Abstract
Purpose
This study was designed to identify strategies for treating bone defects that can be completed on the day of surgery.
Methods
Forty New Zealand white rabbits with unilateral rabbit radius segmental defects (15 mm) were treated with commercially available scaffolds containing either demineralised bone matrix (DBM) or a collagen/beta-tricalcium phosphate composite (Col:β-TCP); each scaffold was combined with either bone marrow aspirate (BMA) or concentrated BMA (cBMA). Bone regeneration was assessed through radiographic and histological analyses.
Results
The concentration of nucleated cells, colony-forming unit-fibroblasts and platelets were increased and haematocrit concentration decreased in cBMA as compared to BMA (p < 0.05). Radiographic analyses of bone formation and defect bridging demonstrated significantly greater bone regeneration in the defects treated with DBM grafts as compared to Col:β-TCP grafts. The healing of bones treated with Col:β-TCP was improved when augmented with cBMA.
Conclusions
Scaffolds containing either DBM or Col:β-TCP with BMA or cBMA are effective same-day strategies available to clinicians for the treatment of bone defects; the latter scaffold may be more effective if combined with cBMA.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The complications associated with impaired bone healing have been well documented [1]. The current gold standard to overcome deficiencies in bone healing is the use of autograft [2]. Significant advances have been made in the field of bone tissue engineering with the goal of replacing the use of autogenous bone grafts to avoid the negative effects associated with them. For example, biomaterials, cells and growth factors are manipulated to create tissue-engineered constructs that have both significantly improved our understanding of regenerating bone and demonstrated the potential of regenerative medicine (for review see [3–5]). Although these advances may result in ideal bone healing in the future, it is important for clinicians to be aware of bone healing alternatives to autograft that are currently at their disposal.
Among the tissue-engineering strategies being developed, those using either demineralised bone matrix (DBM) or beta-tricalcium phosphate (β-TCP) have been extensively explored [6–12]. Given the strong foundation supporting their effectiveness and regulatory status it is not surprising that scaffolds incorporating these materials are commercially available. Experimental investigations to improve their use are ongoing [8–11]; however, the overall objective of the vast majority of studies is to develop or optimise materials that have not yet received regulatory approval. Stem cell-based strategies, especially those using bone marrow aspirate (BMA), have been investigated to minimise the use of autograft [13, 14]. The means to concentrate a relatively less pure, heterogeneous mixture of cells from BMA is also being used in preclinical studies and is commercially available [8, 15]. Similar to the development of biomaterials, much of the research effort is directed towards the advancement of novel stem cell-based strategies rather than improving upon the use of approved methodologies. Collectively, the accessibility of both commercially available scaffolds and devices capable of enriching BMA supports the use of same-day strategies to improve the healing of bone defects.
Due to the paucity of experimental studies using clinically relevant, same-day strategies, the tools that are currently available to clinicians are not always clear. To this end, the objective of our studies was to evaluate bone regeneration using commercially available scaffolds containing DBM or β-TCP when combined with either BMA or concentrated BMA (cBMA).
Materials and methods
Experimental animals
This study was conducted in compliance with the Animal Welfare Act, implementing animal welfare regulations, and in accordance with the principles of the Guide for the Care and Use of Laboratory Animals. All animal procedures performed in this study were approved by the Institutional Animal Care and Use Committee at the US Army Institute of Surgical Research. Adult, female New Zealand white rabbits (∼4 kg) were housed in a vivarium accredited by the Association for Assessment and Accreditation of Laboratory Animal Care International. A total of 40 surgical animals were used for this study, ten animals per group (n = 10/group).
Bone marrow aspiration and concentration
BMA was collected in syringes containing anticoagulant (ACD- A, Anticoagulant Citrate Dextrose, Arteriocyte Medical Systems, Hopkinton, MA, USA) after access to the bone marrow compartment of the tibia and iliac crests of donor animals while under anaesthesia, similar to that described previously [16]. The ratio of ACD-A to aspirate was 1:6.5; a total volume of 29.98 ml ± 1.64 of ACD-A:aspirate was collected per animal. After the pooling and filtration of aspirates from two animals an aliquot of BMA (3 ml) was set aside for cell analysis and scaffold loading, and the remainder was used to generate cBMA using the Magellan® MAR01™ (Arteriocyte Medical Systems, Hopkinton, MA, USA) system as per the manufacturer’s instructions. cBMA was also subjected to cell analyses and used to load scaffolds. Each batch of BMA or cBMA was used to load both a DBM and collagen-β-TCP (Col:β-TCP) scaffold to control for donor variability.
BMA and cBMA analyses
An aliquot of BMA or cBMA (∼1 ml) was subjected to red blood cell lysis using 2 % acetic acid in water and the nucleated cells counted manually using a haemocytometer. The fibroblast colony-forming unit (CFU-F) assay was used as an indicator of progenitor cell content in BMA and cBMA, similar to that described previously [17]. A total of 4 × 105 nucleated cells were plated per well of a six-well plate in triplicate in media consisting of α-Minimum Essential Media, 10 % fetal bovine serum and 1 % penicillin/streptomycin in a humidified incubator (5 % CO2, 37 ° C). Forty-eight hours after seeding media was changed, and media was changed every other day thereafter for 10 days. Cells were then washed with phosphate-buffered saline (PBS) and fixed with a 1:1 mixture of acetone:methanol for ten minutes at room temperature. The plates were allowed to air dry and stained with Giemsa to allow for counting of cell colonies. The CFU-F quantification was performed by an independent, blinded reviewer. An aliquot of each sample was also used for complete blood count analyses for haematocrit and platelet quantification.
Graft preparation
DBM grafts: Rabbit DBM powder (MAROMatch™, Arteriocyte Medical Systems, Hopkinton, MA, USA) was thoroughly rehydrated with either BMA or cBMA and a poloxamer reverse phase medium formulated into a gel-like form (MAROFuse™, Arteriocyte Medical Systems, Hopkinton, MA, USA) in a 1:1:1 ratio (1 cc MAROMatch™ + 1 cc MAROFuse™ + 1 cc BMA or cBMA). The preparation time for DBM grafts with BMA or cBMA was approximately five minutes. DBM grafts were then incubated for ten minutes prior to implantation at room temperature.
Col:β-TCP grafts: Col:-β-TCP composite scaffolds (Integra LifeSciences, Plainsboro, NJ, USA) were pre-cut to approximately 15 mm, placed in a 30-cc syringe joined by a stopcock to another syringe containing either 1 cc BMA or cBMA. The scaffolds were loaded with either BMA or cBMA by passing the aspirate through the syringes under negative pressure. Each graft material was incubated in either BMA or cBMA for ten minutes prior to implantation at room temperature.
Surgical procedures
After a ∼40-mm incision over the mid-diaphysis of the radius, a 15-mm segment was removed using an oscillating bone saw with a copious amount of saline irrigation resulting in a critical-sized defect similar to previously described [16]. The segmental defect was then replaced with either (1) DBM + BMA (DBM-BMA), (2) DBM + cBMA (DBM-cBMA), (3) Col:-β-TCP + BMA (Col:β-TCP-BMA) or (4) Col:-β-TCP + cBMA (Col:β-TCP-cBMA) and the soft tissue layers and skin were both closed with 4–0 Vicryl (Ethicon, Somerville, NJ, USA). Due to the radioulnar syndesmosis that forms the fibrous joint between the radius and the ulna, additional fixation of the defect was not required.
Radiographic analysis
Radiographs were taken immediately and four and eight weeks after surgery (MX-20 X-ray machine, Faxitron Corporation, Tucson, AZ, USA). Each radiograph was taken with an exposure time of 15 seconds and a tube voltage of 35 kVp. Radiographs were scored for bone formation and bridging by five blinded observers (including an orthopaedic surgeon) as described by Bodde et al. [18] using the scoring system presented in Supplemental Table 1.
Histological examination
A subset of samples (n = 5/group) was prepared for histological analyses. Each specimen was dehydrated in a graded series of alcohols and embedded in polymethyl methacrylate without decalcification. Specimens (5 mm) were sectioned along the vertical axis in the middle of the defect site, stained with modified Gomori’s trichrome and scanned using a NanoZoomer Digital Pathology System. The histology slides were used to measure new bone formation using the ImageJ software (National Institutes of Health, Bethesda, MD, USA).
Statistical analysis
SigmaPlot© 12.5 (Systat Software, San Jose, CA, USA) was used to run Student’s t tests, Mann–Whitney or analysis of variance tests with Tukey’s comparison analysis when appropriate to determine differences. Statistical significance was determined when p < 0.05.
Results
Cell analyses
The number of CFU-Fs/well was significantly higher in cBMA than BMA (27.22 ± 3.30 vs 7.67 ± 1.14, respectively; p < 0.001) (Fig. 1a, b). The number of CFU-Fs/ml was also significantly higher in cBMA than BMA (1,900.18 ± 3.30 vs 244.31 ± 33.89, respectively; p < 0.001) (Fig. 1c). One outlier in the cBMA platelet sample was 2.8 standard deviations from the mean and was therefore excluded from the statistical analyses. The number of nucleated cells per millilitre within cBMA was significantly higher than BMA (28.20 ± 6.75×106 vs 13.06 ± 0.74×106, respectively; p < 0.05) (Fig. 1d). The number of platelets per millilitre was increased from 25.53 ± 3.35×106 for BMA to 48.11 ± 10.04×106 for cBMA (p < 0.01) (Fig. 1e). The per cent haematocrit was decreased from 30.78 ± 0.55 % for BMA to 11.84 ± 2.14 % for cBMA (p < 0.001) (Fig. 1f).
Animal surgeries
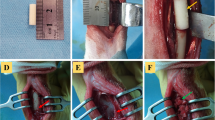
A 15-mm segment was removed from the mid-diaphysis of the radius and replaced with either DBM-BMA, DBM-cBMA, Col:β-TCP-BMA or Col:β-TCP-cBMA (Fig. 2a). No surgical complications, adverse inflammatory response, infection, fractures or graft rejections were observed.
a Photographs of Col:-β-TCP or DBM scaffolds that were loaded with either BMA or cBMA placed in a 15-mm rabbit radial defect. b Representative radiographs of radial segmental defects implanted with Col:β-TCP or DBM scaffolds containing BMA or cBMA at the time of surgery (day 0), 4 or 8 weeks after surgery
Radiographic analyses
At the time of surgery Col:β-TCP could be observed; however, those treated with DBM appeared empty, as the demineralised tissue cannot be detected using radiographs (Fig. 2b). Despite the presence of residual Col:β-TCP, there appeared to be more bone in the DBM group at four and eight weeks, which were evaluated using a standard scoring system [18]. Defects treated with DBM-BMA received a score of 3.62 ± 0.10 and 3.7 ± 0.10 at eight weeks for bone formation (Fig. 3a) and bridging (Fig. 3b), respectively. All defects (10/10) had a score ≥ 3 for both analyses. DBM-cBMA-treated defects received a score of 3.48 ± 0.22 and 3.26 ± 0.29 at eight weeks bone formation (Fig. 3a) and bridging (Fig. 3b), respectively. At eight weeks nine of ten and eight of ten defects treated with DBM-cBMA received a score ≥ 3 for bone formation and bridging by eight weeks, respectively. None of the defects that were treated with Col:β-TCP-BMA achieved a score ≥ 3 for bone formation or defect bridging by eight weeks; average values for the group were 1.56 ± 0.15 and 0.84 ± 0.15, respectively. Defects treated with Col:β-TCP-cBMA achieved a score ≥ 3 in four of ten and two of ten animals at eight weeks for bone bridging and formation; average values for the group were 2.54 ± 0.29 and 2.16 ± 0.37, respectively. Within Col:β-TCP, but not DBM, there was a main effect of aspirate type for both bone formation and bridging (cBMA > BMA; p < 0.05). Within DBM, but not Col:β-TCP, there was an increase in both bone formation and bridging between four and eight weeks (p < 0.05). A summary of the average and individual radiograph scores are found in Supplemental Table 2.
Histological examination
Histology sections (Fig. 4a–d) were used to quantify new bone formation within the area of the defect in a subset of samples (n = 5/group). The results showed a significant increase in per cent bone in the DBM group as compared to Col:β-TCP (0.331 vs 0.229; p < 0.05); however, no differences were seen between the BMA and the cBMA groups (0.289 vs 0.272; p > 0.05).
Histology photographs of representative samples. Col:β-TCP samples with a BMA or c cBMA. DBM samples with b BMA or d cBMA. Photographs taken at ×1.25 magnification match the radiographs shown in Fig. 1. Arrow denotes the site of defect and * denotes new bone formation, also seen in green
Discussion
The primary objective of this study was to identify clinically relevant strategies capable of enhancing bone repair. Grafts were prepared using currently available scaffolds composed of materials that have been well studied, namely DBM and β-TCP, and supplemented with either BMA or cBMA. The major findings of the study are that (1) DBM grafts were capable of achieving union by four weeks post-surgery, regardless of whether BMA or cBMA were used, and (2) the supplementation of Col:β-TCP composite grafts with cBMA improved bone healing as compared to those supplemented with BMA.
At first glance a logical speculation is that the differences in bone repair may be attributed to the main component (i.e. DBM or Col:β-TCP) contained within each scaffold, especially since both types of scaffolds were loaded from the same batches of BMA or cBMA. DBM has been shown to be osteoinductive because it retains measurable levels of bone morphogenetic protein (BMP) and drives stem cell differentiation and new bone formation [19–22]. β-TCP is an osteoconductive material [23] that supports new bone deposition but does not drive cellular differentiation. However, it is also important to take into account the differences in the delivery strategy for each material; Col:β-TCP composite grafts were delivered as strips that were cut to match the defect, while DBM was delivered in a putty form. Future studies that include a direct comparison between β-TCP and DBM using the same form of delivery will be useful to better understand critical parameters for bone repair.
The importance of delivering a sufficient number of progenitor cells to improve bone healing [17] provided the rationale for concentrating the BMA. In line with previous studies, the concentration of nucleated cells, CFU-Fs and platelets increased ∼ 2-, 3.5- and 2-fold with bone marrow concentration, respectively, so that a greater number of progenitor cells and platelets were delivered. The absolute values we observed for these parameters within BMA or cBMA are in general agreement with previously published work where rabbits [24], pigs [15] or humans [8] were used. Since the DBM grafts performed well regardless of whether BMA or cBMA were included, it was not possible to glean any additional information regarding the progenitor cell delivery within DBM. However, the finding that Col:β-TCP was improved with the use of cBMA allows for further speculation. A Pearson correlation test comparing the number of CFUs/well or CFUs/ml to bone formation within the Col:β-TCP demonstrated a significant correlation (p < 0.01) with R 2 values of 0.50 and 0.36, respectively (data not shown). Due to the small sample size it is difficult to effectively determine the minimum number of progenitor cells required to heal this type of defect. Nonetheless, it suggests a relationship between the number of progenitor cells delivered and defect healing may exist under these circumstances, but may require a more challenging osteogenic model to fully elucidate.
Based on these findings, further evaluation of both scaffolds under conditions that may be more challenging and relevant to traumatic injuries is of great interest. This is especially relevant for better evaluating the importance of progenitor cells with DBM. One would expect the augmentation with cells to play a greater role in bone healing if the availability of stem cells and osteogenic growth factors in a defect is low. As just one example, poor fracture healing occurs when there is little soft tissue coverage and a subsequently lesser number of factors available for bone repair [25, 26]. Nonetheless, a conservative estimation based on our findings is that DBM grafts are capable of accelerating bone healing in this model where there is ample soft tissue coverage. Further, our findings are largely based on radiographic analyses using a standardised scoring method. It will be useful to follow up these semi-quantitative analyses with micro-CT, however, this comparison is complicated by the fact that residual β-TCP is difficult to distinguish from regenerated bone.
An obvious limitation to the study is that a group without BMA was not included therefore a definitive conclusion regarding the importance of BMA cannot be made. The goal of the study was to identify strategies currently available to expedite clinical translation. Since previous studies have already documented reduced healing in this model using similar scaffolds without augmentation [27–29], a control group containing either no scaffold or scaffolds without cells was not included to minimise animal use. Overall, based on the relative ease at which bone marrow aspiration can be obtained the cost/risk seems to favour its inclusion.
Many studies are directed towards maximising the regenerative potential of various combinations of biomaterials and stem cells while few experimental studies include both clinically relevant scaffolds and rapid cell isolation procedures. The ability of DBM grafts to achieve union by four weeks when supplemented with BMA suggests that this is one strategy available to clinicians for the treatment of difficult defects. Also, the current findings suggest that either material can be combined with cBMA to effectively heal bone.
References
Gaston MS, Simpson AH (2007) Inhibition of fracture healing. J Bone Joint Surg Br 89(12):1553–1560. doi:10.1302/0301-620x.89b12.19671
Shafiei Z, Bigham AS, Dehghani SN, Nezhad ST (2009) Fresh cortical autograft versus fresh cortical allograft effects on experimental bone healing in rabbits: radiological, histopathological and biomechanical evaluation. Cell Tissue Bank 10(1):19–26. doi:10.1007/s10561-008-9105-0
Chimutengwende-Gordon M, Khan WS (2012) Advances in the use of stem cells and tissue engineering applications in bone repair. Curr Stem Cell Res Ther 7(2):122–126
Patterson TE, Kumagai K, Griffith L, Muschler GF (2008) Cellular strategies for enhancement of fracture repair. J Bone Joint Surg Am 90(Suppl 1):111–119. doi:10.2106/jbjs.g.01572
Peric M, Dumic-Cule I, Grcevic D, Matijasic M, Verbanac D, Paul R, Grgurevic L, Trkulja V, Bagi CM, Vukicevic S (2015) The rational use of animal models in the evaluation of novel bone regenerative therapies. Bone 70:73–86. doi:10.1016/j.bone.2014.07.010
Iwata H, Sakano S, Itoh T, Bauer TW (2002) Demineralized bone matrix and native bone morphogenetic protein in orthopaedic surgery. Clin Orthop Relat Res 395:99–109
Wagoner Johnson AJ, Herschler BA (2011) A review of the mechanical behavior of CaP and CaP/polymer composites for applications in bone replacement and repair. Acta Biomater 7(1):16–30. doi:10.1016/j.actbio.2010.07.012
Zhong W, Sumita Y, Ohba S, Kawasaki T, Nagai K, Ma G, Asahina I (2012) In vivo comparison of the bone regeneration capability of human bone marrow concentrates vs. platelet-rich plasma. PLoS One 7(7):e40833. doi:10.1371/journal.pone.0040833
Walsh WR, Vizesi F, Michael D, Auld J, Langdown A, Oliver R, Yu Y, Irie H, Bruce W (2008) Beta-TCP bone graft substitutes in a bilateral rabbit tibial defect model. Biomaterials 29(3):266–271. doi:10.1016/j.biomaterials.2007.09.035
Wilkins RM, Kelly CM (2003) The effect of allomatrix injectable putty on the outcome of long bone applications. Orthopedics 26(5 Suppl):s567–s570
Lindsey RW, Wood GW, Sadasivian KK, Stubbs HA, Block JE (2006) Grafting long bone fractures with demineralized bone matrix putty enriched with bone marrow: pilot findings. Orthopedics 29(10):939–941
Kim JM, Kim MH, Kang SS, Kim G, Choi SH (2014) Comparable bone healing capacity of different bone graft matrices in a rabbit segmental defect model. J Vet Sci 15(2):289–295
Zhang ZY, Teoh SH, Hui JH, Fisk NM, Choolani M, Chan JK (2012) The potential of human fetal mesenchymal stem cells for off-the-shelf bone tissue engineering application. Biomaterials 33(9):2656–2672. doi:10.1016/j.biomaterials.2011.12.025
Rao RR, Stegemann JP (2013) Cell-based approaches to the engineering of vascularized bone tissue. Cytotherapy 15(11):1309–1322. doi:10.1016/j.jcyt.2013.06.005
Jungbluth P, Hakimi AR, Grassmann JP, Schneppendahl J, Betsch M, Kropil P, Thelen S, Sager M, Herten M, Wild M, Windolf J, Hakimi M (2013) The early phase influence of bone marrow concentrate on metaphyseal bone healing. Injury 44(10):1285–1294. doi:10.1016/j.injury.2013.04.015
Rathbone CR, Guda T, Singleton BM, Oh DS, Appleford MR, Ong JL, Wenke JC (2014) Effect of cell-seeded hydroxyapatite scaffolds on rabbit radius bone regeneration. J Biomed Mater Res A 102(5):1458–1466. doi:10.1002/jbm.a.34834
Hernigou P, Poignard A, Beaujean F, Rouard H (2005) Percutaneous autologous bone-marrow grafting for nonunions. Influence of the number and concentration of progenitor cells. J Bone Joint Surg Am 87(7):1430–1437. doi:10.2106/jbjs.d.02215
Bodde EW, Spauwen PH, Mikos AG, Jansen JA (2008) Closing capacity of segmental radius defects in rabbits. J Biomed Mater Res A 85(1):206–217. doi:10.1002/jbm.a.31549
Bae HW, Zhao L, Kanim LE, Wong P, Delamarter RB, Dawson EG (2006) Intervariability and intravariability of bone morphogenetic proteins in commercially available demineralized bone matrix products. Spine (Phila Pa 1976) 31(12):1299–1306. doi:10.1097/01.brs.0000218581.92992.b7, discussion 1307–1298
Djapic T, Kusec V, Jelic M, Vukicevic S, Pecina M (2003) Compressed homologous cancellous bone and bone morphogenetic protein (BMP)-7 or bone marrow accelerate healing of long-bone critical defects. Int Orthop 27(6):326–330. doi:10.1007/s00264-003-0496-z
Vukicevic S, Oppermann H, Verbanac D, Jankolija M, Popek I, Curak J, Brkljacic J, Pauk M, Erjavec I, Francetic I, Dumic-Cule I, Jelic M, Durdevic D, Vlahovic T, Novak R, Kufner V, Bordukalo Niksic T, Kozlovic M, Banic Tomisic Z, Bubic-Spoljar J, Bastalic I, Vikic-Topic S, Peric M, Pecina M, Grgurevic L (2014) The clinical use of bone morphogenetic proteins revisited: a novel biocompatible carrier device OSTEOGROW for bone healing. Int Orthop 38(3):635–647. doi:10.1007/s00264-013-2201-1
Urist MR (1965) Bone: formation by autoinduction. Science 150(3698):893–899
LeGeros RZ (2002) Properties of osteoconductive biomaterials: calcium phosphates. Clin Orthop Relat Res 395:81–98
Sununliganon L, Peng L, Singhatanadgit W, Cheung LK (2014) Osteogenic efficacy of bone marrow concentrate in rabbit maxillary sinus grafting. J Craniomaxillofac Surg 42(8):1753–1765. doi:10.1016/j.jcms.2014.06.011
Harry LE, Sandison A, Paleolog EM, Hansen U, Pearse MF, Nanchahal J (2008) Comparison of the healing of open tibial fractures covered with either muscle or fasciocutaneous tissue in a murine model. J Orthop Res 26(9):1238–1244. doi:10.1002/jor.20649
Liu R, Schindeler A, Little DG (2010) The potential role of muscle in bone repair. J Musculoskelet Neuronal Interact 10(1):71–76
Yang P, Wang C, Shi Z, Huang X, Dang X, Li X, Lin SF, Wang K (2010) rhVEGF 165 delivered in a porous beta-tricalcium phosphate scaffold accelerates bridging of critical-sized defects in rabbit radii. J Biomed Mater Res A 92(2):626–640. doi:10.1002/jbm.a.32403
Yoneda M, Terai H, Imai Y, Okada T, Nozaki K, Inoue H, Miyamoto S, Takaoka K (2005) Repair of an intercalated long bone defect with a synthetic biodegradable bone-inducing implant. Biomaterials 26(25):5145–5152. doi:10.1016/j.biomaterials.2005.01.054
Zhou J, Lin H, Fang T, Li X, Dai W, Uemura T, Dong J (2010) The repair of large segmental bone defects in the rabbit with vascularized tissue engineered bone. Biomaterials 31(6):1171–1179. doi:10.1016/j.biomaterials.2009.10.043
Acknowledgments
The opinions and assertions contained herein are the private views of the authors and are not to be construed as official or reflecting the views of the Department of the Army or Department of Defense. This study was supported in part by the Armed Forces Institute of Regenerative Medicine (BB). This research was supported in part by an appointment to the Student Research Participation Program at the US Army Institute of Surgical Research administered by the Oak Ridge Institute for Science and Education through an interagency agreement between the US Department of Energy and USAMRMC (JM, SS) and the National Research Council (MP). We would like to thank Drs. Catherine Ward, Jessica Rivera and Randolph Stone for their invaluable contributions.
Conflicts of interest
MAROMatch, MAROFuse, collagen-β-TCP scaffolds, and the Magellan® MAR01™ system and disposables were supplied by Arteriocyte Medical Systems. This study was funded in part by Arteriocyte Medical Systems.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Supplemental Table 1
(PDF 8 kb)
Supplemental Table 2
(PDF 27 kb)
Rights and permissions
About this article
Cite this article
McDaniel, J.S., Pilia, M., Raut, V. et al. Alternatives to autograft evaluated in a rabbit segmental bone defect. International Orthopaedics (SICOT) 40, 197–203 (2016). https://doi.org/10.1007/s00264-015-2824-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00264-015-2824-5