Abstract
Cancer immunotherapy has fewer side effects and higher efficiency than conventional methods. Dendritic cell (DC)-based vaccine, a cancer immunotherapeutic, is prepared by processing mature DCs and pulsing with tumor antigen peptide ex vivo, to induce the activation of tumor-specific T lymphocytes followed by tumor clearance in vivo. Unfortunately, clinical trials of this method mostly failed due to low patient response, possibly due to the absence of novel adjuvants that induce DC maturation through Toll-like receptor (TLR) signals. Interestingly, immune checkpoint inhibitor (ICI) therapy has shown remarkable anti-tumor efficacy when combined with cancer vaccines. In this study, we identified 60S acidic ribosomal protein P2 (RPLP2) through pull-down assay using human cancer cells derived proteins that binds to Toll-like receptor 4 (TLR4). Recombinant RPLP2 induced maturation and activation of DCs in vitro. This DC-based vaccine, followed by pulsing with tumor-specific antigen, has shown to significantly increase tumor-specific CD8+IFN-γ+ T cells, and improved both tumor prevention and tumor treatment effects in vivo. The adjuvant effects of RPLP2 were shown to be dependent on TLR4 using TLR4 knockout mice. Moreover, ICIs that suppress the tumor evasion mechanism showed synergistic effects on tumor treatment when combined with these vaccines.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Antigen-presenting cells (APCs), including dendritic cells (DCs), are immune cells that uptake and present antigens to CD8 + T cells, aiding in tumor clearance [1]. DCs express various pattern recognition receptors (PRRs), which are essential to the innate immune response, and that recognize pattern-associated molecular patterns (PAMPs) or damage-associated molecular patterns (DAMPs) [2]. There are four types of PRRs, including C-type lectin receptors (CLRs), RIG I-like receptors (RLRs), NOD-like receptors (NLRs), and Toll-like receptors (TLRs) [3]. DCs express TLRs that recognize various ligands (e.g., DNA, RNA, and proteins), activate intracellular signaling pathways, and produce pro-inflammatory cytokines [4]. Among the different TLRs, the TLR4 is known to induce a strong immune response after recognizing ligands such as lipopolysaccharide (LPS), a component derived from the cell walls of Gram-negative bacteria [5]. Moreover, HMGB1 is also drawing attention, because it is released from dead cells as DAMPs that stimulate TLR4 to activate an immune response [6].
Surgery, chemotherapy, and/or radiotherapy are commonly used for the treatment of tumors [7]. However, these treatments often lead to side effects, including damage to normal cells or tumor metastasis and recurrence [8]. In a previous case report, immunotherapy through T-cell immunity was shown to be a possible efficient and safe strategy for cancer treatment [9]. As mediators between innate and adaptive immunity, immature DCs can be stimulated by ligands binding to TLRs, resulting in their activation and maturation. In lymph nodes, mature DCs interact with naïve T cells to present tumor-specific antigens, leading to the cells’ activation [10]. Subsequently, educated tumor-specific CD8+ T cells can infiltrate tumors and attack malignant cells by secretion of granzyme B and perforin [11].
Among the various types of cancer vaccines, DC-based vaccines using mature DCs that can induce the secretion of pro-inflammatory cytokines, increase the expression of co-stimulatory molecules, and present tumor-specific antigens to tumors can lead to effective T-cell immune responses [12]. As an example, Provenge (Dendreon Pharmaceuticals; CA, USA) is an FDA-approved DC-based cancer vaccine drug for the treatment of prostate cancer [13]. Despite the potential of DC-based vaccines for effective tumor clearance, there is an important limitation: tumors expressing immune checkpoint proteins, such as PD-L1, can induce effective T cells into a state of energy. Immune checkpoint inhibitors, which block these proteins, could be a promising strategy to overcome this limitation in DC-based vaccines [14]. The combination of immunotherapy, including DNA vaccines, RNA vaccines, or DC-based vaccines, with ICIs’ therapy has been studied for its synergistic anti-tumor effect; however, the adequate timing and the reliable resources like adjuvants still warrant further investigation [15, 16].
For vaccination of mature DCs, an adjuvant that can induce the cells’ activation and maturation is required, such as tumor lysates that stimulate immune receptors (e.g., TLRs) [17]. For example, LPS is a strong stimulator of TLR4, exerting a significant effect through the MyD88 and TRIF signaling pathways. However, LPS is not appropriate for use in humans because of its toxicity [18]. Instead, the other ligands of TLRs such as TLR3 ligand I:C or TLR9 ligand CpG are commonly used, but these ligands need to be expressed at sufficiently high levels to induce maturation and activation of DCs [19]. Therefore, a new adjuvant that can bind to TLRs and effectively induce DC maturation is needed.
In our previous studies, intracellular proteins in human tumor cells were screened through a pull-down assay to identify an efficient adjuvant that binds to TLR4 [20]. About 20 TLR4-binding proteins were found, half of which belonged to ribosomal protein families. Among these proteins, 60S acidic ribosomal protein P2 (RPLP2) was purified and tested for its ability to induce maturation and activate DCs, and it was identified as a possible adjuvant for DC-based vaccines. RPLP2, located in cytoplasm, is a component of the 60S subunit of ribosomes. RPLP2 is acidic, unlike most ribosomal proteins, which are basic, and it plays an important role in the elongation step of protein synthesis. RPLP2, a housekeeping gene, is safe for use in humans, unlike LPS. Moreover, RPLP2 is known to be associated with autophagy; the absence of RPLP2 leads to accumulation of ROS, inducing ER stress [21]. Moreover, this study revealed that RPLP2 has a newly found adjuvant effect.
In this study, we screened out TLR4-binding proteins from human tumor cells and identified RPLP2 as a novel ligand. We examined the effects of RPLP2 on the maturation and activation of DCs. Furthermore, DC vaccination using RPLP2 and tumor-specific peptides confirmed the utility of RPLP2 as an adjuvant. In addition, we showed that the adjuvant effects of RPLP2 were dependent on TLR4. In conclusion, the tumor treatments were effective and survival rates of vaccinated mice were higher when mature DCs were injected together with ICIs as compared with the injection of DC-based vaccination alone.
Materials and methods
Mice
C57BL/6 mice were purchased from Orient Bio (Seongnam, South Korea). TLR4 knockout mice (C57BL/10ScNF) were purchased from Jackson Laboratories (Maine, USA). Female mice were used in the experiments at 6–8 weeks of age and kept under specific pathogen-free conditions, following the animal care guidelines approved by the Institutional Animal Care and Use Committee (IACUC) of Konkuk University (KU18083).
Cells
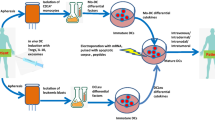
HEK293 cells expressing hTLR4-MD2-CD14 were used in the experiments following the protocol provided by InvivoGen (CA, USA). Mouse monocytes were isolated from bone marrow (BM) and differentiated in culture medium RPMI-1640 supplemented with 10% fetal bovine serum, 50 U/mL penicillin–streptomycin, 1000 μM 2-mercaptoethanol (Gibco, MA, USA), recombinant mouse IL-4, and granulocyte–macrophage colony-stimulating factor (JW CreaGene, Seongnam, South Korea). On day 6, DCs from the bone marrow of mice (BMDCs) were differentiated. Culture medium was replaced with fresh medium every 2 days. For the experiments, DCs were gated as CD11c + through flow cytometry. TC-1 is a transformed mouse lung epithelial cell expressing the HPV-16 E7 gene, highly expressed in cervical cancer cells. The EG.7 cell line, mouse lymphoma EL4 cells transfected with the OVA cDNA, was obtained from the Korean Cell Line Bank. Culture cells were incubated in RPMI-1640, DMEM, or IMEM (Biowest, France), supplemented with 10% fetal bovine serum (Biowest, Rue de la Caille, France) and 50 U/mL penicillin–streptomycin (Biowest, Rue de la Caille, France) at 37 °C and 5% CO2. Human monocytic THP-1 cells were differentiated by treating with PMA (200 μg/mL) (Sigma, Missouri, USA).
Purification of recombinant RPLP2
For the construction of the RPLP2 expression vector, cDNA derived from the human pancreatic adenocarcinoma CFPAC-1 cell line was used as a template. The insert DNA was amplified by PCR using the following primers: forward, 5´-ATGCGCTACGTCGCCTCCTAC-3´; reverse, 5´-TTAATCAAAAAGGCCAAATCCCATGTCA-3´. The amplified DNA was inserted into the pET28b bacterial expression vector. The pET28b-RPLP2 plasmid was transformed into Escherichia coli BL21 competent cells. E. coli BL21 bacterial cells expressing RPLP2 protein were incubated at 37 °C for 5 h, followed by the addition of 1 mM IPTG (isopropyl β-d-1-thiogalactopyranoside, BioBasic, Markham, Canada). The cells were then incubated at 25 °C for 12 h, followed by centrifugation at 6000 rpm for 10 min. The pellet of E. coli was lysed with SoluLyse pH 7.4 (Genlantis, CA, USA), 5 U/mL deoxynuclease I (BioBasic, Markham, ON, Canada), 50 μg/mL lysozyme (BioBasic, Markham, ON, Canada), and 5 mM DTT (BioBasic, Markham, ON, Canada) at 20 °C for 2 h. The lysed E. coli was centrifuged at 12,000 rpm for 20 min. The supernatant was filtered with 0.45 μm and purified by Ni–NTA agarose beads through FPLC. The purified protein was mixed with Triton × −114 Surfact-Amps solution (Thermo Scientific, Massachusetts, USA) to remove endotoxin until its level was less than 0.1 EU/mg using LAL assay kit (Lonza, Basel, Swiss). The purification of RPLP2 was confirmed by SDS-PAGE, gel stained with Coomassie Brilliant Blue, and western blotting detected by anti-RPLP2 antibody (Novus, Colorado, USA) and anti-His antibody (MBL, MA, USA).
The purification of GFP was performed by transforming the pET28a-GFP plasmid into E. coli BL21(DE3) competent cells (NEB, MA, USA). The rest of the processes were the same as those described above for the purification of the recombinant RPLP2.
Pull-down assay
A total of 50 μL of Ni–NTA beads (Qiagen, Hilden, Germany) and 10 μg of hTLR4-His (Sino, New Jersey, USA) were added to 50 μg of SKOV3 lysate. For negative control, 50 μL of Ni–NTA beads were added to 50 μg of SKOV3 lysate. The resin was washed out five times with 1 × PBS buffer (pH 6.0). The proteins were stained with silver stain assay after electrophoresis on 12% SDS-PAGE gel. The TLR4-binding proteins were identified and analyzed using the MALDI-TOF MS analysis system and the MASCOT search program (Korea Basic Science Institute, Daejeon, South Korea).
Luciferase assay
For the assay, 293/hTLR4A-MD2-CD14 (2 X 104/well) cells were seeded in a 96-well white plate (SPL, Pocheon, Korea). The pGL4.32[luc2P/NF-κB-RE/Hygro] vector (Promega) and the pRL-TK vector (Promega) were transfected into 293/hTLR4A-MD2-CD14 cells. NF-κB activity was assessed using the Dual-Glo Luciferase Assay System (Promega, Wisconsin, USA). RPLP2 (5 μg/mL), GFP (5 μg/mL), or LPS (100 ng/mL) were added to the cells and incubated for 2 h. Firefly luminescence, measuring NF-κB activity, and Renilla luminescence, measuring the normalization value, were assessed according to the assay kit protocol (Promega). Luciferase activity was presented by the ratio of firefly luminescence to Renilla luminescence for each well.
Enzyme-linked immunosorbent assay (ELISA)
Mouse BMDCs were treated with RPLP2 (5 μg/mL), GFP (5 μg/mL), or LPS (100 ng/mL), and the supernatant was collected after 16 h for assessment of the cytokine levels. The levels of cytokines secreted by mouse BMDCs were measured using assay kit according to the manufacturer’s instructions for mouse TNF-α, IL-1β, IL-6, IL-10, IL-12p70 (ebioscience, MA, USA), and IFN-β (pbl, NJ, USA).
Flow cytometry analysis
The wild type or TLR4−/− Mouse BMDCs were treated with RPLP2 (5 μg/mL), GFP (5 μg/mL), or LPS (100 ng/mL) for 16 h. Cells were stained with the surface markers for 1 h and washed out with PBS; FITC labeled anti-CD11c, PE labeled anti-CD40, PE labeled anti-CD80, and PE labeled anti-MHC-I (BioLegend). The maturation of BMDCs was analyzed through an FACsCaliber flow cytometer with CELLQuest (Becton Dickinson, New Jersey, USA). Splenocytes were isolated from wild type or TLR4−/− vaccinated mice and stained with PE labeled anti-CD8a and FITC labeled anti-IFN-γ (BioLegend). While FITC labeled anti-IFN-γ staining, intracellular staining was performed for 30 min according to the assay kit (BC Bioscience). After staining, the generation of CD8+ T cells in vivo was assessed by FACs analysis.
Western blotting
The wild type or TLR4−/− mice BMDCs were treated with RPLP2 (5 μg/mL) over a time course (0, 20, 40, and 60 min). Cells were harvested and lysed using RIPA buffer (0.5% NP-40, 1 mM EDTA, 50 mM Tris–Cl pH 8.0, 120 mM NaCl, protease inhibitor cocktail, and 100 mM PMSF, 0.5 M NaF) on ice for 1 h. The concentration of protein was determined by the Bradford protein assay and distributed equally with SDS loading buffer (250 nmol/L Tris–HCl, pH 6.8, 0.5 mol/L DTT, 10% SDS, 0.5% bromophenol blue, and 50% glycerol). Protein was separated by 12% SDS-PAGE and transferred to polyvinylidene difluoride membranes (Roche, Ltd). The membrane was first probed with antibodies against ERK, p-ERK, JNK, p-JNK, p38, p-p38, AKT, −AKT, IκB-α (Cell Signaling Technology), and β-actin (Sigma) diluted to 1:1000 in 5% bovine serum albumin, followed by antibody anti-mouse IgG (Abbiotec) conjugated to horseradish peroxidase. Bands were visualized by immunoreaction with chemiluminescence using LAS 4000 (Luminescent Image Analyzer).
Antigen uptake quantification
Phagocytic activity was performed as described by Jung et al. [22]. Briefly, 2 × 106 BMDCs were equilibrated at 37 °C or 4 °C for 45 min and then pulsed with 1 mg/ml of FITC-conjugated dextran (40,000 Da; Sigma-Aldrich). The reaction was stopped by cold staining buffer and washed by PBS several times. The cells were stained with PE-conjugated anti-CD11c for 1 h and analyzed using flow cytometry.
In vivo experiments
Mice were vaccinated with 2 × 106 of wild type or TLR4−/− BMDCs on the footpad two times in a week. In each experiment, mice were separated into groups; no vaccination, PBS-treated BMDCs, BMDCs loaded with tumor antigen peptide, RPLP2-treated BMDCs, RPLP2-treated BMDCs loaded with tumor antigen peptide, and LPS-treated BMDCs loaded with tumor antigen peptide. The tumor-specific antigen peptides were OVA (SIINFEKL) and E7 (RAHYNIVTE), purchased from Anygen (Gwangju, Korea). For generation of CD8+IFN-γ+ T cells, splenocytes from each vaccination group were isolated 1 week after last injection and incubated with 1 μg/mL OVA or E7 peptide and 1 μl/mL GolgiPlug (BD Cytofix/Cytoperm Kit) for 16 h. After staining, CD8+IFN-γ+ T cells were analyzed by the FACSCalibur flow cytometer. For the tumor prevention experiment, mouse cancer cells (1 × 106 EG.7 cells and 2 × 105 TC-1 cells) were injected subcutaneously 7 days after last vaccination. For the tumor treatment experiments, mouse BMDCs were vaccinated 3 days after subcutaneous injection of mouse cancer cells (1 × 106 EG.7 cells and 2 × 105 TC-1 cells). Tumor size was measured every 2–3 days and the tumor mass was calculated by the formula (length × width2)/2. Mice were sacrificed when tumor diameter was over 2 cm. To assess the combination of therapeutic effects in treatment experiments, first vaccination was delivered 5 days after cancer cells (1 × 106 EG.7 cells and 2 × 105 TC-1 cells) were subcutaneously injected. A total of 200 μg anti-PD-1 and 200 μg anti-PD-L1 antibodies were administered via intraperitoneal injection a day before the second vaccination, at 2 day intervals.
Statistical analysis
T test represents statistical significance as shown as *P < 0.01, **P < 0.05, ***P < 0.001.
Results
The human tumor cell-derived-RPLP2 binds to Toll-like receptor 4
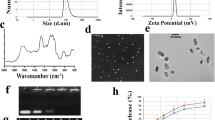
To select the candidate, SKOV3 lysate was pulled down with recombinant TLR4 (Fig. 1a) and BMDCs were treated with different ribosomal family proteins, to measure their activity. The 60S acidic ribosomal protein (RPLP2) was selected in our experiments, because it induced the activation and maturation of BMDCs. The recombinant RPLP2-His protein was purified from E. coli and confirmed by Coomasie Brilliant Blue (CBB) staining and western blotting detected with RPLP2 or histidine antibody (Fig. 1b). The activity of NF-kB was increased in TLR4-MD2 expressing HEK293 cells after treatment with RPLP2 compared to a control (Fig. 1c). To confirm the binding affinity between RPLP2 and TLR4, the BLITZ assay was performed, and the KD value was significantly lower compared to the KD value of the interaction between BSA and TLR4 (Fig. 1d). LPS was used as a positive control. It is concluded that RPLP2, one of the ribosomal family proteins, resides in all cells and binds to TLR4.
The association of TLR4 with the recombinant RPLP2. a Identification of TLR4-binding proteins using silver staining. b Coomasie Brilliant Blue (CBB) staining and western blotting results showing the purification of recombinant RPLP2 protein. c NF-кB activity of TLR4-MD2 expressing HEK293 cells treated with RPLP2 (1.5 μg/mL), GFP (5 μg/mL), or LPS (100 ng/mL). d BLITZs analysis of binding affinity of RPLP2 protein or LPS with TLR4. All experiments were performed three times. Data are represented as mean ± SD. *P < 0.01, **P < 0.05, ***P < 0.001
The recombinant RPLP2 induces activation and maturation of BMDCs and activation of TLR4 downstream signaling pathways
To confirm the effects of RPLP2 on BMDCs expressing TLR4, mice BMDCs were treated with PBS, GFP, RPLP2, or LPS. The supernatant was collected for ELISA assessment and cells were analyzed by flow cytometry. The level of pro-inflammatory cytokines and IFN-β was increased after BMDCs were treated with RPLP2, compared to PBS treatment (Fig. 2a). LPS was used as a positive control. In addition, RPLP2 induced IL-10 secretion at a lower rate than LPS, which is known to characterize immune suppression in tumor microenvironment. The expression of co-stimulatory molecules on BMDCs was also increased after treatment with RPLP2 compared to PBS (Fig. 2b). It was confirmed that RPLP2 induces the maturation of BMDCs as much as LPS. To identify the activation of TLR4, BMDCs were treated with RPLP2 in a time course. The downstream of TLR4 signaling pathways was activated for 20–40 min and IkB-α was degraded as NF-kB was activated (Fig. 2c). β-actin was used as a loading control, as it is expressed in all cells. To examine whether the RPLP2 has an equivalent functional activity against human cells, we used THP-1 that is human monocyte (Supplementary Fig 3). These results suggested that RPLP2 can induce the activation and maturation of DCs and activate TLR4 signaling pathways.
The activation and maturation of dendritic cell and downstream of TLR4 signaling pathways. BMDCs were treated with PBS, GFP (5 μg/mL), RPLP2 (1.5 μg/mL), or LPS (100 ng/mL) for 16 h. a After collecting the supernatant of culture cells, ELISA was performed to assess the level of pro-inflammatory cytokines (TNF-α, IL-1β, IL-6, IL-10, and IL-12p70) and IFN-β. b Expression of co-stimulatory molecules (CD40, CD80, CD86, and MHC I) on cells was assessed by flow cytometer. c DCs were treated with RPLP2 for 0, 20, 40, 60 min and downstream MAPKs (EKR, P38, and JNK), AKT, and IκB-α, were detected by western blotting. β-actin, expressed equally in cells, was used to confirm loading control. All experiments were performed three times. Data are represented as mean ± SD. *P < 0.01, **P < 0.05, ***P < 0.001
DC-based vaccines using RPLP2 as an adjuvant lead to the generation of tumor-specific CD8+ T cells and tumor prevention and treatment effects in vivo
We next assessed the effects of DC-based vaccines, following DC ex vivo maturation by RPLP2, namely if the activation of T-cell immunity and tumor prevention were achieved. Before injection in mice, 2 × 106 BMDCs were treated with either RPLP2 or LPS and pulsed with tumor-specific antigen peptides (OVA or E7). Pre-activated BMDCs were then injected into footpads of mice two times. After a week from last injection, the spleen of each animal was isolated and the number of IFNγ+ CD8+ T cells was measured by flow cytometric analysis. As shown in Fig. 3a, b, the number of IFNγ+ CD8+ T cells was significantly increased in the group vaccinated with BMDCs treated with RPLP2 and pulsed with OVA or E7 peptide, compared to that of the group vaccinated with immature BMDCs. Next, we assessed the tumor prevention effects of the DC-based vaccines. After a last vaccination with 2 × 106 BMDCs, the mice received a subcutaneous injection of 1 × 106 EG.7 or 2 × 105 TC-1 cells. The animals vaccinated with BMDCs treated with RPLP2 and pulsed with either OVA or E7 peptide remained all tumor-free, while tumors were formed in mice vaccinated with immature BMDCs (Fig. 3c, d).
In vivo study showing the generation of tumor-specific CD8+ T cells and tumor prevention effects by DC-based vaccines using RPLP2 protein as an adjuvant. In in vivo experiments, vaccination groups were the following: no vaccination, PBS-treated BMDCs, PBS-treated BMDCs loaded with tumor antigen peptides (OVA or E7), RPLP2 (5 μg/mL) treated BMDCs, RPLP2 (5 μg/mL) treated BMDCs loaded with tumor antigen peptides (OVA or E7), and LPS (100 ng/ml) treated BMDCs loaded with tumor antigen peptides (OVA or E7). a, b The generation of tumor-specific CD8+ T cells in the animals’ spleen was assessed by flow cytometry after DCs were injected into the footpads twice in 1 week. c, d Tumor-free mice were observed after tumor cells (EG.7 or TC-1) were subcutaneously injected, followed by DC vaccines. All experiments were performed three times. IBM SPSS Statistics Base 22.0 was used as a statistical method to analyze differences between experimental groups. *P < 0.01, **P < 0.05, ***P < 0.001
Next, we assessed the tumor treatment effects of DC-based vaccines using RPLP2 as an adjuvant and tumor-specific antigen peptide (OVA or E7). After 1 × 106 EG.7 or 2 × 105 TC-1 cells were injected subcutaneously in mice, immature or mature BMDCs pulsed with or without peptide were injected into the footpads of mice on day 3 after the tumor mass was formed. Tumor growth was suppressed and the long-term survival improved in mice vaccinated with BMDCs treated with RPLP2 and pulsed with either OVA or E7 peptide (Fig. 4a, c). In contrast, tumor growth was not suppressed and long-term survival did not improve in mice vaccinated with immature BMDCs or mature BMDCs without pulsing with a peptide. The number of IFNγ+ CD8+ T cells was significantly increased in the group vaccinated with BMDCs treated with RPLP2 and pulsed with OVA or E7 peptide, compared to that of the group vaccinated with immature BMDCs (Fig. 4b, d). Overall, DC-based vaccines using RPLP2 as an adjuvant for DC maturation were seen to have great effects on boosting T-cell immunity, tumor prevention, and tumor treatment.
In vivo study showing the tumor treatment effects of DC-based vaccines using RPLP2 as an adjuvant. The vaccination groups in in vivo experiments were as follows: no vaccination, PBS-treated BMDCs, PBS-treated BMDCs loaded with tumor antigen peptides (OVA or E7), RPLP2 (5 μg/mL) treated BMDCs, RPLP2 (5 μg/mL) treated BMDCs loaded with tumor antigen peptides (OVA or E7), and LPS (100 ng/mL) treated BMDCs loaded with tumor antigen peptides (OVA or E7). Tumor cells (EG.7 or TC-1) were injected subcutaneously in mice. After 3 days, when tumor mass was measurable, DCs were injected into footpads of mice two times in 1 week. a, b The tumor mass was measured until diameter was over 2 cm or mice died (left panel) and the survival of mice was observed for up to 30 days (right panel). All experiments were performed three times. IBM SPSS Statistics Base 22.0 was used as a statistical method to analyze differences between experimental groups. *P < 0.01, **P < 0.05, ***P < 0.001
The activation and maturation effects of RPLP2 as an adjuvant are dependent on TLR4 expression
We then examined that if the RPLP2 effects on the activation and maturation of BMDCs, followed by suppression of the tumor growth and longer survival of mice, were dependent on TLR4. BMDCs were differentiated from monocytes isolated from the bone marrow of wild type or TLR4−/− mice. To identify the activation and maturation, wild type or TLR4−/− BMDCs were treated with RPLP2 and incubated for 16 h. The level of the pro-inflammatory cytokines (TNF-α, IL-1β, IL-6, IL-10, and IL-12p70) was significantly increased in wild-type BMDCs treated with RPLP2, but not in TLR4−/− BMDCs (Fig. 5a). Also, the expression of co-stimulatory molecules (CD40, CD80, CD86, and MHC I) on wild-type BMDCs was increased after treatment with RPLP2, but this increase was not observed in TLR4−/− BMDCs (Fig. 5b). In addition, the signaling pathways in TLR4−/− BMDCs were not activated even after treatment with RPLP2. On the other hand, in wild-type BMDCs, downstream proteins were phosphorylated and IkB-α was degraded (Fig. 5c). Next, DC-based vaccines were injected into the footpads of mice after wild-type BMDCs or TLR4−/− BMDCs were treated with RPLP2 and pulsed with E7 tumor-specific antigen peptide. The generation of tumor-specific IFNγ+ CD8+ T cells was increased in mice vaccinated with wild-type BMDCs, but this increase was not observed in mice vaccinated with TLR4−/− BMDCs (Fig. 5d). In addition, tumor treatment effect and survival were decreased in mice vaccinated with TLR4−/− BMDCs compared to mice vaccinated with wild-type BMDCs (Fig. 5e). To exclude a possibility of an intrinsic defect in TLR4-deficient DCs, we used poly I:C, a stimulator for TLR3 for activation of TLR4−/− BMDCs (Supplementary Fig. 2). In conclusion, RPLP2 induced the activation and maturation of BMDCs through TLR4. Moreover, the adjuvant effect of RPLP2 on DC-based vaccines, specifically the generation of IFNγ+ CD8+ T cells and tumor treatment effects, is dependent on TLR4.
The TLR4-dependent activity of RPLP2 in vitro and in vivo. Monocytes isolated from wild type or TLR4−/− mice were differentiated and treated with RPLP2 (1.5 μg/mL), GFP (5 μg/mL), or LPS (100 ng/mL). After collecting supernatant, (a) the levels of pro-inflammatory cytokines (TNF-α, IL-1β, IL-6, IL-10, and IL-12p70) were assessed by ELISA assay. b The expressions of co-stimulatory molecules (CD40, CD80, CD86, and MHC I) were assessed by flow cytometry. c DCs were treated with RPLP2 in a time course (0, 20, 40, and 60 min) and downstream MAPKs (EKR, P38, and JNK), AKT, and IκB-α, were detected by western blotting. β-actin, expressed equally in cells, was used to confirm loading control. In in vivo, (d) the generation of tumor-specific CD8+ T cells in the animals’ spleen was assessed by flow cytometer, after RPLP2-treated BMDCs (wild type or TLR4−/−) loaded with the E7 peptide were injected into the footpads of mice two times in 1 week. e Tumor mass was measured and survival rates were observed. After TC-1 cells were injected subcutaneously on mice, RPLP2-treated BMDCs (wild type or TLR4−/−) loaded with the E7 peptide were injected into the footpads. All experiments were performed three times. IBM SPSS Statistics Base 22.0 was used as a statistical method to analyze differences between experimental groups. *P < 0.01, **P < 0.05, ***P < 0.001
DC-based vaccines are synergistically effective in tumor treatment when used with ICIs.
In these studies, we proposed that DC-based vaccines and ICIs therapy would have a synergistic effect on tumor treatment. After 1 × 106 EG.7 or 2 × 105 TC-1 cells were injected subcutaneously in mice, immature or mature BMDCs pulsed with or without peptide were injected into the footpads of mice. To identify the different effects of DC-based vaccines with ICIs therapy and DC-based vaccines only, DCs were injected on day 5 when tumor mass was measurable compared to tumor mass on day 3. Then, anti-PD-1 or anti-PD-L1 was injected intraperitoneally every third day. The tumor growth was significantly suppressed and long-term survival was observed in the mice vaccinated with BMDCs treated with RPLP2 and pulsed with OVA or E7, together with anti-PD-1 or anti-PD-L1, compared to DC-based vaccines only (Fig. 6a, c). The number of IFNγ+ CD8+ T cells was significantly increased in the group vaccinated with BMDCs treated with RPLP2 and pulsed with OVA or E7 peptide, together with anti-PD-1 or anti-PD-L1, compared to DC-based vaccines only (Fig. 6b, d). In conclusion, DC-based vaccines could be an effective therapy against tumor growth, more so in combination with ICIs.
The synergistic effects of DC-based vaccines and ICIs on tumor treatment. In in vivo experiments, vaccination groups were the following: no vaccination; anti-PD-1; anti-PD-L1; RPLP2 (5 μg/ml) treated BMDCs loaded with tumor antigen peptides (OVA or E7); RPLP2 (5 μg/ml) treated BMDCs loaded with tumor antigen peptides (OVA or E7) with anti-PD-1; RPLP2 (5 μg/ml) treated BMDCs loaded with tumor antigen peptides (OVA or E7) with anti-PD-L1. Tumor cells (EG.7 or TC-1) were injected subcutaneously on mice. After 3 days, in which tumor mass was measurable, DCs were injected into footpads of mice for 2 times in a week and anti-PD-1 or anti-PD-L1 were injected every third day. a, b The tumor mass was measured until diameter was over 2 cm or mice died (left panel) and the survival of mice was observed until 30 days (right panel). All experiments were performed three times. IBM SPSS Statistics Base 22.0 was used as a statistical method to analyze differences between experimental groups. *P < 0.01, **P < 0.05, ***P < 0.001
Discussion
Cancer vaccines, a type of immunotherapy that harnesses the strength of our own immune system stimulates a strong immune reaction via the activation of T-cell immunity, which induces an anti-tumor response. However, there are limitations to this treatment, such as low efficiency due to tumor cells’ ability to counteract the function of T cells with inhibitory checkpoint molecules. To overcome this, ICIs have been developed and shown to have significant efficiency even in monotherapy. Moreover, several trials show that cancer vaccines combined with ICIs have synergistic effects in the treatment of cancer patients. In particular, DC-based vaccines can stimulate the significant generation of functional T cells, leading to an anti-tumor immune response potentiated by ICIs that suppress inhibitory checkpoint molecules expressed on tumors [23]. Furthermore, the activation of DCs might be extended by blocking inhibitory ICIs like PD-L1, which is also known to be expressed in DCs [24]. Indeed, in our study, the combination of DC-based vaccines with ICIs showed synergistic therapeutic effects on the prevention and treatment of tumors.
For DC-based vaccines, DCs undergo maturation and activation ex vivo and then induce the generation and activation of CD8+ T cells in vivo following vaccination. In fact, multiple clinical trials have been carried out targeting different cancers using DC-based vaccines, despite the overall low responses of cancer patients [25]. One of the problems is the absence of a promising adjuvant to stimulate TLR signals in DCs and increase the activation and migratory capacity of CD8+ T cells to lymph nodes, leading to anti-tumor immunity. For example, poly I:C is a mimic of virus RNA that induces immune response through TLR3 signaling; however, it is well-known that immune response through TLR4 signaling is much stronger. Even though LPS is a well-known stimulator for TLR4 that induces strong immune response, it is not appropriate for human, because it is endotoxin from cell wall of Gram-negative bacteria. Here, we sought to find a new adjuvant from human cell-derived proteins that would stimulate TLR4 signals. After extraction from human cancer cells, various ribosomal family proteins were screened out and analyzed for association with TLR4 [20]. Among various candidates, we found that 60S acidic ribosomal protein P2 (RPLP2) can induce the activation of NF-кB, which is increased in a TLR4-MD2 expressing HEK293 cells. Moreover, the recombinant RPLP2 protein induced the activation and maturation of DCs in a TLR4-dependent manner. In conclusion, RPLP2 as a novel TLR4 ligand could be a promising adjuvant for DC-based vaccination.
In addition to the maturation of DCs, the antigen-presenting process is also necessary for the activation of naïve T cells into tumor-specific T cells [26]. However, cancer vaccines conventionally use self-antigen that can induce side effects like an insufficient amount of tumor-specific T cells and increased self-immunity leading to immune tolerance [27]. In our study, we pulsed the DCs with the E7 antigen peptide ex vivo that is mostly expressed in HPV-16 or 17-related cervical cancer [28]. As E7 is non-self and it is necessary to maintain the transformed phenotype of cervical cancer cells, it can evade immune tolerance and induce a stronger anti-tumor immune response [29]. Afterward, neo-antigen as a personalized therapy can overcome various hurdles that each tumor has [30]. Moreover, it is a breakthrough that the neo-antigen is taken up by DCs and induces significant CTL (Cytotoxic T lymphocyte) response against tumor cells with decreased immune tolerance of the DC-based vaccine [31]. Future studies may show that ICIs can support the combination therapy of DC-based vaccines with neo-antigen to reverse the immune suppression induced by tumors, achieving remarkable effects on tumor treatment [32,33,34].
In this study, we found that RPLP2 is a novel adjuvant for DC-based vaccines that binds to TLR4, inducing the activation and maturation of DCs. As a human cell-based adjuvant, the ex vivo treatment of DCs with the recombinant RPLP2 induces less side effects. However, the effects of RPLP2 on tumor cell or immune cell in vivo remain uncertain, as this protein is present in all cells and can be released as a DAMP molecule. Therefore, the immunological function of RPLP2 in cancer therapy should be further investigated. ICIs, anti-PD-1, or anti-PD-L1, together with DC-based vaccines were shown to inhibit immune checkpoint molecules expressed on either tumor cells or DCs, recover the function of tumor-specific T lymphocytes, and sustain the antigen presenting ability of DCs by suppressing the immune evasion mechanism in the tumor microenvironment. The combination of DC-based vaccines with a novel adjuvant and ICIs is a promising new strategy for cancer therapy.
Code availability
Not applicable.
References
Wculek SK, Cueto FJ, Mujal AM, Melero I, Krummel MF, Sancho D (2020) Dendritic cells in cancer immunology and immunotherapy. Nat Rev Immunol 20(1):7–24. https://doi.org/10.1038/s41577-019-0210-z
Seong S-Y, Matzinger P (2004) Hydrophobicity: an ancient damage-associated molecular pattern that initiates innate immune responses. Nat Rev Immunol 4(6):469–478. https://doi.org/10.1038/nri1372
Thompson MRK, Kurt-Jones JJ, Fitzgerald EA (2011) Pattern recognition receptors and the innate immune response to viral infection. Viruses 3:920–940
Takeuchi O, Akira S (2010) Pattern recognition receptors and inflammation. Cell 140(6):805–820. https://doi.org/10.1016/j.cell.2010.01.022
Palsson-McDermott EM, O’Neill LA (2004) Signal transduction by the lipopolysaccharide receptor, toll-like receptor-4. Immunology 113(2):153–162. https://doi.org/10.1111/j.1365-2567.2004.01976.x
Yang H, Wang H, Ju Z, Ragab AA, Lundback P, Long W, Valdes-Ferrer SI, He M, Pribis JP, Li J, Lu B, Gero D, Szabo C, Antoine DJ, Harris HE, Golenbock DT, Meng J, Roth J, Chavan SS, Andersson U, Billiar TR, Tracey KJ, Al-Abed Y (2015) MD-2 is required for disulfide HMGB1-dependent TLR4 signaling. J Exp Med 212(1):5–14. https://doi.org/10.1084/jem.20141318
Breugom AJ, Swets M, Bosset JF, Collette L, Sainato A, Cionini L, Glynne-Jones R, Counsell N, Bastiaannet E, van den Broek CB, Liefers GJ, Putter H, van de Velde CJ (2015) Adjuvant chemotherapy after preoperative (chemo)radiotherapy and surgery for patients with rectal cancer: a systematic review and meta-analysis of individual patient data. Lancet Oncol 16(2):200–207. https://doi.org/10.1016/s1470-2045(14)71199-4
Carelle N, Piotto E, Bellanger A, Germanaud J, Thuillier A, Khayat D (2002) Changing patient perceptions of the side effects of cancer chemotherapy. Cancer 95(1):155–163. https://doi.org/10.1002/cncr.10630
Sanmamed MF, Chen L (2018) a paradigm shift in cancer immunotherapy: from enhancement to normalization. Cell 175(2):313–326. https://doi.org/10.1016/j.cell.2018.09.035
Palucka K, Banchereau J (2012) Cancer immunotherapy via dendritic cells. Nat Rev Cancer 12(4):265–277. https://doi.org/10.1038/nrc3258
Lanzavecchia A, Sallusto F (2001) Regulation of T cell immunity by dendritic cells. Cell 106(3):263–266. https://doi.org/10.1016/S0092-8674(01)00455-X
Santos PM, Butterfield LH (2018) Dendritic cell-based cancer vaccines. J Immunol 200(2):443. https://doi.org/10.4049/jimmunol.1701024
Cheever MA, Higano CS (2011) PROVENGE (Sipuleucel-T) in prostate cancer: the first FDA-approved therapeutic cancer vaccine. Clin Cancer Res 17(11):3520. https://doi.org/10.1158/1078-0432.CCR-10-3126
Darvin P, Toor SM, Sasidharan Nair V, Elkord E (2018) Immune checkpoint inhibitors: recent progress and potential biomarkers. Exp Mol Med 50(12):1–11. https://doi.org/10.1038/s12276-018-0191-1
Lopes A, Vanvarenberg K, Kos Š, Lucas S, Colau D, Van den Eynde B, Préat V, Vandermeulen G (2018) Combination of immune checkpoint blockade with DNA cancer vaccine induces potent anti-tumor immunity against P815 mastocytoma. Sci Rep 8(1):15732. https://doi.org/10.1038/s41598-018-33933-7
Kyi C, Postow MA (2016) Immune checkpoint inhibitor combinations in solid tumors: opportunities and challenges. Immunotherapy 8(7):821–837. https://doi.org/10.2217/imt-2016-0002
Ho NI, Huis I, Veld LGM, Raaijmakers TK, Adema GJ (2018) Adjuvants enhancing cross-presentation by dendritic cells: the key to more effective vaccines? Front Immunol 9:2874–2874. https://doi.org/10.3389/fimmu.2018.02874
Sharifi AM, Hoda FE, Noor AM (2010) Studying the effect of LPS on cytotoxicity and apoptosis in PC12 neuronal cells: role of Bax, Bcl-2, and Caspase-3 protein expression. Toxicol Mech Methods 20(6):316–320. https://doi.org/10.3109/15376516.2010.486420
Weber C, Müller C, Podszuweit A, Montino C, Vollmer J, Forsbach A (2012) Toll-like receptor (TLR) 3 immune modulation by unformulated small interfering RNA or DNA and the role of CD14 (in TLR-mediated effects). Immunology 136(1):64–77. https://doi.org/10.1111/j.1365-2567.2012.03559.x
Park HJ, Jang G-Y, Kim YS, Park JH, Lee SE, Vo M-C, Lee J-J, Han HD, Jung ID, Kang TH, Park Y-M (2019) A novel TLR4 binding protein, 40S ribosomal protein S3, has potential utility as an adjuvant in a dendritic cell-based vaccine. J Immunother Cancer 7(1):60–60. https://doi.org/10.1186/s40425-019-0539-7
Artero-Castro A, Perez-Alea M, Feliciano A, Leal JA, Genestar M, Castellvi J, Peg V, Ramon YCS, Lleonart ME (2015) Disruption of the ribosomal P complex leads to stress-induced autophagy. Autophagy 11(9):1499–1519. https://doi.org/10.1080/15548627.2015.1063764
Jung ID, Jeong SK, Lee CM, Noh KT, Heo DR, Shin YK, Yun CH, Koh WJ, Akira S, Whang J, Kim HJ, Park WS, Shin SJ, Park YM (2011) Enhanced efficacy of therapeutic cancer vaccines produced by co-treatment with Mycobacterium tuberculosis heparin-binding hemagglutinin, a novel TLR4 agonist. Cancer Res 71(8):2858–2870. https://doi.org/10.1158/0008-5472.can-10-3487
Versteven M, Van den Bergh JMJ, Marcq E, Smits ELJ, Van Tendeloo VFI, Hobo W, Lion E (2018) Dendritic cells and programmed death-1 blockade: a joint venture to combat cancer. Front Immunol 9:394–394. https://doi.org/10.3389/fimmu.2018.00394
Sponaas A-M, Moharrami NN, Feyzi E, Standal T, Holth Rustad E, Waage A, Sundan A (2015) PDL1 expression on plasma and dendritic cells in myeloma bone marrow suggests benefit of targeted anti PD1-PDL1 therapy. PLoS ONE 10(10):e0139867–e0139867. https://doi.org/10.1371/journal.pone.0139867
Gilboa E (2007) DC-based cancer vaccines. J Clin Investig 117(5):1195–1203. https://doi.org/10.1172/JCI31205
Hughes CE, Benson RA, Bedaj M, Maffia P (2016) Antigen-presenting cells and antigen presentation in tertiary lymphoid organs. Front Immunol 7:481. https://doi.org/10.3389/fimmu.2016.00481
Saupe F, Huijbers EJ, Hein T, Femel J, Cedervall J, Olsson AK, Hellman L (2015) Vaccines targeting self-antigens: mechanisms and efficacy-determining parameters. FASEB J Off Pub Am Soc Exp Biol 29(8):3253–3262. https://doi.org/10.1096/fj.15-271502
Burd EM (2003) Human papillomavirus and cervical cancer. Clin Microbiol Rev 16(1):1–17. https://doi.org/10.1128/cmr.16.1.1-17.2003
Hung C-F, Wu TC, Monie A, Roden R (2008) Antigen-specific immunotherapy of cervical and ovarian cancer. Immunol Rev 222:43–69. https://doi.org/10.1111/j.1600-065X.2008.00622.x
Guo Y, Lei K, Tang L (2018) Neoantigen vaccine delivery for personalized anticancer immunotherapy. Front Immunol 9:1499–1499. https://doi.org/10.3389/fimmu.2018.01499
Zhang R, Yuan F, Shu Y, Tian Y, Zhou B, Yi L, Zhang X, Ding Z, Xu H, Yang L (2020) Personalized neo-antigen-pulsed dendritic cell vaccines show superior immunogenicity to neoantigen-adjuvant vaccines in mouse tumor models. Cancer Immunol Immunother 69(1):135–145. https://doi.org/10.1007/s00262-019-02448-z
van Willigen WW, Bloemendal M, Gerritsen WR, Schreibelt G, de Vries IJM, Bol KF (2018) Dendritic cell cancer therapy: vaccinating the right patient at the right time. Front Immunol 9:2265. https://doi.org/10.3389/fimmu.2018.02265
Garg AD, Coulie PG, Van den Eynde BJ, Agostinis P (2017) Integrating next-generation dendritic cell vaccines into the current cancer immunotherapy landscape. Trends Immunol 38(8):577–593. https://doi.org/10.1016/j.it.2017.05.006
Dolcetti R, López-Soto A, Dal Col J (2020) Editorial: dendritic cell-based immunotherapy in solid and haematologic tumors. Front Immunol 11:507–507. https://doi.org/10.3389/fimmu.2020.00507
Acknowledgments
This study was supported by the National Research Foundation of Korea (NRF) grant funded by the Korea government (NRF-2016R1A5A2012284 and NRF-2018R1A2B6008455). This paper was supported by the KU Research Professor Program of Konkuk University.
Funding
This study was supported by the National Research Foundation of Korea (NRF) grant funded by the Korea government (NRF-2016R1A5A2012284 and NRF-2018R1A2B6008455).
Author information
Authors and Affiliations
Contributions
GYJ, YSK, HDH, THK, and YMP designed the experiments. GYJ, YSK, SEL, and JWL carried out the experiments. GYJ and THK analyzed the data and wrote the manuscript. HDH verified the statistical methods. All authors provided critical feedback and contributed to the final manuscript. THK and YMP supervised the project.
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethics approval
All animal studies were approved by the Institutional Animal Care and Use Committee (IACUC) of Konkuk University (KU18083).
Consent to participate
Not applicable.
Consent for publication
Not applicable.
Availability of data and material
Not applicable.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Jang, GY., Kim, Y.S., Lee, S.E. et al. Improvement of DC-based vaccines using adjuvant TLR4-binding 60S acidic ribosomal protein P2 and immune checkpoint inhibitors. Cancer Immunol Immunother 70, 1075–1088 (2021). https://doi.org/10.1007/s00262-020-02759-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00262-020-02759-6