Abstract
Purpose
The purpose of this study was to examine calcitonin gene-related peptide (CGRP) concentrations in serum and synovial fluid of patients with primary knee osteoarthritis (OA) and healthy controls and to explore their relationship with clinical and radiographic severity of OA.
Methods
Sixty-five patients with primary knee OA and 21 healthy controls were recruited. CGRP concentrations in the serum and synovial fluid were measured using enzyme-linked immunosorbent assays. The radiographic severity of OA was evaluated using the Kellgren and Lawrence (KL) classification. The Western Ontario and McMaster University Osteoarthritis Index (WOMAC) was used to assess pain, stiffness and physical function.
Results
Serum and synovial fluid CGRP concentrations tended to be higher with the increase in KL grades (r = 0.565 and r = 0.441, P < 0.001, respectively), and were significantly positively correlated with KL grades, total WOMAC score and each subscale (pain, stiffness and physical function).
Conclusions
The result demonstrated that CGRP in serum and synovial fluid was related to progressive joint damage in knee OA. CGRP can be selected as a biomarker for monitoring disease severity and could be a predictive role on prognosis and progression of knee OA.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Osteoarthritis (OA) is the most common joint-affecting disease that characterised by the progressive destruction of articular cartilage, subchondral bone sclerosis or cyst and osteophyte formation at joint margins. Knee joints are most likely involved and the clinical features of OA include severe pain, swelling, stiffness, functional limitation and mild, chronic non-specific synovial inflammation [1]. OA has been reported as the leading cause of lower extremity disability among older people with an estimated lifetime risk for knee OA being approximately 40 % in men and 47 % in women [2].
The diagnosis of OA is generally based on clinical and radiographic changes, which reflect the severity of disease by grading the joint destruction [3]. But the clinical severity of OA does not always accurately correspond to the radiographic performance. In order to identify patients with a high risk of destructive OA and test the efficacy of drug treatment, more sensitive techniques than plain X-rays are needed [4]. Recently, more and more researchers have focused on measuring the concentrations of some biomarkers in serum, synovial fluid and articular cartilage in order to judge the severity and progression of OA, such as interleukin-15 [5], osteopontin [6], BMP-7 [7] and bradykinin [8].
The aetiology and pathophysiology of OA are still both poorly understood, though age, gender, genetics, obesity, occupation, physical activity, knee injuries and so on were proved to be risk factors [2]. In addition, another important consensus achieved is that knee OA is an inflammatory disease different from what we thought before. Many proinflammatory cytokines have been reported to contribute to OA pathogenesis by increasing the cartilage degradation and sensitivity to pain via a number of direct and indirect actions [5, 9, 10].
Calcitonin gene-related peptide (CGRP) is a 37-amino acid peptide, which is primarily localised to C and A sensory fibres [11]. These fibres display a wide innervation throughout the body including synovium of the knee [12], and is known to have a variety of pro-inflammatory effects via its influence on immune cells [13]. Raap et al. [14] proved that CGRP promoted the release of IL-6 and IL-8 from synovial fibroblasts in patients with rheumatoid arthritis. Hernanz et al. [15] identified that CGRP produced a significant stimulation of IL-1, IL-6 and TNF-α production by whole blood cells from OA patients. And Yaraee et al. [16] made similar conclusions.
On the other hand, recent researches have focused on the influence of CGRP for OA-related pain, which is the major factor affecting the quality of life and the most complained about by OA patients. Animal experiments that application of CGRP-receptor antagonists and gene knock out had confirmed that [17–19].
Materials and methods
Study subjects
The present study was approved by the Ethical Committee of the No. 3 Hospital of Hebei Medical University and was conducted in accordance with the Declaration of Helsinki. Written informed consent was obtained from the patients and healthy volunteers prior to their participation in this study.
Sixty-five patients aged 48–77 years with primary knee OA (54 women and 11 men; mean age 61.8 ± 6.8 years) according to the criteria of the American College of Rheumatology who visited our hospital for total knee arthroplasty or knee arthroscopy were enrolled in the study. We also recruited 21 gender- and age-matched volunteers (17 females and four males; mean age 59.1 ± 6.6 years) with normal knee radiographs as controls. All participants were excluded on the basis of having serious cardiovascular disease, diabetes, rheumatoid arthritis, post-traumatic arthritis, previous joint infection, history of taking painkillers in three months, other types of arthropathy, and histories of corticosteroids medication. Patients with any kind of systemic inflammatory or autoimmune disorder, or any type of malignant or chronic illness, were not included in this study.
Radiographic grading of OA
The severity of the disease was determined using weight-bearing anteroposterior radiographs of the affected knee. Knee radiographs were evaluated according to the Kellgren and Lawrence (KL) classification: grade 0, no X-ray changes; grade 1, doubtful narrowing of joint space and possible osteophyte lipping; grade 2, definite osteophytes and possible narrowing of joint space; grade 3, moderate multiple osteophytes, definite narrowing of joint space, some sclerosis and possible deformity of bone contour; grade 4, large osteophytes, remarkable narrowing of joint space, severe sclerosis and definite deformity of bone contour [20]. OA patients were defined as having radiographic knee OA of KL grade ≥2 in at least one knee, and controls as having KL grades of 0. The grading scale used for analysis was the higher one of the two knees.
Severity evaluation of OA
The functional condition and pain severity of each patient were evaluated using the Western Ontario McMaster University Osteoarthritis Index (WOMAC) score when the patients admitted to hospital. The WOMAC score was used to evaluate self-reported pain, knee stiffness and physical function of OA. Five questions were asked about the severity of pain they felt owing to the knee OA during the following activities: walking on flat ground, walking up and down the stairs, staying in bed at night, sitting or lying, and standing upright. For stiffness, two questions were asked about the severity of stiffness when first awakening in the morning and later after some activities like sitting or resting. Seventeen questions about the difficulty of activities in daily living were asked to assess physical function of knee joint. Each question was scored according to the degree of difficulty to complete the actions or pain severity from 0 (none) to 4 (extreme) [21]. A higher WOMAC score means poorer physical function and greater pain [21]. The data were arranged according to the KL grade, and the correlations between the subgroup were analysed. Correlations between the CGRP concentrations and WOMAC score were also analysed.
Laboratory examinations
One to two millilitres of synovial fluid was aspirated from the affected knee using the sterile knee puncture technique prior to surgery, and centrifuged to remove cells and joint debris. The specimen was stored immediately at −80 °C until the day of measurement. Synovial fluid was not extracted from the healthy controls due to ethical concern. Venous blood samples were collected from the antecubital vein of all participants in the fasting state at 7.00-7.30 a.m. one day before surgery. After clotting, centrifuged to remove cells and debris, the resulting serum was removed and stored at −80 °C until used. Double-blind quantitative detection of CGRP in serum and synovial fluid were measured using commercially available sandwich enzyme-linked immunosorbent assay (ELISA) kits (Phoenix Pharmaceuticals, Belmont, CA, USA) according to the manufacturer’s protocol.
Statistical analysis
Statistical analysis was carried out with the Statistical Package for Social Sciences (IBM SPSS, New York, USA) software, version 19.0 for Windows. The normality of continuous variables was assessed using the Kolmogorov-Smirnov test. Differences between the two groups were analysed using unpaired t-test, Mann–Whitney U test, chi-squared test when appropriate. Differences among groups were analysed by one-way analysis of variance (ANOVA), followed by Tukey post hoc analysis, Kruskal-Wallis analysis or chi-squared test when appropriate. Spearman rank correlation coefficient was calculated to determine the relationship between CGRP concentrations in serum/synovial fluid and KL grades/WOMAC scores. Multivariate linear regression modelling was used to evaluate the independent predictors of WOMAC total scores, pain score, stiff score and physical function score. All values are expressed as mean ± standard deviation (SD). P values < 0.05 (two-tailed) were considered statistically significant.
Results
Baseline clinical characteristics
The baseline clinical data of the healthy controls and OA patients were shown in Table 1. The OA patients and controls were similar in age (61.2 ± 6.8, 47–77 vs 59.1 ± 6.6, 47–75 years), gender (11/54 in patients, 4/17 in controls) and body mass index (BMI) (26.8 ± 3.4, 18.7–35.9 vs 26.1 ± 2.3, 21.7–32.5 kg/m2). According to the Kellgren and Lawrence (KL) classification, 17 patients were KL grade 2, whereas 22 patients were KL grade 3 and 26 patients were KL grade 4. There was also no significant difference in baseline clinical characteristics among the subgroup based on KL grade (P > 0.05).
The CGRP concentrations in serum and synovial fluid
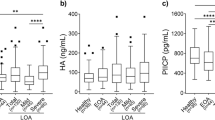
As shown in Fig. 1, OA patients had higher serum CGRP concentrations compared with healthy controls (2.43 ± 0.74 vs 1.95 ± 0.69 ng/mL, P = 0.006). CGRP concentrations in synovial fluid were significantly lower than in paired serum samples (1.24 ± 0.51 vs 2.43 ± 0.74 ng/mL, P < 0.001). And, it also showed a positive correlation between serum CGRP concentration and synovial fluid CGRP concentration (r = 0.606, P < 0.001) (Fig. 2). Serum and synovial fluid CGRP concentrations of subgroups based on KL grade and healthy controls are shown in Table 2. The serum CGRP concentration of KL grade 2 was 1.90 ± 0.33 ng/mL; for KL grade 3, 2.33 ± 0.82 ng/mL; for KL grade 4, 2.87 ± 0.61 ng/mL. The healthy controls were 1.95 ± 0.69 ng/mL. These results showed that serum CGRP concentration in KL grade 4 was significantly higher than KL grades 2 and 3, and also the controls (P < 0.001). Additionally, serum CGRP concentration in KL grade 3 was higher than in KL grade 2 and healthy controls, but the difference was not statistically significant. The synovial fluid CGRP concentration of KL grade 2 was 0.99 ± 0.34 ng/mL; KL grade 3, 1.16 ± 0.34 ng/mL; KL grade 4, 1.47 ± 0.63 ng/mL. The data demonstrated that synovial fluid CGRP concentration tended to be higher with the increease in KL grade, the difference between KL grade 4 and KL grades 2 and 3 was statistically significant (P < 0.05), whereas the difference between KL grade 2 and KL grade 3 was not.
Correlation of CGRP concentrations with KL grades and WOMAC scores
We analysed the correlation between CGRP concentrations in serum and synovial fluid and KL grades of OA. In knee OA group, CGRP concentrations in serum and synovial fluid were significantly correlated with KL grades respectively (r = 0.565, P < 0.001; r = 0.441, P < 0.001, respectively) (Figs. 3 and 4).
We further examined the correlations between the CGRP concentrations in serum and synovial fluid and the WOMAC score. Serum CGRP concentration and synovial fluid CGRP concentration respectively presented a significant positive correlation with the total WOMAC score and with each subscale (pain, stiffness and physical function). The precise statistical values are shown in Table 3.
Multinomial logistic regression analysis demonstrated that CGRP concentrations in serum and synovial fluid were correlated with KL grades and WOMAC score after adjusting for other variables such as age, BMI and gender (P < 0.001).
Discussion
In this research, we examined the relationship between serum/synovial fluid CGRP concentrations and WOMAC score and radiographic severity of patients with primary knee OA. This study provides evidence that CGRP concentrations in serum and synovial fluid were both positively correlated with WOMAC total score, pain score, stiffness score, physical function score and radiographic severity of knee OA patients. It means that higher concentrations of CGRP in serum and synovial fluid are indicative of worsening pain, more severe stiffness and poorer physical function, and are usually associated with more serious image presentations in knee OA patients. It indicated that CGRP in serum and synovial fluid might predict clinical severity for OA patients.
Biomarkers could reflect physical condition and symptoms of patients and predict the progression and prognosis of disease. Besides, one reliable biomarker that can accurately predict the severity of disease should be chosen based on the pathophysiological process of OA. In other words, biomarkers are usually involved in the development of knee OA; this also provides new and potential targets for OA interventions [22]. Although the exact cause of OA is still uncertain, there is growing evidence that inflammation plays a key role in the development and progression of the disease. Previous studies have confirmed a clear correlation between the progression of cartilage damage and the presence of a reactive or inflammatory synovial membrane [23–25]. Therefore, inflammation-related cytokines are explored for the potential role as OA-related biomarkers owing to the inflammatory nature of the disease [10, 25, 26]. Apart from the above mentioned factors, macrophage migration inhibitory factor (MIF) [27], interleukin-10 [28], interleukin-8 [29] and connective tissue growth factor [30], have also been shown to be potential biomarkers for knee OA, and are associated with severity and clinical symptom of OA.
CGRP is one kind of major pro-inflammatory sensory neuropeptide. Sensory neurons expressing CGRP innervate most joint structures, particularly on the synovial membrane, ligaments and subchondral bone [12, 19, 31]. The peripheral sensory nervous system will release neuropeptides in local surroundings when acute and chronic inflammatory processes occur. And neuropeptides play an important role in modulating of inflammation. A wealth of studies showed that CGRP promoted the production of pro-inflammatory cytokines. The study of Yaraee et al. [16] indicated that CGRP enhanced the secretion of IL-1β and tumour necrosis factor (TNF) by HSV-infected and uninfected macrophages. IL-1β is over-expressed during OA in the cartilage, as well as in the synovial tissue [32]. TNF-α was proved to promote resorption and inhibit the synthesis of proteoglycan in cartilage [32]. Raap et al. [14] found the phenomenon that CGRP evoke the release of IL-6 and IL-8 from synovial fibroblasts in patients with rheumatoid arthritis. Dallas et al. [33] proved that CGRP is able to induce an up-regulation of IL-1α, TNF-α and IL-8 production in cultured human keratinocytes. IL–1β and TNF are considered as the main proinflammatory cytokines that are involved in the pathophysiology of OA [10], and they also induce the production of IL-6 and IL-8. These cytokines exert the effect on chondrocytes by up-regulating the expression of the MMP family of catabolic enzymes such as MMP-1, 3 and 13 that induce extracellular matrix degradation and digest extracellular matrix in the process of OA [32, 34–36]. Therefore, CGRP may contribute to the development and progression of OA indirectly by promoting the release of inflammatory cytokines, and inducing a chain reaction of inflammation.
On the other hand, CGRP is associated with pain transmission, pain modulation and plays an important role in OA-related pain. The study showed that the expression of CGRP-positive and Substance P-positive neurons in the joints of OA patients is increasing, which revealed the potential function of neuropeptides in the painful degenerative joint disease [12, 37]. The study of Craig et al. [18] showed that CGRP was able to acutely sensitise the responses of joint nociceptor to stimulation of the knee joint, and it also certified that the CGRP knockout mice reduced nociceptive hypersensitivity [17]. Benschop et al. [38] conducted a study that there was a dose-dependent and time-dependent effect of antibody to CGRP on pain-related behaviour and it may be useful to alleviate pain in a patient with knee OA. Additionally, the role for CGRP in OA pain was implied by a phenomenon that an increased innervation density of CGRP-positive nerve fibre is only seen in hip joints suffering from OA-pain and not in non-painful joints [37]. Also, it is known that CGRP contributes to the generation of migraine pain, and recent research also showed that CGRP antagonist was useful for the treatment and prevention of migraine [39], and effectively reduced the pain. Bullock and Kelly [31] pointed out the view that similar to migraine, elevated levels of CGRP, activation of peripheral neurons and peripheral sensitisation are all features of OA, prompting the vital function of CGRP in OA-related pain.
In this study, several potential limitations should be taken into account. Firstly, our study was based on a relatively small size of patients who visited our hospital, so multi-centre prospective longitudinal study with a larger population is needed to further confirm the results. Secondly, it was not possible to extract synovial fluid from healthy controls because of ethical concerns, which may induce some potential bias. Thirdly, WOMAC score is a subjective rating, so the results may be affected by subjects’ personal factors such as education level and so on. Fourthly, synovial fluid samples may be polluted when extract from the knee with aspirations technique though we had take all efforts to avoid it, which may confound the results.
In summary, this study showed that patients with primary knee OA had elevated concentrations of serum and synovial fluid CGRP compared with healthy controls, meanwhile CGRP concentrations tend to be higher with the increase in KL grade. The study also demonstrated that increased CGRP concentrations were independently correlated with worsening pain, more severe stiffness and poorer physical function in knee OA patients. All results in this study suggest that CGRP is involved in the progression of knee OA and might play a vital role in the pathogenesis of OA. CGRP can be selected as a biomarker for determining disease severity and could predict the progression of knee OA. It also provides a potential therapeutic target to delay the progression and relieve the symptoms of OA, which need further systematic investigation.
References
Berenbaum F (2013) Osteoarthritis as an inflammatory disease (osteoarthritis is not osteoarthrosis!). Osteoarthritis Cartilage 21:16–21
Johnson VL, Hunter DJ (2014) The epidemiology of osteoarthritis. Best Pract Res Clin Rheumatol 28:5–15
Melikoglu M, Yildirim K, Senel K (2009) Relationship between radiographic grading of osteoarthritis and serum beta-2 microglobulin. Ir J Med Sci 178:151–154
Mohammed FI, Abd El-Azeem MI, KamalElDin AM (2012) Plasma and synovial fluid osteopontin levels in patients with knee osteoarthritis: relation to radiological grade. Egypt Rheumatol 34:131–136
Sun JM, Sun LZ, Liu J, Su BH, Shi L (2013) Serum interleukin-15 levels are associated with severity of pain in patients with knee osteoarthritis. Dis Markers 35:203–206
Honsawek S, Tanavalee A, Sakdinakiattikoon M, Chayanupatkul M, Yuktanandana P (2009) Correlation of plasma and synovial fluid osteopontin with disease severity in knee osteoarthritis. Clin Biochem 42:808–812
Honsawek S, Chayanupatkul M, Tanavalee A, Sakdinakiattikoon M, Deepaisarnsakul B, Yuktanandana P, Ngarmukos S (2009) Relationship of plasma and synovial fluid BMP-7 with disease severity in knee osteoarthritis patients: a pilot study. Int Orthop 33:1171–1175
Bellucci F, Meini S, Cucchi P, Catalani C, Nizzardo A, Riva A, Guidelli GM, Ferrata P, Fioravanti A, Maggi CA (2013) Synovial fluid levels of bradykinin correlate with biochemical markers for cartilage degradation and inflammation in knee osteoarthritis. Osteoarthritis Cartilage 21:1774–1780
Wang Y, Xu D, Long L, Deng X, Tao R, Huang G (2014) Correlation between plasma, synovial fluid and articular cartilage Interleukin-18 with radiographic severity in 33 patients with osteoarthritis of the knee. Clin Exp Med 14:297–304
Kapoor M, Martel-Pelletier J, Lajeunesse D, Pelletier JP, Fahmi H (2011) Role of proinflammatory cytokines in the pathophysiology of osteoarthritis. Nat Rev Rheumatol 7:33–42
Russell FA, King R, Smillie SJ, Kodji X, Brain SD (2014) Calcitonin gene-related peptide: physiology and pathophysiology. Physiol Rev 94:1099–1142
Saito T, Koshino T (2000) Distribution of neuropeptides in synovium of the knee with osteoarthritis. Clin Orthop Relat Res (376):172–182
Bowler KE, Worsley MA, Broad L, Sher E, Benschop R, Johnson K, Yates JM, Robinson PP, Boissonade FM (2013) Evidence for anti-inflammatory and putative analgesic effects of a monoclonal antibody to calcitonin gene-related peptide. Neuroscience 228:271–282
Raap T, Justen HP, Miller LE, Cutolo M, Scholmerich J, Straub RH (2000) Neurotransmitter modulation of interleukin 6 (IL-6) and IL-8 secretion of synovial fibroblasts in patients with rheumatoid arthritis compared to osteoarthritis. J Rheumatol 27:2558–2565
Hernanz A, Medina S, de Miguel E, Martı́n-Mola E (2003) Effect of calcitonin gene-related peptide, neuropeptide Y, substance P, and vasoactive intestinal peptide on interleukin-1β, interleukin-6 and tumor necrosis factor-alpha production by peripheral whole blood cells from rheumatoid arthritis and osteoarthritis patients. Regul Pept 115:19–24
Yaraee R, Ebtekar M, Ahmadiani A, Sabahi F (2003) Neuropeptides (SP and CGRP) augment pro-inflammatory cytokine production in HSV-infected macrophages. Int Immunopharmacol 3:1883–1887
Zhang L, Hoff AO, Wimalawansa SJ, Cote GJ, Gagel RF, Westlund KN (2001) Arthritic calcitonin/α calcitonin gene-related peptide knockout mice have reduced nociceptive hypersensitivity. Pain 89:265–273
Bullock CM, Wookey P, Bennett A, Mobasheri A, Dickerson I, Kelly S (2014) Peripheral calcitonin gene-related peptide receptor activation and mechanical sensitization of the joint in rat models of osteoarthritis pain. Arthrit Rheumatol 66:2188–2200
Fernihough J, Gentry C, Bevan S, Winter J (2005) Regulation of calcitonin gene-related peptide and TRPV1 in a rat model of osteoarthritis. Neurosci Lett 388:75–80
Kellegren J, Lawrence J (1957) Radiological assessment of osteoarthritis. Ann Rheum Dis 16:494–501
Bellamy N, Buchanan WW, Goldsmith CH, Campbell J, Stitt LW (1988) Validation study of WOMAC: a health status instrument for measuring clinically important patient relevant outcomes to antirheumatic drug therapy in patients with osteoarthritis of the hip or knee. J Rheumatol 15:1833–1840
Chen G, Hao J, Xi Y, Wang W, Wang Z, Li N, Li W (2008) The therapeutic effect of vasoactive intestinal peptide on experimental arthritis is associated with CD4+CD25+ T regulatory cells. Scand J Immunol 68:572–578
Požgan U, Caglič D, Rozman B, Nagase H, Turk V, Turk B (2010) Expression and activity profiling of selected cysteine cathepsins and matrix metalloproteinases in synovial fluids from patients with rheumatoid arthritis and osteoarthritis. Biol Chem 391:571–579
Ayral X, Pickering E, Woodworth T, Mackillop N, Dougados M (2005) Synovitis: a potential predictive factor of structural progression of medial tibiofemoral knee osteoarthritis—results of a 1 year longitudinal arthroscopic study in 422 patients. Osteoarthritis Cartilage 13:361–367
Sokolove J, Lepus CM (2013) Role of inflammation in the pathogenesis of osteoarthritis: latest findings and interpretations. Ther Adv Musculoskelet Dis 5:77–94
Zhou SJ, Sun ZX, Liu J (2013) Neopterin concentrations in synovial fluid may reflect disease severity in patients with osteoarthritis. Scand J Clin Lab Invest 73:344-348
Liu M, Hu C (2012) Association of MIF in serum and synovial fluid with severity of knee osteoarthritis. Clin Biochem 45:737–739
Scrivo R, Conigliaro P, Riccieri V, Di Franco M, Alessandri C, Spadaro A, Perricone R, Valesini G (2015) Distribution of interleukin-10 family cytokines in serum and synovial fluid of patients with inflammatory arthritis reveals different contribution to systemic and joint inflammation. Clin Exp Immunol 179:300–308
Mabey T, Honsawek S, Saetan N, Poovorawan Y, Tanavalee A, Yuktanandana P (2014) Angiogenic cytokine expression profiles in plasma and synovial fluid of primary knee osteoarthritis. Int Orthop 38:1885–1892
Honsawek S, Yuktanandana P, Tanavalee A, Chirathaworn C, Anomasiri W, Udomsinprasert W, Saetan N, Suantawee T, Tantavisut S (2012) Plasma and synovial fluid connective tissue growth factor levels are correlated with disease severity in patients with knee osteoarthritis. Biomarkers 17:303–308
Bullock CM, Kelly S (2013) Calcitonin gene-related peptide receptor antagonists: beyond migraine pain—a possible analgesic strategy for osteoarthritis? Curr Pain Headache Rep 17:375
Haseeb A, Haqqi TM (2013) Immunopathogenesis of osteoarthritis. Clin Immunol 146:185–196
Dallos A, Kiss M, Polyanka H, Dobozy A, Kemeny L, Husz S (2006) Effects of the neuropeptides substance P, calcitonin gene-related peptide, vasoactive intestinal polypeptide and galanin on the production of nerve growth factor and inflammatory cytokines in cultured human keratinocytes. Neuropeptides 40:251–263
Kobayashi M, Squires GR, Mousa A, Tanzer M, Zukor DJ, Antoniou J, Feige U, Poole AR (2005) Role of interleukin-1 and tumor necrosis factor α in matrix degradation of human osteoarthritic cartilage. Arthrit Rheuma 52:128–135
Inoue K, Masuko-Hongo K, Okamoto M, Nishioka K (2005) Induction of vascular endothelial growth factor and matrix metalloproteinase-3 (stromelysin) by interleukin-1 in human articular chondrocytes and synoviocytes. Rheumatol Int 26:93–98
Rowan A, Koshy P, Shingleton W, Degnan B, Heath J, Vernallis A, Spaull J, Life P, Hudson K, Cawston T (2001) Synergistic effects of glycoprotein 130 binding cytokines in combination with interleukin-1 on cartilage collagen breakdown. Arthrit Rheuma 44:1620–1632
Saxler G, Loer F, Skumavc M, Pfortner J, Hanesch U (2007) Localization of SP- and CGRP-immunopositive nerve fibers in the hip joint of patients with painful osteoarthritis and of patients with painless failed total hip arthroplasties. Eur J Pain 11:67–74
Benschop RJ, Collins EC, Darling RJ, Allan BW, Leung D, Conner EM, Nelson J, Gaynor B, Xu J, Wang XF, Lynch RA, Li B, McCarty D, Oskins JL, Lin C, Johnson KW, Chambers MG (2014) Development of a novel antibody to calcitonin gene-related peptide for the treatment of osteoarthritis-related pain. Osteoarthritis Cartilage 22:578–585
Bigal ME, Walter S, Rapoport AM (2013) Calcitonin gene-related peptide (CGRP) and migraine current understanding and state of development. Headache 53:1230–1244
Conflicts interest
The authors declare that there are no conflicts of interest.
Funding
This study was supported by the National Natural Science Foundation of China (Grant No. 81401789).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Dong, T., Chang, H., Zhang, F. et al. Calcitonin gene-related peptide can be selected as a predictive biomarker on progression and prognosis of knee osteoarthritis. International Orthopaedics (SICOT) 39, 1237–1243 (2015). https://doi.org/10.1007/s00264-015-2744-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00264-015-2744-4