Abstract
Purpose of the study
Adequate treatment of forearm nonunion should achieve both biological stimulation of the bone and mechanical stability. The use of bone graft could enhance the healing of a nonunion providing osteogenic, osteoconductive and osteoinductive stimulation and an optimal stability of the fixation. We retrospectively reviewed two cohorts of patients affected by forearm nonunion and treated with plate and opposite bone graft to determine whether the use of autograft versus allograft differs in terms of (1) rate of healing of the nonunion and (2) time of healing.
Materials and methods
Thirty-four patients were treated for aseptic forearm nonunion with cortical graft strut with opposite plate and intercalary graft in case of segmental bone defect. In 20 patients an autograft harvest from the fibula (group A) and in 14 (group B) an allograft provided by the bone bank of our institution were used.
Results
All the nonunions healed in a mean of four months in both groups, ranging from two to 12 months in group A and from three to ten months in group B. At the latest follow up forearm function and pain were satisfactory in both groups.
Conclusion
The use of plate and opposite bone graft demonstrated to be effective in promoting the healing of forearm nonunions, without significant differences in terms of rate and time of healing in the two groups. Considering the higher surgical time and the comorbidity of the donor site, if a bone bank is available, we suggest to use homologous cortical bone strut graft with opposite plate and screw fixation for the treatment of aseptic forearm nonunion rather than autograft.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Multiple factors like fracture location and complexity, patient characteristics and surgical technique are involved in the development of nonunion [1]. Forearm nonunions are usually associated with complex injury, inadequate initial reduction of the fracture, unstable fixation, or too early limb mobilization. Because of the peculiar anatomical and functional relationships between radius and ulna the forearm represents a sophisticated functional unit so fractures of the radius and the ulna should be considered like articular fractures [2].
The function of the forearm is to assist the hand in space positioning and to support the hand movements. Pronation and supination of the forearm occur at the radiohumeral, proximal radioulnar and distal radioulnar joints. Failure of the relationship between the radius and ulna due to changes in the length or in the spatial mutual dealing lead to alterations in the proximal or distal articulation or both with impairment of the forearm function and the ability to position the hand in space. When nonunion occur in the forearm this modifies the normal relationship between radius and ulna, with impairment of forearm, elbow and wrist motion. In these cases fracture healing is a necessary condition to recover a physiological function of the upper limb.
Although there is still no consensus about diagnostic criteria, a forearm nonunion can be considered when there are no signs of both endosteal and periosteal reaction and no evidence of radiographic progression of healing for six months after the initial treatment.
Based on the characteristic of the bone ends, aseptic nonunions can be distinguished into atrophic and hypertrophic [3]. The first seems to recognize the biological impairment as the main pathogenetic cause, while the second is mainly considered a consequence of mechanical failure [4]. Adequate treatment of forearm nonunion must therefore consider providing both biological stimulation and mechanical stability.
Bone graft and bone substitutes have long been used in post traumatic skeletal conditions such as nonunion to stimulate bone healing and fill bone defects [5]. Currently different options include cancellous or cortical bone, both autologous and homologous, autologous bone marrow and calcium phosphate-based bone graft substitutes [6, 7]. Each of these grafts differs in terms of osteogenesis, osteoconduction, osteoinduction, mechanical properties and vascularity [8] (Table 1).
Autologous bone graft (autograft) is the transplantation of bone of the same individual from an anatomical donor site to one receiver [8]. Sources include fibula, ribs and iliac crest. This type of graft has osteogenic, osteoconductive and osteoinductive properties, especially the cancellous form while the cortical one has little or no osteoinductive properties and is mostly osteoconductive and little osteogenics because of the surviving osteoblast [6]. Autologous cortical graft can provide good mechanical strength, but due to the initial resorption phase it still must be supported by internal or external fixation. The main advantages of autologous graft are a complete histocompatibility, no risk of transmitting disease and a reported good rate of satisfactory results [9], but factors such as donor site morbidity, limited quantity of available bone and prolonged surgical time justifies considering possible alternatives [10].
Homologous bone graft (allograft) represents the transplant of bone from different individuals of the same species. Allograft can provide mechanical support with satisfactory osteoinductive capacity but poor osteogenic and osteoinductive properties. Compared to autograft, allograft are easier to use, require less surgical time and are potentially unlimited in amount, although allograft presents disadvantages such as higher risk of infection, viral disease transmission, and the need of a bone bank available to the hospital [11, 12].
The aim of this study was to compare the results of the treatment of aseptic forearm nonunion with plate and opposite bone strut, using either autograft or allograft. We retrospectively reviewed two groups of patients and compared the results determining whether the use of autograft versus allograft differs in terms of (1) rate of healing of the nonunion, (2) time of healing and (3) functional results.
Materials and methods
From 1987 to 2008 a total of 47 patients have been treated for aseptic forearm nonunion with cortical graft strut with opposite plate and intercalary graft in case of segmental bone defect. From 1987 to 1996 a first group (A) of 31 patients was treated using an autograft taken from the fibula. Later, from 1997 to 2008 the same technique was performed in a second group (B) of 16 patients, using homologous bone graft provided by the bone bank of our institute. In both groups we included patients with a diaphyseal nonunion of one or both forearm bones. We considered a nonunion when there were no signs of healing at a minimum of six months after the initial treatment. Exclusion criteria were: (1) presence of other fractures in the same limb at the time of the primary forearm injury and (2) septic nonunion.
Nine patients in group A and two in group B did not satisfy inclusion criteria and therefore were excluded from the study. Two patients of group A were lost at follow up. This left a total of 34 patients in the two groups. The initial treatment of the fracture which evolved into nonunion consisted of open reduction and internal fixation with plate and screws, intramedullary rod, external fixation or cast immobilization (Table 2).
Nonunion evolved in radius alone in two patients of both groups, in the ulna alone in 14 patients in group A and in eight of group B, and nonunion of the radius and ulna evolved in four patients of both groups (Table 3).
The mean age of patients at the time of surgery was 31 years in both the group (range 17–48 in group A and 18–45 in group B); there were four women and 16 men in group A and four women and ten men in group B. Nonunion occurred in the right arm in 16 patients in group A and in eight in group B and in the left arm respectively in eight and six patients; the dominant limb was involved in 16 patients in group A and nine in group B.
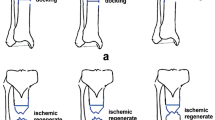
The same surgical procedure was performed in the two groups with the only difference related to the type of graft that consisted in a fibular cortical autograft strut, combined with a fibular intercalary autograft in case of segmental bone defect, in group A, and in a cortical strut and intercalary graft of homologous bone provided by the bone bank of our institute in group B. As homologous bone graft we used tibial, fibular, radial or ulnar cortical strut and a fibular or radial cylinder for segmental defect. In both groups surgical procedures were performed with patients positioned supine on the operating table with the elbow 90° flexed and the forearm leaning on the table, with ischemic tourniquet inflated at the arm. Radius exposure was performed through dorsal Thompson approach [13], while ulna was expose using a direct posterior approach. Fixation devices, when present, were removed. Bone ends were freshened, removing fibrous and/or necrotic tissue and hypertrophic bone, in order to obtain healthy bone and medullary canal of the bone was opened. Once adequate alignment of the bone was obtained, fixation was performed using a plate and applying a cortical bone strut opposite to the plate. When a segmental bone defect was also present, an intercalary graft was added between bone ends (Fig. 1). Segmental bone defects was quantified and length of the bones were measured using the image intensifier according to the method described by Szabo and Weber [14]. In case of both radius and ulna nonunion, fixation of the ulna was performed first in order to properly restore length of the forearm and alignment of the bones [15].
After surgery a long-arm cast with elbow 90° flexed and forearm in intermediate rotation was applied and maintained for four weeks. In group B in order to protect the donor site we applied an ambulatory boot cast for four weeks. After cast removal physiotherapy was prescribed.
All patients were checked monthly until there was radiographic evidence of bone healing, and thereafter, they were checked yearly for a minimum of two and 12 years postoperatively, respectively, in groups B and A.
To evaluate the time of healing of the fractures, a combined clinical and radiographic assessment was used. The clinical parameters evaluated were (1) absence of pain or tenderness on palpation, (2) absence of pain on grip strength and (3) full range of motion at the adjacent joint. The radiographic parameters used were (1) bridging of the fracture site by bone, callus or trabeculae, (2) bridging of the fracture seen at three cortices and (3) obliteration of the fracture line or cortical continuity.
We rated forearm functional results using the modified Anderson system, which classifies the results as follows: an excellent result is defined as a united fracture with loss less than 10° of elbow or wrist flexion-extension or loss less than 25 % of elbow pronation-supination; a satisfactory result is defined as a healed fracture with loss less than 20° of elbow or wrist flexion-extension or loss less than 50 % of elbow pronation-supination; an unsatisfactory result is defined as a healed fracture with a loss greater than 20° of elbow or wrist flexion-extension or loss greater than 50 % of elbow pronation-supination; and failure is defined as malunion, nonunion or osteomyelitis [16].
Patient’s grip strength was evaluated and the outcome divided into three groups: normal, slight, and severe limitation. We also evaluated the return to activities of daily living (ADLs) split up: no ADLs limitation, slight, and severe limitation. In group A the donor-site functional results assessing the following parameters were also evaluated: hindfoot alignment—normal (5–7° valgus), minimal malalignment (7–10° valgus), and severe (valgus greater then 10°); ankle stability—stable or unstable; and limitation of ankle movement—no limitation, slight and severe limitation. Pain was rated using the visual analog scale, ranging from 0 (absence of pain) and 10 (maximum pain).
Results
No intra-operative complications occurred. One patient in group B suffered a transient palsy of posterior interosseous nerve that completely resolved spontaneously three months after surgery. There were no early or late infections in either group. All the nonunions healed (Figs. 2 and 3). Average time of healing of the nonunion was four months in both groups, ranging from two to 12 months in group A and from three to ten months in group B. Forearm functional results according to the Anderson scale were excellent in 12 (60 %) patients in group A and in eight patients (57.1 %) in group B, satisfactory in seven patients (35 %) in group A and in four patients (28.6 %) in group B and unsatisfactory in one patient (5 %) in group A and two patients (14.3 %) in group B (Table 4).
Radiographic aspect of ulna nonunion developed after open reduction and internal fixation with plate and screws (a, b). Postoperative radiographs showing new synthesis with opposite omologous cortical strut graft (c, d). Anteroposterior (e) and lateral (f) radiographs show complete remodeling of the graft at 2 years follow-up
Lateral (A) and anteroposterior (B) radiographs show atrophic nonunion of the radius. Postoperative radiographs (C, D) show new synthesis with opposite autologous cortical strut graft and intercalary graft. Lateral (E) and anteroposterior (F) radiographs show complete remodeling of the graft at 12-years follow-up. Postoperative AP radiographs of the fibula donor site (G, H)
Patients resumed ADLs two months after surgery and their original work activity at a mean of three months after surgery for sedentary work and a mean of four months for strenuous jobs with no differences in both groups. In group A we evaluated the donor site morbidity. Nineteen patients had normal hindfoot alignment (5–7° valgus) and one patient had minimal malalignment (7–10° valgus). The ankle was stable in all patients. Eighteen patients had no limitation of ankle motion, and two patients had slight limitation. At last followup mean value of pain according to VAS was 1 in both group A (range 0–2) and group B (range 0–3).
Discussion
Nonunions of the forearm are rare and difficult to treat. Modern fixation techniques with application of the AO principles in forearm fractures have been shown to be relatively secure to achieve healing. Large series have reported nonunion rates below 5 % [16–19]. Enhancement of healing of a long bone fracture nonunion is a multifactorial process and it depends on the type of nonunion. Thus, for the hypertrophic forms, associated with excessive motion, the first step is to obtain a stable fixation often by the revision of the method of fixation. Conversely, in the atrophic forms, the goal must be the restoration of a good biological environment [20]. Various surgical techniques have been proposed for treatment of forearm nonunions [21–35].
Shelton and Sage reported an union rate of 87 % using a compression plate and iliac graft [33]. Spira obtained an union rate of 73 % with the use of iliac graft combined with intramedullary nail [34]. Grace and Eversmann using plate and iliac bone graft reported a success rate of 67 % [23]. Some studies reported success rates of 100 % [24, 26, 27], but the described techniques have significant disadvantages. Han et al. [24] and Jupiter [27] reported high success rates using vascularized bone graft which requires longer surgical time and specialized equipment. As does the Ilizarov technique [26] which also requires a demanding postoperative management.
Most of the authors use autologous iliac crest bone graft due to its rich source of progenitor cells and growth factors [36]. This type of graft assures osteoconduction and osteoinduction but lacks strength and it still requires stable fixation. We do not recommend the iliac autograft because it is difficult to obtain an adequate opposite cortical strut from the iliac crest. We decided to use something that could bring both stability and biological enhancement so we preferred the fibula as a massive cortical graft because its curvature ray is similar to that of the forearm bones. Using a cortical graft opposite to a metal plate provides more stability to the implant compared with a metal plate alone. If good stability is obtained, bone healing occurs earlier and more easily allowing active motion and rapid recovery of activities. It is important to be careful in choosing the morphological characteristics of the graft, because if it is very short, it may not provide stability. Also, the screws should not be placed close to the nonunion or the intercalary graft when present so that healing would not be compromised; also, neither the cortical strut nor the plate should be too close to the interosseous membrane because of the risk of conflict during the pronation-supination. We initially used a fibular cortical autograft strut but this technique involves some difficulties including (1) comorbidity in the donor site, (2) increased surgical time, considering the time necessary to harvest the graft from the donor site, and (3) the need to adapt to the characteristics of the patient’s fibula such as length, diameter and cortical thickness. For these reasons we have therefore thought of resorting to the bone bank of our institute and to use homologous bone that responded case-by-case to our needs. The greatest benefits that we found were (1) short surgical time, (2) possibility to customize the graft according to the patient’s characteristics in terms of length, thickness and width and (3) negligible difference in osteoinductive and osteoconductive properties due to the fact that both were cortical bone type and that in the majority of the cases we need more the stability than the biological enhancement. We didn’t find in literature procedures such as ours with homologous bone strut graft so we were not able to compare our results.
We are also aware of the limitations of this study since non-homogeneous groups of patients are compared and since the presence of bone cells and growth factors in autologous graft is difficult to be quantified as well as their contribution in promoting the healing of the nonunion that may be postulated rather than demonstrated.
In our experience both techniques were found to be valid in achieving the fractures consolidation and recovery of the elbow and wrist motion without major complications.
All patients treated for aseptic forearm nonunion achieved bone healing using the described approaches and recovered quickly.
We believe that ensuring adequate coverage of the graft by muscles could promote graft vascularization, surgical wound healing and avoid infection. We assumed that the excellent rate of wound healing and the low rate of infection in our series may be due to the adequate position of the implant. Due to the lack of literature concerning only aseptic forearm nonunions we were not able to compare our results about wound healing and rate of infections with other studies.
Moreover we avoided placing the plate and the graft too close to the interosseous membrane in order to avoid impingement and pronosupination impairment. We believe this could explain the good functional results observed in our series. Nevertheless, it was not always possible to completely avoid the impingement between soft tissues and the two forearm bones due to the presence of the hardware on one side and the opposite graft on the other side of the bone. The encumbrance of the hardware and the bone graft could have affected the movement of the forearm (both flexion-extension and pronosupination) so we assumed that lower functional results may be ascribed to this problem. In the presented series, up to the time of last follow up, none of the patients underwent further surgery to remove fixation devices, so we weren’t able to evaluate if hardware removal could actually affect the functional results, reducing the impingement between radius, ulna and soft tissues, and thus improving forearm movement. Anyway, we believe that bone remodeling over time, after nonunion healing, may partially reduce the impingement.
Similarly, in these series we could not evaluate the potential risk of iterative fracture after removing the plate and the screws. However, since an obvious integration and remodeling of the bone graft was observed in both groups, without significant difference between autologous and homologous, we assume that the bone should not present a higher risk of new fracture once it is completely healed and remodeled even after hardware removal.
High success rate was achieved in terms of forearm alignment and function with both techniques without significant differences. So, if a bone bank is available, we suggest to use homologous cortical bone strut graft with opposite plate and screw fixation for the treatment of aseptic forearm nonunion.
References
Kloen P, Buijze GA, Ring D (2012) Management of forearm nonunions: current concepts. Strateg Trauma Limb Reconstr 7:1–11. doi:10.1007/s11751-011-0125-0
Richard MJ, Ruch DS, Aldridge JM (2007) Malunions and nonunions of the forearm. Hand Clin 23:235–243. doi:10.1016/j.hcl.2007.02.005, vii
Naimark A, Miller K, Segal D, Kossoff J (1981) Nonunion. Skelet Radiol 6:21–25
Giannoudis PV, Einhorn TA, Marsh D (2007) Fracture healing: the diamond concept. Injury 38(Suppl 4):S3–S6
Hernigou P (2015) Bone transplantation and tissue engineering. Part II: bone graft and osteogenesis in the seventeenth, eighteenth and nineteenth centuries (Duhamel, Haller, Ollier and MacEwen). Int Orthop 39:193–204. doi:10.1007/s00264-014-2578-5
Finkemeier CG (2002) Bone-grafting and bone-graft substitutes. J Bone Joint Surg Am 84-A:454–464
Hinsenkamp M, Collard J-F (2015) Growth factors in orthopaedic surgery: demineralized bone matrix versus recombinant bone morphogenetic proteins. Int Orthop 39:137–147. doi:10.1007/s00264-014-2562-0
Khan SN, Cammisa FP, Sandhu HS et al (2005) The biology of bone grafting. J Am Acad Orthop Surg 13:77–86
Precheur HV (2007) Bone graft materials. Dent Clin N Am 51:729–746. doi:10.1016/j.cden.2007.03.004, viii
Nodzo SR, Kaplan NB, Hohman DW, Ritter CA (2014) A radiographic and clinical comparison of reamer-irrigator-aspirator versus iliac crest bone graft in ankle arthrodesis. Int Orthop 38:1199–1203. doi:10.1007/s00264-014-2348-4
Stevenson S (1998) Enhancement of fracture healing with autogenous and allogeneic bone grafts. Clin Orthop 355(Suppl):239–246
Faldini C, Miscione MT, Acri F et al (2011) Use of homologous bone graft in the treatment of aseptic forearm nonunion. Musculoskelet Surg 95:31–35. doi:10.1007/s12306-011-0117-8
Thompson JE (1918) Anatomical methods of approach in operations on the long bones of the extremities. Ann Surg 68:309–329
Szabo RM, Weber SC (1988) Comminuted intraarticular fractures of the distal radius. Clin Orthop 230:39–48
Bronstein AJ, Trumble TE, Tencer AF (1997) The effects of distal radius fracture malalignment on forearm rotation: a cadaveric study. J Hand Surg 22:258–262. doi:10.1016/S0363-5023(97)80160-8
Anderson LD, Sisk D, Tooms RE, Park WI (1975) Compression-plate fixation in acute diaphyseal fractures of the radius and ulna. J Bone Joint Surg Am 57:287–297
Chapman MW, Gordon JE, Zissimos AG (1989) Compression-plate fixation of acute fractures of the diaphyses of the radius and ulna. J Bone Joint Surg Am 71:159–169
Kloen P, Wiggers JK, Buijze GA (2010) Treatment of diaphyseal non-unions of the ulna and radius. Arch Orthop Trauma Surg 130:1439–1445. doi:10.1007/s00402-010-1071-x
Tzioupis C, Giannoudis PV (2007) Prevalence of long-bone non-unions. Injury 38(Suppl 2):S3–S9
Kanakaris NK, Paliobeis C, Nlanidakis N, Giannoudis PV (2007) Biological enhancement of tibial diaphyseal aseptic non-unions: the efficacy of autologous bone grafting, BMPs and reaming by-products. Injury 38(Suppl 2):S65–S75
Christensen NO (1973) Küntscher intramedullary reaming and nail fixation for non-union of fracture of the femur and the tibia. J Bone Joint Surg (Br) 55-B:312–318
Dabezies EJ, Stewart WE, Goodman FG, Deffer PA (1971) Management of segmental defects of the radius and ulna. J Trauma 11:778–788
Grace TG, Eversmann WW (1980) The management of segmental bone loss associated with forearm fractures. J Bone Joint Surg Am 62:1150–1155
Han CS, Wood MB, Bishop AT, Cooney WP (1992) Vascularized bone transfer. J Bone Joint Surg Am 74:1441–1449
Hong G, Cong-Feng L, Hui-Peng S et al (2006) Treatment of diaphyseal forearm nonunions with interlocking intramedullary nails. Clin Orthop 450:186–192. doi:10.1097/01.blo.0000214444.87645.75
Ilizarov GA, Kaplunov AG, Degtiarev VE, Lediaev VI (1972) Treatment of pseudarthroses and ununited fractures, complicated by purulent infection, by the method of compression-distraction osteosynthesis. Ortop Travmatol Protez 33:10–14
Jupiter JB (1990) Complex non-union of the humeral diaphysis. Treatment with a medial approach, an anterior plate, and a vascularized fibular graft. J Bone Joint Surg Am 72:701–707
Moroni A, Caja VL, Sabato C et al (1995) Composite bone grafting and plate fixation for the treatment of nonunions of the forearm with segmental bone loss: a report of eight cases. J Orthop Trauma 9:419–426
Moroni A, Rollo G, Guzzardella M, Zinghi G (1997) Surgical treatment of isolated forearm non-union with segmental bone loss. Injury 28:497–504
Nicoll EA (1956) The treatment of gaps in long bones by cancellous insert grafts. J Bone Joint Surg (Br) 38-B:70–82
Ring D, Allende C, Jafarnia K et al (2004) Ununited diaphyseal forearm fractures with segmental defects: plate fixation and autogenous cancellous bone-grafting. J Bone Joint Surg Am 86-A:2440–2445
Scaglietti O, Stringa G, Mizzau M (1965) Bone grafting in nonunion of the forearm. Clin Orthop 43:65–76
Shelton WR, Sage FP (1981) Modified Nicoll-graft treatment of gap non-unions in the upper extremity. J Bone Joint Surg 63:226–231
Spira E (1954) Bridging of bone defects in the forearm with iliac graft combined with intramedullary nailing. J Bone Joint Surg (Br) 36-B:642–646
Williamson DM, Copeland SA, Landi A (1989) Pseudarthrosis of the radius treated by free vascularised bone graft. J Hand Surg Br 14:221–225
Sen MK, Miclau T (2007) Autologous iliac crest bone graft: should it still be the gold standard for treating nonunions? Injury 38(Suppl 1):S75–S80. doi:10.1016/j.injury.2007.02.012
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
(MPG 13540 kb)
Rights and permissions
About this article
Cite this article
Faldini, C., Traina, F., Perna, F. et al. Surgical treatment of aseptic forearm nonunion with plate and opposite bone graft strut. Autograft or allograft?. International Orthopaedics (SICOT) 39, 1343–1349 (2015). https://doi.org/10.1007/s00264-015-2718-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00264-015-2718-6