months after surgery, the intensities in the bone tunnel wall were 1.36 ± 0.63 and 1.21 ± 0.87, respectively, and those in the tendon graft were 4.25 ± 1.87 and 3.44 ± 1.99, respectively.
Conclusion
Blood flow was seen around the bone tunnel on the femoral and tibial sides two months after ACL reconstruction and in the tendon graft parenchyma three months after surgery. The remodeling process continued after seven months.
Level of evidence IV
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Hamstring tendons are one of the most commonly used grafts in anterior cruciate ligament (ACL) reconstruction [1–6]. The harvested hamstring is passed through bone tunnels made in the tibia and the femur, and is then fixed. However, the tendon graft tends to necrose immediately after surgery because there are no blood vessels in the hamstring, and more than a year may be necessary to achieve consolidation of the bone tunnel walls and tendon graft in the bone tunnels as well as maturation of the tunnels and joint [7]. As this remodeling process influences the time to return to sports after ACL reconstruction, post-surgical evaluation of blood flow to the bone tunnel walls is important.
While many studies involving such evaluation of blood flow have been performed in animal models [8, 9], Arai et al. initially succeeded in achieving visualization of blood flow to the bone tunnel walls and tendon graft in the bone tunnels after ACL reconstruction in human subjects using magnetic resonance angiography (MRA) [10]. They showed that blood flow re-started from the middle and inferior genicular arteries toward the bone tunnel walls, and supplied blood flow to the tendon graft in the bone tunnels. Using this technique, Terauchi et al. quantified blood flow to the bone tunnel walls and tendon graft in the tunnels from two to six months after ACL reconstruction in 30 patients, and showed that blood flow was present in the bone tunnel walls two months after surgery [11]. They also evaluated the signal intensities of the wall and inside of the bone tunnel using a single image generated by superimposition of oblique sagittal images and maximum intensity projection (MIP) processing, and suggested that the intensity of the bone tunnel wall may have affected the signal intensity inside the bone tunnel, including the tendon graft parenchyma. Tendon graft remodeling remains incomplete up to six months after surgery. Previous studies have not demonstrated changes in the signal intensity of tendon grafts on MRA at seven months after surgery or thereafter.
The purpose of the study was to quantitatively assess the vascular ingrowth of the bone tunnel walls and tendon graft parenchyma in the bone tunnel after ACL reconstruction. We hypothesized that the signal intensity of the bone tunnel wall increases first, followed by the tendon graft parenchyma, and that this enhancement continues during the course of remodeling after ACL reconstruction.
Materials and methods
Subjects
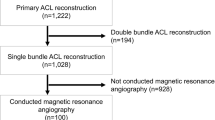
The study population consists of 100 patients (100 knees; 36 men and 64 women) that underwent single-bundle ACL reconstruction with multi-stranded hamstring between 2005 and 2012. The mean age of the patients was 23.7 (range 14–47) years. The inclusion criteria were single-bundle reconstruction with multi-stranded hamstring and initial injury. The exclusion criteria were double-bundle reconstruction, bone-tendon-bone graft, revision, and complex ligament injury. Ethical approval for this study was obtained from the Ethical Review Board of our hospital (ERB-C-159-1).
Surgical technique
All reconstructive procedures were performed using the inside-out technique under arthroscopic observation. The semitendinosus tendon was harvested and arranged into a fourfold loop with a diameter of 9–10 mm. One end of the stump was sutured with 0 Surgilon thread (Kendall, Hampshire, UK) and four sutures were placed for fixation. The Endobutton CL fixation system (Smith & Nephew, Andover, MA) was attached to the looped end of the graft for femoral fixation. An artificial ligament (Telos, Tokyo, Japan) made of polyester was connected to the other sutured end of the graft for tibial fixation. A tibial tunnel, 10 mm in diameter, and a femoral tunnel, 10 mm in diameter and 25 mm in length, were drilled.
Nylon threads attached to the endobutton CL–semitendinosus tendon complex were connected to the end of the passing pin and inserted into the tunnel. The ceramic button was inserted into the tibial tunnel. The Telos artificial ligament with the semitendinosus tendon was ligated through the hole of the ceramic button in the 20° flexion position with maximum manual pulling. The grafts in the bone tunnels of femoral and tibial sides were 10–15 mm in length. After insertion of the graft, we confirmed the absence of roof impingement. Post-operatively, the knee was fixed with a splint (Light Splint; ALCARE, Tokyo, Japan) in the 20° flexion position. Knee range-of-motion exercises were begun on the second day after surgery. Partial weight bearing was initiated seven days after surgery. At four weeks post-operatively, walking was permitted; jogging was encouraged eight weeks after reconstruction. Return to sports was permitted six months after surgery, following evaluation of muscle strength.
MRA
All patients underwent MRA after ACL reconstruction. The patients were retrospectively divided into those undergoing MRA two, three, four to six, and ≥ seven months after ACL reconstruction (46, 17, 16, and 21 patients, respectively) (Table 1). The timing of image acquisition was set in consideration of the post-operative rehabilitation protocol. As return to playing sports was permitted 6 months after surgery, images were acquired at two, three and four to six months during medical and athletic rehabilitation. As remodeling continues beyond seven months after surgery, evaluation was performed for up to two years after surgery. MRA was performed after four months in patients treated between August 2005 and March 2008, and up to three months after surgery in those treated between April 2008 and December 2012.
Imaging was performed using a 1.5-T magnetic resonance imager (Gyroscan; Philips Medical Systems, Best, The Netherlands) with a knee coil, as described previously [11, 12]. For fat suppression images, the water selective excitation technique was used. Contrast-enhanced MRA was performed after intravenous injection of Gd-DTPA (0.1 mmol/kg body weight). To reduce the background tissue signal, a mask image was obtained immediately after administration of the contrast agent. The imaging parameters were as follows: repetition time (TR) = 13 ms, echo time (TE) = 5.2 ms, flip angle (FA) = 25°, field of view = 150 mm, slice thickness = 6 mm, gap between slices = 3 mm, with a 224 × 180 matrix. Digital subtraction angiography was performed every 24 s. Imaging was confined to oblique and sagittal sections to allow for alignment of the popliteal artery. The total imaging time was 140 s. In the first step of reconstruction, the mask image data set was subtracted from the contrast-enhanced data set.
Analysis
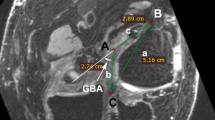
MRA DICOM images were quantified using image processing software (OsiriX®). To set the region of interest (ROI), slices in which the tendon graft and bone tunnel walls were most linear in the longitudinal direction in an oblique sagittal plane were used. On the femoral side, the inner wall of the bone tunnel, 5 mm anterior to the center of the tendon graft on Blumensaat’s line and parallel to this line, was regarded as the bone tunnel wall, which was manually enclosed as a linear region with a width of 2.0 mm. The tendon graft parenchyma in the bone tunnel on the femoral side was enclosed manually as a linear region with a width of 10 mm set on the boundary of the joint surface side parallel to Blumensaat’s line. On the tibial side, the inner wall of the bone tunnel, 5 mm anterior to the center of the tendon graft at the bone tunnel opening on the joint surface side parallel to the joint surface, was regarded as the bone tunnel wall, and the wall was manually enclosed as a linear region with a width of 2 mm. The tendon graft parenchyma in the bone tunnel on the tibial side was enclosed as a linear region with a width of 10 mm, setting the boundary on the joint surface side parallel to the joint surface at the bone tunnel opening. The signal intensity was measured independently by two separate orthopaedic surgeons in a blinded manner, and each measurement was repeated twice with the average values regarded as the results. The measured signal intensity was divided by the signal intensity of air at 2 cm anterior to the tibial tuberosity to obtain the signal-to-noise ratio (SNR), and the signal intensity was normalized (Fig. 1).
Statistical analysis
Measurements were expressed as the means ± standard deviation. Two-way analysis of variance (ANOVA) was used to analyze the mean SNR with group (bone tunnel wall or tendon graft parenchyma) and post-operative period (2, 3, 4–6, ≥ 7 months) as between-subject factors; Tukey’s post hoc test was performed for multiple comparisons. Intraclass correlation coefficients (ICCs) were calculated with a two-way random model. Statistical analyses were conducted using SPSS (version 21.0 for Windows; IBM, Chicago, IL). In all analyses, p < 0.05 was taken to indicate statistical significance.
Results
Bone tunnel wall and tendon graft in the femur
The mean signal intensity of the femoral bone tunnel wall was highest at two months after surgery (12.6 ± 3.41), and it decreased significantly to 2.56 ± 1.29 at three months (p < 0.001) (Fig. 2). The intensity was 1.76 ± 0.73 at four to six months (p = 0.25, compared with 3 months) and 1.36 ± 0.63 at ≥ seven months (p = 0.21, compared with 4–6 months), showing no significant change. In the tendon graft parenchyma in the femoral tunnel, the mean signal intensity increased significantly from 2.65 ± 1.94 at two months after surgery to 6.25 ± 2.18 at three months (p < 0.001). The intensity was 5.01 ± 2.11 at four to six months (p = 0.32, compared with 3 months) and 4.25 ± 1.87 at ≥ seven months (p = 0.58, compared with 4–6 months), showing no significant change. The signal intensity was significantly higher in the femoral bone tunnel wall compared with the tendon graft parenchyma at two months after surgery, but was significantly higher in the tendon graft parenchyma at ≥ three months.
Bone tunnel wall and tendon graft in the tibia
The mean signal intensity of the tibial bone tunnel wall was highest at two months after surgery (10.7 ± 3.04), and decreased significantly to 2.50 ± 1.11 at three months (p < 0.001) (Fig. 3). The intensity decreased significantly to 1.62 ± 0.72 at four to six months, compared with that at three months (p = 0.046). The intensity at ≥ seven months was 1.21 ± 0.87, showing no significant change (p = 0.18). The mean signal intensity of the tendon graft parenchyma in the tibial bone tunnel increased significantly from 2.50 ± 2.02 at two months after surgery to 5.77 ± 1.57 at three months (p < 0.001). The signal intensity was 4.01 ± 2.35 at four to six months (p = 0.10, compared with 3 months) and 3.44 ± 1.99 at ≥ seven months (p = 0.65, compared with 4–6 months), showing no significant change. The signal intensities of the tibial bone tunnel wall and the tendon graft parenchyma were similar to those in the femur—the intensity was significantly higher in the tibial bone tunnel wall than in the tendon graft parenchyma at two months after surgery, but was significantly higher in the tendon graft parenchyma at ≥ three months.
The inter-observer reliability of the two orthopaedic surgeons for measurement of signal intensity was 0.925. Intra-observer reliabilities of observers 1 and 2 were 0.964 and 0.902, respectively.
Discussion
Blood flow was present in the bone tunnels around the tendon graft two months after surgery, and flowed into the graft in the bone tunnels three months after surgery. Time to return to sport is still a matter of controversy [12]. While muscular strength and endurance level are both important factors, the tendon graft remodeling might also play a crucial role in the rehabilitation period. If the tendon graft remodeling process can be evaluated noninvasively, it may be useful to adjust the exercise intensity during post-operative rehabilitation and permit a return to sports. However, no index is currently available for such evaluation. We performed MRA in 100 patients at two to seven months or later after ACL reconstruction, and demonstrated that the signal intensities of the bone tunnel wall and tendon graft parenchyma in the tunnel changed with the progression of remodeling from early after surgery. Restarting of blood flow from the bone tunnel wall to the tendon graft parenchyma in the bone tunnel was shown to be important for the early remodeling process after ACL reconstruction. These findings suggest that MRA is useful to evaluate the remodeling process after ACL reconstruction.
In 1995, Howell et al. followed up hamstring grafts by contrast MRI for two years after surgery, and reported that the intensity of the tendon graft parenchyma was low, but enhancement of the region around the tendon graft was observed [13]. Ntoulia et al. evaluated BTB grafts, and observed that while the intensity of the tendon graft was reduced three days after surgery, the area surrounding the graft showed high intensity and the tendon graft showed heterogeneous high intensity six months after surgery, with the intensity of the tendon graft decreasing to a low level thereafter [14]. These reports indicated that tendon grafts exhibit heterogeneous low intensity immediately after transplantation, but this changes with time to heterogeneous high intensity, which decreases thereafter, and blood flow reaches around the tendon graft in the remodeling process. However, as the images were acquired when the tendon graft was sufficiently enhanced after intravenous contrast medium injection, only the venous phase was evaluated on contrast MRI, and the route by which blood flow reached the tendon graft may not have been evaluated.
In the present study, to reduce the influence of the intensity of the bone tunnel wall and to individually assess the bone tunnel wall and parenchyma in the bone tunnel, MIP processing was not applied, and the slices in which the bone tunnel walls and tendon graft were most linear in the longitudinal direction in the oblique sagittal plane were used. The tendon graft and bone tunnel walls were directly enclosed, and the intensity was measured in each region. From two to three months after surgery, the signal intensity of the bone tunnel wall decreased significantly on both femoral and tibial sides, but the intensity of the tendon graft in the tunnel increased significantly. When the time course of the intensity was compared between the bone tunnel wall and tendon graft parenchyma, the intensity of the bone tunnel wall was greater two months after surgery, whereas the intensity of the tendon graft parenchyma was greater three months after surgery. The intensity was greater in the tendon graft parenchyma after four months. These findings indicated blood flow around the bone tunnel wall two months after ACL reconstruction and the tendon graft parenchyma three months after surgery, and showed that the remodeling process continued after seven months. We suggest that active tendon graft remodeling occurs from three months after surgery.
Malinin et al. clarified the presence of blood vessels connecting the tendon graft in the bone tunnel and bone tunnel wall in humans in a study in which the grafts were retrieved from ten patients after ACL reconstruction [15]. Dovan et al. reported that blood vessels were regenerated between the FDP tendon placed in a bone tunnel and surrounding the bone tunnel, and promoted bone tunnel–tendon graft fusion in dogs [8]. These results along with our findings indicated blood flow from the middle and inferior genicular arteries around the bone tunnel wall on the femoral and tibial sides two months after ACL reconstruction, with formation of new blood vessels connecting the bone tunnel and tendon graft in the tunnel, the blood flow reached the tendon graft parenchyma three months after surgery, and the remodeling process continued after seven months.
This study had several limitations. First, there was bias in the number of patients between each group. Second, the period of seven to 24 months represents a long time in comparison with previous studies. Third, time course measurement of MRA could not be performed in the same patients. While it may be ideal to apply MRA several times in each patient, there may be problems due to the invasiveness of the use of contrast medium and cost. Fourth, it could not be determined when the feeding vessels reached the graft. Blood flow was observed around the bone tunnel two months after surgery, suggesting that vascularization occurred between zero and two months after surgery.
Conclusion
Blood flow was seen around the bone tunnel on the femoral and tibial sides two months after ACL reconstruction and the tendon graft parenchyma three months after surgery. The remodeling process continued after seven months.
References
Amano H, Toritsuka Y, Uchida R, Mae T, Ohzono K, Shino K (2015) Outcome of anatomical double-bundle ACL reconstruction using hamstring tendons via an outside-in approach. Knee Surg Sports Traumatol Arthrosc 23:1220–1230
Hara K, Arai Y, Ohta M, Minami G, Urade H, Hirai N, Watanabe N, Kubo T (2005) A new double-bundle anterior cruciate ligament reconstruction using the posteromedial portal technique with hamstrings. Arthroscopy 21:1274.e1–1274.e6
Koga H, Muneta T, Yagishita K, Watanabe T, Mochizuki T, Horie M, Nakamura T, Sekiya I (2014) Effect of femoral tunnel position on graft tension curves and knee stability in anatomic double-bundle anterior cruciate ligament reconstruction. Knee Surg Sports Traumatol Arthrosc 22:2811–2820
Kondo E, Yasuda K, Miyatake S, Kitamura N, Tohyama H, Yagi T (2012) Clinical comparison of two suspensory fixation devices for anatomic double-bundle anterior cruciate ligament reconstruction. Knee Surg Sports Traumatol Arthrosc 20:1261–1267
Ohkawa S, Adachi N, Deie M, Nakamae A, Nakasa T, Ochi M (2012) The relationship of anterior and rotatory laxity between surgical navigation and clinical outcome after ACL reconstruction. Knee Surg Sports Traumatol Arthrosc 20:778–784
Andrei BI, Niculescu M, Popescu G (2015) Position of anterior cruciate ligament after single-bundle arthroscopic reconstruction. Int Orthop doi:10.1007/s00264-015-2964-7
Falconiero RP, DiStefano VJ, Cook TM (1998) Revascularization and ligamentization of autogenous anterior cruciate ligament grafts in humans. Arthroscopy 14:197–205
Dovan TT, Ritty T, Ditsios K, Silva MJ, Kusano N, Gelberman RH (2005) Flexor digitorum profundus tendon to bone tunnel repair: a vascularization and histologic study in canines. J Hand Surg [Am] 30:246–257
Unterhauser FN, Bail HJ, Hoher J, Haas NP, Weiler A (2003) Endoligamentous revascularisation of an anterior cruciate ligament graft. Clin Orthop Relat Res 414:276–288
Arai Y, Hara K, Takahashi T, Urade H, Minami G, Takamiya H, Kubo T (2008) Evaluation of the vascular status of autogenous hamstring tendon grafts after anterior cruciate ligament reconstruction in humans using magnetic resonance angiography. Knee Surg Sports Traumatol Arthrosc 16:342–347
Terauchi R, Arai Y, Hara K, Minami G, Nakagawa S, Takahashi T, Ikoma K, Ueshima K, Shirai T, Fujiwara H, Kubo T (2014) Magnetic resonance angiography evaluation of the bone tunnel and graft following ACL reconstruction with a hamstring tendon autograft. Knee Surg Sports Traumatol Arthrosc doi:10.1007/s00167-014-3358-x
Shabani B, Bytyqi D, Lustig S, Cheze L, Bytyqi C, Neyret P (2015) Gait knee kinematics after ACL reconstruction: 3D assessment. Int Orthop 39:1187–1193
Howell SM, Knox KE, Farley TE, Taylor MA (1995) Revascularization of a human anterior cruciate ligament graft during the first two years of implantation. Am J Sports Med 23:42–49
Ntoulia A, Papadopoulou F, Ristanis S, Argyropoulou M, Georgoulis AD (2011) Revascularization process of the bone-patellar tendon-bone autograft evaluated by contrast-enhanced magnetic resonance imaging 6 and 12 months after anterior cruciate ligament reconstruction. Am J Sports Med 39:1478–1486
Malinin TI, Levitt RL, Bashore C, Temple HT, Mnaymneh W (2002) A study of retrieved allografts used to replace anterior cruciate ligaments. Arthroscopy 18:163–170
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
None.
Rights and permissions
About this article
Cite this article
Kanamura, H., Arai, Y., Hara, K. et al. Quantitative evaluation of revascularization at bone tunnels and grafts with contrast-enhanced magnetic resonance angiography after anterior cruciate ligament reconstruction. International Orthopaedics (SICOT) 40, 1531–1536 (2016). https://doi.org/10.1007/s00264-015-3092-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00264-015-3092-0