Abstract
CD47 is a widely expressed cell-surface protein that regulates phagocytosis mediated by cells of the innate immune system, such as macrophages and dendritic cells. CD47 serves as the ligand for a receptor on these innate immune cells, signal regulatory protein (SIRP)-α, which in turn inhibits phagocytosis. Several targeted CD47 therapeutic antibodies have been investigated clinically; however, how to improve its therapeutic efficacy remains unclear. Herein, we developed a CD47 blocking antibody, named IBI188, that could specifically block the CD47-SIRP-α axis, which transduces the "don’t eat me" signal to macrophages. In vitro phagocytosis assays demonstrated the pro-phagocytosis ability of IBI188. Furthermore, several in vivo models were chosen to evaluate the anti-tumor efficacy of IBI188. IBI188 treatment upregulated cell movement- and inflammation-related genes in macrophages. Synergism was observed when combined with an anti-CD20 therapeutic antibody, whose function depends on antibody-dependent cellular cytotoxicity/phagocytosis (ADCC/ADCP). CD47 expression was evaluated following azacytidine (AZA) treatment, a standard-of-care for patients with multiple myeloma; enhanced anti-tumor efficacy was observed in the combination group in AML xenograft models. Notably, IBI188 treatment increased vascular endothelial growth factor-A (VEGF-A) levels in a solid tumor model, and combined treatment with an anti-VEGF-A antibody and IBI188 resulted in an enhanced anti-tumor effect. These data indicate that IBI188 is a therapeutic anti-CD47 antibody with anti-tumor potency, which can be enhanced when used in combination with standard-of-care drugs for cancer treatment.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Immunotherapy is a powerful tool for the treatment of cancer. Directly targeting the immune system triggers a strong memory immune response than conventional chemotherapy, which leads to substantial survival benefits [1]. The overall response rate observed with programmed cell death protein (PD)-1-targeted therapy varies between cancer types and generally remains low [2]; therefore, new combination therapies are needed to maximize the anti-tumor efficacy of these therapies.
CD47, also known as integrin-associated protein (IAP), is a widely expressed transmembrane protein. Tumor cells expressing CD47 directly inhibit macrophage or dendritic cell phagocytosis of tumor cells via interaction with signal regulatory protein (SIRP)-α expressed on phagocytes. High expression of CD47 has been reported in numerous hematologic and solid cancers [3,4,5,6,7], suggesting that CD47 participates in tumor immune escape. The clinical prognostic outcome is strongly negatively correlated with CD47 expression [4]. Blocking the CD47/SIRP-α interaction may enhance the phagocytotic function of antigen-presenting cells, and has demonstrated strong anti-tumor potency in multiple preclinical models, either through macrophages or dendritic cells [5, 8, 9]. Based on this, therapeutic antibodies and fusion proteins targeting the CD47-SIRP-α pathway have been identified and tested clinically.
Vascular endothelial growth factor (VEGF)-A regulates blood vessel development and homeostasis. VEGF-A is secreted by tumor cells and the surrounding stromal cells, promoting endothelial cell proliferation or survival and subsequent angiogenesis [10,11,12]. These blood vessels then provide tumor cells with nutrients. In addition, VEGF-A was recently shown to possess immune-suppressive function. VEGF-A can directly inhibit the maturation of dendritic cells and the cytotoxic function of T cells [13,14,15]. Moreover, CD47 deficiency in T cells or tumor stromal cells increases VEGF-A expression in T cells and at tumor sites, which contributes to the state of immune suppression. It is unclear whether blocking the CD47 pathway in tumor cells would elevate VEGF-A expression inside the tumor.
In this study, we screened a highly potent anti-CD47 blocking antibody named IBI188, which can promote the phagocytosis of tumor cells by macrophages in vitro. The anti-tumor efficacy of IBI188 has been demonstrated in NHL and AML/MDS xenograft mouse models, when administered as monotherapy and in combination with an anti-CD20 antibody or azacytidine (AZA). During AZA treatment, negative feedback was observed with upregulation of CD47, which inhibited the phagocytotic ability of macrophages. Moreover, in a solid tumor model, VEGF-A expression was elevated following anti-CD47 antibody treatment, which suggests that angiogenesis limits the efficacy of this antibody in solid tumors.
Materials and methods
Cell line, cell line construction, and transfection
Raji, MDA-MB-231, MV-4-11, CCRF-CEM, and HL-60 cells were purchased from ATCC (Manassas, VA). CHO-S expression cell lines were generated according to the manufacturer’s instructions using the Freedom® CHO-S® Kit (Invitrogen). Full-length human CD47 coding sequences (CDS) were inserted into the pCHO 1.0 vector to generate CHO-S cells overexpressing CD47.
Antibody expression and purification
Hu5F9 is a human immunoglobulin (Ig)G4 CD47 antibody that utilizes heavy and light chain sequences from a publicly available source (World Health Organization Proposed INN List 120). IBI301 is a bio-similar of Rituximab (World Health Organization Proposed INN List 77). IBI305 is a bio-similar of bevacizumab (World Health Organization Proposed INN List 83). All functional antibodies, including IBI188, Hu5F9, IBI301 (biosimilar to Rituximab) and IBI305, used in the study were generated internally from HEK293F culture supernatant (Innovent Biologics Co., Ltd., Suzhou, China). Cleared cell supernatants were then applied to a Protein A affinity column (GE Healthcare). Antibodies were purified according to manufacturer’s protocols. The purity of antibodies was analyzed by analytic size exclusion chromatography (SEC).
Affinity measurement by Biolayer interferometry (BLI)
The binding affinity of human CD47–IBI188 and Hu5F9 was measured using Octet Red96 (Fortebio, USA). Briefly, IBI188 and Hu5F9 were loaded onto AHC sensor tips (Fortebio, USA). Then, the sensor tips were dipped into serially diluted human CD47-his tag (0, 1.25, 2.5, 5, 10, and 20 nM) (Acro Biosystems, USA) for association, and then transferred into running buffer for dissociation. The binding time was 180 s, and the dissociation time was 300 s. The dilutions and experiments were performed at 30 °C in running buffer (1 × phosphate-buffered saline [PBS], 0.1% bovine serum albumin [BSA], 0.05% Tween-20). All data were analyzed with Fortebio Octet data analysis software (version 7.0) using a 1:1 binding model.
Flow cytometry
All antibodies used in this study were purchased from BD Biosciences, eBioscience, BioLegend, or SouthernBiotech. Flow cytometry was performed on a BD FACSCELESTA (BD Biosciences), FlowJo software (TreeStar) was used for further analyses, and mean fluorescence intensity (MFI) was calculated accordingly.
For the cell-based binding assay, human CD47-expressing cells (CHO-hCD47, Raji, and MDA-MB-231 cells, 2 × 105 cells/well) were incubated with serial dilutions of IBI188 and Hu5F9 for 30 min in PBS on ice. The cells were washed twice with PBS, followed by incubation with a secondary antibody (PE anti-human IgG, SouthernBiotech) in PBS for 30 min on ice (protected from light). Cells were washed at least twice with PBS and analyzed via flow cytometry.
For the cell-based blocking assay, CHO-hCD47, Raji, and MDA-MB-231 cells were used as the CD47 source. Serial antibody dilutions were added in the presence of the human SIRP-α/mouse Fc protein (human SIRP-α/mouse Fc protein, 200 nM). The mixture was then incubated with CD47-expressing cells (2 × 105 cells/well) for 30 min in PBS on ice. Then, the cells were washed with PBS twice, followed by incubation with a secondary antibody (APC goat anti-mouse IgG, BioLegend) in PBS for 30 min on ice (protected from light). Cells were washed at least twice with PBS and analyzed via flow cytometry.
To determine the level of CD47 expression, MV-4-11 cells were seeded onto a 96-well-plate, and serial dilutions of AZA (synthesis by shanghai 9dingchem Co., Ltd, CAS #: 320-67-2) were added to the culture medium, 6 and 24 h later, MV-4-11 cells were incubated with anti-human CD47 antibody (clone CC2C6, BioLegend) for 30 min on ice. Cells were washed at least twice with PBS and analyzed via flow cytometry.
For endothelial cell staining, tumor tissues were digested with an enzyme mixture (Liberase from Roche with DNase, Sigma) for 30 min. The digestion was washed with cold PBS and filtered through a 70-μm cell strainer (Biologix Group). A single cell suspension was then stained with LIVE/DEAD Fixable Yellow (Invitrogen), mouse CD45 antibody (clone 30-F11, BioLegend), and mouse CD31 antibody (clone 390, eBioscience). Cells were washed at least twice with PBS and analyzed via flow cytometry.
In vitro phagocytosis assay
CD14+ monocytes were isolated from peripheral blood mononuclear cells (PBMCs, Allcells) and then differentiated into macrophages following incubation for 7 days with granulocyte-macrophage colony-stimulating factor (GM-CSF; 10 ng/mL). Macrophages were obtained following stimulation with interferon (IFN)-γ (20 ng/mL) for 1 h on day 5, and with lipopolysaccharide (LPS; 100 ng/mL) for 48 h. The human tumor cell line CCRF-CEM was chosen as the target cell line. CCRF-CEM was labeled with the CellTrace™ CFSE kit (Invitrogen). The labeled tumor cells were co-incubated with human macrophages in serum-free medium and antibody at 37 °C for 3 h. The tumor cell-to-macrophage ratio was 4:1. Cells were washed three times with PBS, followed by incubation with APC mouse anti-human CD14 antibody (BD Biosciences) in PBS for 30 min on ice (protected from light). Cells were washed three times with PBS and analyzed via flow cytometry. Phagocytosis index denotes the ratio of CD14+ CFSE+ phagocytic macrophages to total CD14+ macrophages.
For the imaging-based phagocytosis assay, IFN-γ- and LPS-stimulated macrophages were labeled with CellTracker Red CMTPX (Invitrogen), seeded onto a 96-well-plate, and cultured overnight. The next day, CellTracker Green CMFDA (Invitrogen) labeled CCRF-CEM cells were incubated with or without IBI188 for 30 min, and then free antibody was removed after several washes. After the antibody-bound dye-labeled tumor cells were added to macrophages, plates were washed with pre-warmed culture medium after 2 h of co-culture. Images were captured using an Olympus IX53 with 200 × magnification.
In vivo xenograft tumor model
To generate a B cell lymphoma xenograft model, Raji cells were subcutaneously implanted on the right flank of female NOD.SCID mice (Beijing Vital River Laboratory Animal Technology). When tumor volume reached 80 mm3, around days 7–8 post-inoculation, tumor-bearing mice were randomly grouped and dosed according to Fig.3. IBI188 was administered intraperitoneally every other day for 2 weeks. IBI301 (biosimilar of rituximab) was administered intraperitoneally twice a week for 2 weeks. Body weight, maximum length of the major axis (L), and maximum length of the minor axis (W) of tumors were measured twice a week. The tumor volume in mm3 was calculated using the formula: (width)2 × length/2. Mice were euthanized when the tumor volume reached 2000 mm3, or the percentage of body weight loss exceeded 20%. For the AML xenograft model, MV-4-11 and HL60 cells were subcutaneously implanted in the right flank of NOD.SCID mice (Beijing Vital River Laboratory Animal Technology). Around day 7 post-inoculation, tumor-bearing mice were randomly grouped and dosed. IBI188 was administered intraperitoneally every other day for 2 weeks, and AZA was administered intraperitoneally for 5 days. Tumor volume and body weight were measured twice per week.
NOG mice (Beijing Vital River Laboratory) were used to generate the MDA-MB-231 and A431 humanized model. Two-million PBMCs were intravenously injected into each mouse 5 days before tumor cells implantation. In the same day of tumor implantation, tumor-bearing mice were randomly grouped and dosed according to the figure legend indicated. IBI188 was administered intraperitoneally every other day for 2 weeks, and IBI305 (anti-VEGF-A) was administered intraperitoneally twice a week for 2 weeks. Tumor volume and body weight were measured twice per week.
All mice were maintained under pathogen-free conditions in the Experimental Animal Center of Innovent Biologics Co., Ltd. (Suzhou, China). All mice-related experiments were approved by the Animal Use and Care Committee of Innovent Biologics.
RNA sequencing and data analysis
Single tumor immune cells were acquired or RNA sequencing as follows: tumor tissues were first digested with enzyme mixture (Liberase, Roche with DNase, Sigma) for 30 min and then subjected to CD45 microbead enrichment. After single cells were acquired, TRIzol (Thermo) was added. RNA extraction, cRNA library construction, and sequencing were performed by Genergy Biotechnology (Shanghai, China), according to the manufacturer’s instructions. A cDNA library was constructed using the VAHTS Stranded mRNA-seq Library Prep Kit for Illumina (Vazyme NR602). The cDNA library was sequenced using Illumina Nova-seq with a 150 bp paired end.
Raw reads were filtered to obtain clean data using Trimmomatic (v0.35) [16]. The cleaned data were then mapped to the human GRCm38 reference genome using STAR aligner (v2.7.2a) [17]. htseq-count command was used to count reads mapped to each gene [18]. The R package DESeq2 was used to identify differentially expressed genes (DEGs) [19]. About 125 DEGs were chosen based on these criteria (|log2FC| > 0.5, p value < 0.1, total 125 genes), and further analyses were performed using DAVID [20, 21].
Statistical analysis
Statistical analyses were performed using GraphPad Prism (version 8 GraphPad Software Inc., San Diego, California, USA). Statistical significance for tumor volume between groups was determined by two-way ANOVA, and p values of less than 0.05 were considered statistically significant. An unpaired Mann–Whitney test was used for two-group comparisons. Data are presented as the mean ± standard error of the mean (SEM; *p < 0.05, **p < 0.01, ***p < 0.001, and ****p < 0.0001).
Results
Characterization of the fully human anti-CD47 antibody, IBI188
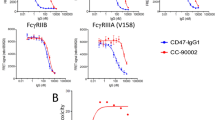
A therapeutic antibody targeting CD47 was discovered from a synthetic fully human antibody library using a yeast display platform (Adimab LLC). A panel of 25 anti-human CD47 candidate antibodies were obtained after three rounds of screening. The final lead molecule, IBI188, was selected based on its appropriate level (nM) of affinity, and its ability to block ligand and receptors. Biolayer interferometry (BLI) technology was used to measure the affinity of IBI188 and Hu5F9 (Fig. 1a). The KD of IBI188 and Hu5F9 to human CD47 was 5.3 and 4 nM, respectively. A CD47-overexpressing CHO cell line (CHO-hCD47) and primary tumor cells (Raji and MDA-MB-231) were used to measure the binding ability of IBI188 to hCD47. As shown in Fig. 1b, IBI188 could efficiently bind to cell-surface hCD47, similar to Hu5F9.
IBI188 potently blocks the CD47/SIRP-α interaction. a The binding affinity of IBI188 and Hu5F9 was measured by Fortebio using Octet Red96. b The binding of IBI188 to cell-surface human CD47 was detected by flow cytometry. c The blocking activity toward human CD47 was measured by flow cytometry. hIgG was used as the control antibody. Data in b and c represent the mean ± SEM
IBI188 promotes phagocytosis through CD47-SIRP-α blockade
High CD47 expression was found in tumor cells to escape macrophage surveillance through CD47/SIRP-α interaction [5, 22]. Next, we tested the ability of IBI188 to block the CD47/SIRP-α interaction. The blocking potency of IBI188 was demonstrated in both CHO-hCD47 and primary tumor cell lines (Raji and MDA-MB-231) (Fig. 1c). We then examined whether IBI188 could restore the phagocytic ability of macrophages. Red-dye-labeled macrophages and green-dye-labeled tumor cells were co-cultured in the presence of IBI188 or control antibody, and significant phagocytosis (red and green color overlay) was observed in the IBI188 group when compared to control group (Fig. 2a). Flow cytometry was used to quantify the phagocytosis index, CFSE-labeled tumor cells, and CD14-positive macrophages were co-cultured in the presence of the indicated antibodies. CFSE and CD14 double-positive cells were considered to be macrophages that phagocytose tumor cells. IBI188 efficiently enhanced the phagocytic ability in a dose-dependent manner (Fig. 2b).
IBI188 promotes macrophage phagocytosis through tumor surface CD47 blockade. a Red dye-labeled macrophage and green dye-labeled CCRF-CEM tumor cells were co-cultured for 2 h. A representative phagocytosis image with red-green co-staining is shown in the right. Magnification was 200x. b A phagocytosis assay performed by flow cytometry and experimental results from two different donors are presented. CCRF-CEM tumor cells were used here. Phagocytosis index denotes the ratio of CD14+ CFSE+ phagocytic macrophages to total CD14+ macrophages. Threefold serial drug concentrations were prepared with dilutions from 100 to 0.045 µg/mL
IBI188 treatment upregulates pro-inflammatory genes and eradicates tumor cells in a B cell lymphoma model
We next investigated the anti-tumor efficacy of IBI188 both in vivo and in Raji cells. Once the Raji tumor was palpable (around 1 week after implantation), mice were treated with the indicated antibodies every 2 days. As shown in Fig. 3a, the tumor started to regress following antibody treatment in a significant dose-dependent manner. Similar to previous data [4], IBI188 with IBI301, a biosimilar of rituximab, showed better tumor suppression than monotherapy at the high doses of IBI188 (Fig. 3b).
IBI188 demonstrates anti-tumor efficacy in NHL xenograft tumors as both monotherapy and combination therapy. Raji cells were subcutaneously implanted in NOD/Scid mice, and mice were then treated with different doses of IBI188 a and IBI188 in combination with IBI301 (biosimilar of rituximab). b Tumor volume curves were plotted, and two-way ANOVA was used for analysis. Data are presented as the mean ± standard error of the mean (SEM; *p < 0.05, **p < 0.01, ***p < 0.001, and ****p < 0.0001)
For the mechanism study, Raji-bearing mice were treated with IBI188 or control hIgG, tumor immune cells were isolated 8 days after the first dose, and bulk RNA was extracted and further sequenced. Compared with the hIgG group, the expression of anti-inflammatory genes, including IL11 [23], PTX3 [24], and SFRP4 [25], was downregulated in the IBI188 groups (Supplementary Fig. 1). In addition, phagocytosis is a dynamic process that requires cytoskeleton remodeling [26]. IBI188 treatment upregulated the expression of cytoskeleton-related genes, including ACTN3, ACTA1, and MYBPC2 (Supplementary Fig. 1). Together, these data suggest that IBI188 might promotes macrophage phagocytosis by inhibiting negative signals for inflammation and remodeling macrophages to enhance phagocytosis.
AZA treatment induces tumor cells expressing CD47
Next, we characterized the in vivo efficacy of IBI188 in two xenograft models of acute myeloid leukemia AML, a common hematologic malignancy. HL60 and MV-4-11 were injected subcutaneously into the right flank of NOG mice. Drugs were administered when the tumor reached 100–150 mm3, and the efficacy and safety were evaluated based on tumor volume and body weight, respectively. IBI188 monotherapy demonstrated a significant dose-dependent response in both models. AZA is the standard-of-care for AML patients. Enhanced anti-tumor efficacy was observed following combination treatment compared with monotherapy, suggesting an additional effect between these two drugs (Fig. 4a–d). We considered whether AZA can upregulate CD47 expression, since AZA is a genome-wide hypomethylating agent. MV-4-11 cells were treated with various concentrations of AZA and elevated CD47 expression was observed in AML cell lines after 6 and 24 h. The extent of CD47 upregulation was correlated with AZA concentration (Fig. 4e). Taken together, these data support the potential use of an anti-CD47 antibody combined with AZA in clinical practice.
IBI188 demonstrates anti-tumor efficacy in AML xenograft tumor as both monotherapy and combination therapy. HL60 (a and b) and MV-4-11 (c and d) tumor cells were subcutaneously implanted in NOD/Scid mice, and tumor-bearing mice were treated with IBI188 as monotherapy (a and c) or as combination (b and d) therapy. Tumor volume was plotted. Data are presented as the mean ± standard error of the mean (SEM; *p < 0.05, **p < 0.01, ***p < 0.001, and ****p < 0.0001). e MV-4-11 tumor cells were treated with serial concentrations of AZA, and the surface CD47 expression was measured by flow cytometry after 6 and 24 h treatment
IBI188 demonstrates better anti-tumor effect when combine with an anti-VEGF-A antibody in solid tumor models
Compared to hematopoietic cancers, anti-CD47 antibodies activate ineffective phagocytosis in solid tumor cell lines [27, 28], the similar in vitro phenomenon was also observed (Supplementary Fig. 2). Therefore, we next tested the anti-tumor capacity of IBI188 in a MDA-MB-231 humanized triple negative breast tumor model. Human PBMC-reconstituted NOG mice with MDA-MB-231 tumor cells were treated with IBI188 and control antibody, and limited tumor suppression efficacy was observed in the IBI188 group (Fig. 5a). Because previous studies have shown that CD47 signal blockade in tumor stromal cells increased VEGF-A expression at the tumor site, we wondered whether CD47 blockade in tumor cells would upregulate VEGF-A expression and subsequently inhibit the immune response. Therefore, we isolated tumor tissues and evaluated the levels of soluble VEGF-A at the tumor site by ELISA. As shown in Fig. 5b, elevated VEGF-A expression was observed after IBI188 treatment. VEGF-A stimulation can promote endothelial proliferation and homeostasis. Compared to the control group, more endothelial cells (CD45-CD31+ cells) were observed following IBI188 treatment (Fig. 5c). Therefore, we investigated whether combining an anti-CD47 antibody with IBI305, a biosimilar of bevacizumab, would demonstrate enhanced anti-tumor efficacy in the MDA-MB-231 humanized model. As shown in Fig. 5d, IBI188 monotherapy demonstrated limited efficacy, which was significantly improved when IBI188 was used in combination with IBI305, indicating an additional effect. The similar trend was observed in another solid tumor A431 humanized model (Supplementary Fig. 3).
Increasing expression of VEGF-A by solid tumor cells restricts the anti-tumor effect of IBI188 a MDA-MB-231 was subcutaneously implanted into PBMC-reconstituted NOG mice, who were then treated with IBI188. Tumor volume was measured and plotted. b Tumor tissue was collected and the concentration of VEGF-A in the tumor was measured and normalized to the total tumor weight. c The tumor was isolated, a single cell suspension was acquired by enzyme digestion, and CD31 +CD45- endothelial cells were compared between the IBI188 and control groups. Data from two independent experiments were pooled. d MDA-MB-231 humanized tumor-bearing mice were treated with IBI188 and IBI305 (anti-VEGF-A antibody, biosimilar of bevacizumab). An unpaired Mann–Whitney test was used for two-group comparisons. Tumor volume curves were plotted, and two-way ANOVA was used for analysis. Data are presented as the mean ± standard error of the mean (SEM; *p < 0.05, **p < 0.01, ***p < 0.001, and ****p < 0.0001)
Discussion
Immunotherapy, including PD-1/PD-L1-targeting therapies, represents a new era of cancer treatment. However, the low overall response rate achieved with such therapies suggests that a variety of combination strategies are needed. Multiple tumor cells express high levels of CD47, which inhibits phagocytosis by sending the "don’t eat me" signal. Increasing evidence supports the use of CD47 as a novel innate immune checkpoint target to enhance the efficacy of immunotherapy. Here, we identified a novel CD47 monoclonal antibody that efficiently blocks CD47 and SIRP-α signaling pathways in vitro, and enhances the phagocytosis of tumor cells by macrophages. Anti-tumor efficacy was demonstrated in multiple in vivo tumor models both in monotherapy and in combination with standard of care therapies. Moreover, in a solid tumor model, IBI188 could upregulate VEGF-A production, which contributes to adaptive resistance by promoting tumor neovascularization and suppressing the immune response. Combined therapy with anti-angiogenic drugs can overcome this resistance.
In addition to tumor cells, CD47 is expressed in many normal tissues; therefore, a therapeutic anti-CD47 antibody needs to overcome the peripheral antigen to enter the tumor site and enhance phagocytosis. Therefore, the affinity of the CD47 antibody must be moderate. An appropriate level of affinity efficiently blocks the interaction between CD47 and SIRP-α, and avoids an excessive antigen sink on the periphery. The affinity between CD47 and SIRP-α is about 10–7 M [29, 30], and the affinity of IBI188 with CD47 is 10–9 M, which efficiently blocks the binding of CD47 and SIRP-α. To reduce the toxicity associated with Fc engagement, human IgG4 was selected as the final form. Compared with human IgG1, the human IgG4 anti-CD47 antibody causes less RBC agglutination [31]. In addition, complete activation of phagocytosis requires both the SIRP-α and the FcR signal. To maximize efficacy and minimize toxicity, combination treatment with an anti-CD47 antibody and a tumor-specific targeting antibody is preferred [22]. Synergism between IBI188 and anti-CD20 antibodies (IBI301) has been demonstrated in vivo. Different from previously study [4], NOD.SCID mice were used here rather than NOG mice, because the remaining nature killer cells in NOD.SCID mice can be used to evaluate the ADCC function of IBI301. Compared with the human IgG1 anti-CD47 antibody, this mechanism helps to improve the safety and effectiveness of anti-CD47. These results support the potential combination of IBI188 with other ADCC-dependent antibodies.
In order to delineate the mechanism through which anti-CD47 regulates macrophage function, we compared changes in gene expression in tumor immune cells between the IBI188 and hIgG groups. Actin binding-related genes (Myh4, Actn3, Acta1, Mybpc2, Tnnt3, and Tnni2) were upregulated after IBI188 treatment (Supplementary Fig. 1). The functions of these genes in regulating macrophages remain unknown. We inferred that these genes might contribute to the formation of phagocytosis synapses after the release of "don’t eat me" signal mediated by anti-CD47. However, further investigation is needed.
AZA is a hypomethylating agent available for the treatment of elderly patients with myelodysplastic syndromes (MDS) or AML. The major anti-tumor mechanisms are as follows: AZA inhibits DNA methyltransferase and causes hypomethylation of DNA at low doses, and directly kills abnormal hematopoietic cells in the bone marrow through its incorporation into DNA and RNA at high doses. The relationship between AZA and macrophage phagocytosis remains unclear. Recently, forty-seven company have reported that AZA could induce endogenous expression of surface calreticulin [32], which deliver "eat me" signals to macrophage. Herein, we found that the AML cell line upregulates its cell-surface CD47 in 6 h after AZA treatment in vitro. CD47 expression increased continuously after 24 h of treatment. Upregulating CD47 might inhibit macrophage phagocytosis and thus limit the anti-tumor efficacy of AZA. We demonstrated the anti-tumor potency of the two AML/MDS xenografts in vivo. The clinical efficacy of combination treatment with AZA and IBI188 in AML is under evaluation in China.
The induction of angiogenesis is one of the hallmarks of cancer, and VEGF-A plays an essential role in this process [33]. Previous research has shown that CD47 ablation in tumor stromal cells can increase VEGF-A expression in tumors. However, tumor cells express higher levels of CD47 than normal cells, and are major contributors of VEGF-A at tumor sites. To our knowledge, no study has described whether blocking the CD47 pathway in tumor cells would increase VEGF-A secretion. Herein, we found elevated VEGF-A expression at the tumor site after IBI188 treatment. In our humanized model, tumor cells were the major IBI188 effector cells. These data suggest that anti-CD47 blockade might enhance the expression of VEGF-A by tumor cells. This negative regulation inhibits anti-tumor efficacy through neovascularization and immunosuppression. Finally, our data suggest the necessity of a combination of anti-angiogenesis drugs when running anti-CD47 clinical trials.
Abbreviations
- ADCC:
-
Antibody-dependent cellular cytotoxicity
- ADCP:
-
Antibody-dependent cellular phagocytosis
- AZA:
-
Azacytidine
- IBI188:
-
A fully human anti-CD47 therapeutic antibody
- VEGF-A:
-
Vascular endothelial growth factor A
References
Vokes EE, Ready N, Felip E et al (2018) Nivolumab versus docetaxel in previously treated advanced non-small-cell lung cancer (CheckMate 017 and CheckMate 057): 3-year update and outcomes in patients with liver metastases. Ann Oncol 29:959–965. https://doi.org/10.1093/annonc/mdy041
El-Khoueiry AB, Sangro B, Yau T et al (2017) Nivolumab in patients with advanced hepatocellular carcinoma (CheckMate 040): an open-label, non-comparative, phase 1/2 dose escalation and expansion trial. Lancet 389:2492–2502. https://doi.org/10.1016/S0140-6736(17)31046-2
Jaiswal S, Jamieson CHM, Pang WW, Park CY, Chao MP, Majeti R, Traver D, van Rooijen N, Weissman IL (2009) CD47 is upregulated on circulating hematopoietic stem cells and leukemia cells to avoid phagocytosis. Cell 138:271–285. https://doi.org/10.1016/j.cell.2009.05.046
Chao MP, Alizadeh AA, Tang C et al (2010) Anti-CD47 antibody synergizes with rituximab to promote phagocytosis and eradicate non-Hodgkin lymphoma. Cell 142:699–713. https://doi.org/10.1016/j.cell.2010.07.044
Majeti R, Chao MP, Alizadeh AA, Pang WW, Jaiswal S, Gibbs KD Jr, van Rooijen N, Weissman IL (2009) CD47 is an adverse prognostic factor and therapeutic antibody target on human acute myeloid leukemia stem cells. Cell 138:286–299. https://doi.org/10.1016/j.cell.2009.05.045
Willingham SB, Volkmer J-P, Gentles AJ et al (2012) The CD47-signal regulatory protein alpha (SIRPa) interaction is a therapeutic target for human solid tumors. Proc Natl Acad Sci 109:6662–6667. https://doi.org/10.1073/pnas.1121623109
Chao MP, Alizadeh AA, Tang C, Jan M, Weissman-Tsukamoto R, Zhao F, Park CY, Weissman IL, Majeti R (2011) Therapeutic antibody targeting of CD47 eliminates human acute lymphoblastic leukemia. Cancer Res 71:1374–1384. https://doi.org/10.1158/0008-5472.CAN-10-2238
Li Y, Zhang M, Wang X, Liu W, Wang H, Yang Y-G (2020) Vaccination with CD47 deficient tumor cells elicits an antitumor immune response in mice. Nat Commun. https://doi.org/10.1038/s41467-019-14102-4
Vonderheide RH (2015) CD47 blockade as another immune checkpoint therapy for cancer. Nat Med 21:1122–1123. https://doi.org/10.1038/nm.3965
Ferrara N (2010) Binding to the extracellular matrix and proteolytic processing: two key mechanisms regulating vascular endothelial growth factor action. Mol Biol Cell 21:687–690. https://doi.org/10.1091/mbc.E09-07-0590
Ferrara N (2010) Vascular endothelial growth factor and age-related macular degeneration: from basic science to therapy. Nat Med 16:1107–1111. https://doi.org/10.1038/nm1010-1107
Jain RK (2003) Molecular regulation of vessel maturation. Nat Med 9:685–693. https://doi.org/10.1038/nm0603-685
Basu A, Hoerning A, Datta D, Edelbauer M, Stack MP, Calzadilla K, Pal S, Briscoe DM (2010) Cutting edge: Vascular endothelial growth factor-mediated signaling in human CD45RO+ CD4+ T cells promotes Akt and ERK activation and costimulates IFN-gamma production. J Immunol 184:545–549. https://doi.org/10.4049/jimmunol.0900397
Gabrilovich DI, Chen HL, Girgis KR, Cunningham HT, Meny GM, Nadaf S, Kavanaugh D, Carbone DP (1996) Production of vascular endothelial growth factor by human tumors inhibits the functional maturation of dendritic cells. Nat Med 2:1096–1103. https://doi.org/10.1038/nm1096-1096
Ziogas AC, Gavalas NG, Tsiatas M et al (2012) VEGF directly suppresses activation of T cells from ovarian cancer patients and healthy individuals via VEGF receptor Type 2. Int J Cancer 130:857–864. https://doi.org/10.1002/ijc.26094
Bolger AM, Lohse M, Usadel B (2014) Trimmomatic: a flexible trimmer for Illumina sequence data. Bioinformatics 30:2114–2120. https://doi.org/10.1093/bioinformatics/btu170
Dobin A, Davis CA, Schlesinger F, Drenkow J, Zaleski C, Jha S, Batut P, Chaisson M, Gingeras TR (2013) STAR: ultrafast universal RNA-seq aligner. Bioinformatics 29:15–21. https://doi.org/10.1093/bioinformatics/bts635
Anders S, Pyl PT, Huber W (2015) HTSeq–a Python framework to work with high-throughput sequencing data. Bioinformatics 31:166–169. https://doi.org/10.1093/bioinformatics/btu638
Love MI, Huber W, Anders S (2014) Moderated estimation of fold change and dispersion for RNA-seq data with DESeq2. Genome Biol. https://doi.org/10.1186/s13059-014-0550-8
da Huang W, Sherman BT, Lempicki RA (2009) Bioinformatics enrichment tools: paths toward the comprehensive functional analysis of large gene lists. Nucleic Acids Res 37:1–13. https://doi.org/10.1093/nar/gkn923
da Huang W, Sherman BT, Lempicki RA (2009) Systematic and integrative analysis of large gene lists using DAVID bioinformatics resources. Nat Protoc 4:44–57. https://doi.org/10.1038/nprot.2008.211
Veillette A, Chen J (2018) SIRPα-CD47 immune checkpoint blockade in anticancer therapy. Trends Immunol 39:173–184. https://doi.org/10.1016/j.it.2017.12.005
Trepicchio WL, Wang L, Bozza M, Dorner AJ (1997) IL-11 regulates macrophage effector function through the inhibition of nuclear factor-kappaB. J Immunol 159:5661–5670
Shiraki A, Kotooka N, Komoda H, Hirase T, Oyama JI, Node K (2016) Pentraxin-3 regulates the inflammatory activity of macrophages. Biochem Biophys Rep 5:290–295. https://doi.org/10.1016/j.bbrep.2016.01.009
Bai J, Liu Z, Xu Z et al (2015) Epigenetic downregulation of SFRP4 contributes to epidermal hyperplasia in psoriasis. J Immunol 194:4185–4198. https://doi.org/10.4049/jimmunol.1403196
May RC, Machesky LM (2001) Phagocytosis and the actin cytoskeleton. J Cell Sci 114:1061–1077
Sockolosky JT, Dougan M, Ingram JR, Ho CC, Kauke MJ, Almo SC, Ploegh HL, Garcia KC (2016) Durable antitumor responses to CD47 blockade require adaptive immune stimulation. Proc Natl Acad Sci USA 113:E2646–E2654. https://doi.org/10.1073/pnas.1604268113
Weiskopf K, Ring AM, Ho CCM et al (2013) Engineered SIRPα variants as immunotherapeutic adjuvants to anticancer antibodies. Science 341:88–91. https://doi.org/10.1126/science.1238856
Kwong LS, Brown MH, Barclay AN, Hatherley D (2014) Signal-regulatory protein α from the NOD mouse binds human CD47 with an exceptionally high affinity– implications for engraftment of human cells. Immunology 143:61–67. https://doi.org/10.1111/imm.12290
Ho CCM, Guo N, Sockolosky JT, Ring AM, Weiskopf K, Özkan E, Mori Y, Weissman IL, Garcia KC (2015) “Velcro” engineering of high affinity CD47 ectodomain as signal regulatory protein α (SIRPα) antagonists that enhance antibody-dependent cellular phagocytosis. J Biol Chem 290:12650–12663. https://doi.org/10.1074/jbc.M115.648220
Pietsch EC, Dong J, Cardoso R et al (2017) Anti-leukemic activity and tolerability of anti-human CD47 monoclonal antibodies. Blood Cancer J. https://doi.org/10.1038/bcj.2017.7
Feng D, Gip P, McKenna KM et al (2018) Combination treatment with 5F9 and azacitidine enhances phagocytic elimination of acute myeloid leukemia. Blood. https://doi.org/10.1182/blood-2018-99-120170
Hanahan D, Weinberg RA (2011) Hallmarks of cancer: the next generation. Cell 144:646–674. https://doi.org/10.1016/j.cell.2011.02.013
Author information
Authors and Affiliations
Contributions
BP, HB and JG provided the yeast platform; ZW performed the affinity measurement experiments; SZ and LW designed and prepared all the needed protein; HN, XG, YG and HJ designed and performed the in vitro assays; LC, MW, YL, JD, PZ, YZ, WW and BC designed and performed all the in vivo mouse experiment; YX and JS performed the bioinformatics analysis, MY, WW and JL designed the study. All authors read and approved the final manuscript.
Corresponding authors
Ethics declarations
Conflicts of interest
Bianka Prinz, Hemanta Baruah and James Geoghegan have no potential conflicts of interest to disclose. All other authors are employees of Innovent Biologics (Suzhou).
Ethical approval
All mice were maintained under pathogen-free conditions in the Experimental Animal Center of Innovent Biologics Co., Ltd. (Suzhou, China). All mice-related experiments were approved by the Animal Use and Care Committee of Innovent Biologics.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Ni, H., Cao, L., Wu, Z. et al. Combined strategies for effective cancer immunotherapy with a novel anti-CD47 monoclonal antibody. Cancer Immunol Immunother 71, 353–363 (2022). https://doi.org/10.1007/s00262-021-02989-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00262-021-02989-2