Abstract
High-dose IL-2 induces cancer regression but its therapeutic use is limited due to high toxicities resulting from its broad cell targeting. In one strategy to overcome this limitation, IL-2 has been modified to selectively target the intermediate affinity IL-2R that broadly activates memory-phenotypic CD8+ T and NK cells, while minimizing Treg-associated tolerance. In this study, we modeled an alternative strategy to amplify tumor antigen-specific TCR transgenic CD8+ T cells through limited application of a long-acting IL-2 fusion protein, mIL-2/mCD25, which selectively targets the high-affinity IL-2R. Here, mice were vaccinated with a tumor antigen and high-dose mIL-2/mCD25 was applied to coincide with the induction of the high affinity IL-2R on tumor-specific T cells. A single high dose of mIL-2/mCD25, but not an equivalent amount of IL-2, amplified the frequency and function of tumor-reactive CD8+ T effector (Teff) and memory cells. These mIL-2/mCD25-dependent effects relied on distinctive requirements for TLR signals during priming of CD8+ tumor-specific T cells. The mIL-2/mCD25-amplified tumor-reactive effector and memory T cells supported long-lasting antitumor responses to B16-F10 melanoma. This regimen only transiently increased Tregs, yielding a favorable Teff–Treg ratio within the tumor microenvironment. Notably, mIL-2/mCD25 did not increase non-tumor-specific Teff or NK cells within tumors, further substantiating the specificity of mIL-2/mCD25 for tumor antigen-activated T cells. Thus, the selectivity and persistence of mIL-2/mCD25 in conjunction with a tumor vaccine supports antitumor immunity through a mechanism that is distinct from recombinant IL-2 or IL-2-based biologics that target the intermediate affinity IL-2R.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Recombinant IL-2 was the first cancer immunotherapy approved for the treatment of metastatic melanoma and renal cell carcinoma (RCC) [1]. The antitumor activity of IL-2 is due to the expansion of T effector (Teff) cells, including tumor-specific cytotoxic T cells, CD8+ memory-phenotypic T cells, and NK cells, the latter of which can attack MHC-I low tumors [2]. Tumor antigen-specific Teff cells that have recently encountered antigen express the high-affinity IL-2R, consisting of CD25 (IL-2Rα), CD122 (IL-2Rβ), and the common gamma chain (γc) [3]; whereas, memory-phenotypic CD8+ T and NK cells express the intermediate affinity IL-2R, consisting of CD122 and γc [4, 5]. Another favorable activity of IL-2 is that the duration and intensity of IL-2R signaling promote development of memory T cells, which have the potential to protect against tumor relapse [6]. Nevertheless, the application of recombinant IL-2 in cancer patients has been complicated by its short half-life (< 10 min) [7], which necessitates frequent administrations with high amounts of IL-2, leading to severe toxicities [8, 9]. In addition, regulatory T cells (Tregs) also express the high affinity IL-2R and readily expand in response to IL-2. Increased amounts of Tregs are a negative prognostic factor in melanoma and other tumors [10], and contribute to the low response rates to IL-2 therapy in patients with these cancers [1, 11]. Consequently, IL-2 is no longer the standard treatment for patients with metastatic melanoma or RCC [5].
In an effort to take advantage of the antitumor activity of IL-2 while circumventing these drawbacks, new formulations of IL-2 are being developed that show efficacy in pre-clinical models. Many of these seek to extend the half-life of IL-2 while endowing selectivity toward the intermediate affinity IL-2R to avoid stimulation of Tregs. Examples include IL-2-containing polyethylene glycol groups, IL-2/anti-IL-2 mAb complexes that hinder cell-associated CD25 from binding to IL-2, and IL-2 muteins harboring mutations that interfere with binding to CD25 [12,13,14,15]. These approaches broadly target memory-phenotypic CD8+ T and NK cells, but are not designed to selectively enhance the tumor-specific CD8+ Teff cells that may be most effective in tumor immunotherapy.
We recently developed a novel fusion protein (FP) where mouse IL-2 is covalently linked to mouse CD25 (mIL-2/mCD25) via a non-cleavable glycine–serine linker [16]. This FP exists predominately as an inactive dimer, where IL-2 from one FP binds non-covalently in trans to CD25 on another FP. The off-rate for the dissociation of the mIL-2/mCD25 dimer into a monomer, which can bind to cell-associated IL-2Rs, is approximately 1000-fold slower when compared to the dissociation of recombinant IL-2 from CD25. This mechanism of slow dimer dissociation results in concentrations of monomer mIL-2/mCD25 in vitro and in vivo that show high selectivity toward cells expressing the high affinity IL-2R, and contributes to its in vivo half-life of approximately 16 h. At a low dose, mIL-2/mCD25 readily stimulates Tregs and prevents diabetes in NOD mice [16].
In this proof of concept study, we investigated the potential of a single application of a high dose of this mIL-2/mCD25 FP to amplify tumor antigen-specific TCR transgenic effector and memory T cells, to promote antitumor immunity to B16-F10 melanoma, while limiting effects on Tregs. The high affinity IL-2R was induced on tumor-reactive TCR transgenic CD8+ T cells by immunization with cognate antigen, and IL-2R signaling was enhanced by administering mIL-2/mCD25 shortly thereafter. Our data show that a single administration of high-dose mIL-2/mCD25 substantially amplified tumor-reactive CD8+ effector and memory T cells, and these responses were accompanied by more effective antitumor immunity toward B16-F10 melanoma.
Materials and methods
Mice
C57BL/6 J (JAX stock #000664), CD45.1-congenic C57BL/6 (B6.SJL-Ptprca Pepcb/BoyJ; JAX stock #002014), OT-I/Rag1−/− TCR transgenic (C57BL/6-Tg(TcraTcrb)1100Mjb/J; JAX stock #003831) [17], and Thy-1.1+ Pmel-1 TCR transgenic (B6.Cg-Thy1a/Cy Tg(TcraTcrb)8Rest/J; JAX stock #005023) [18] mice were purchased from The Jackson Laboratory. Subsequently, CD45.1-congenic C57BL/6, OT-I, and Pmel-1 mice were bred in our colony at the University of Miami. Experiments were initiated with 7- to 8-week-old female or male mice. Mice were housed in a specific pathogen-free facility. Animal studies were approved by the Institutional Animal Care and Use Committee at the University of Miami (Protocol 18-147).
Synthetic peptides and TLR agonists
OVA257–264 (OVA; SIINFEKL) [17] and human gp10025–33 (hgp100; KVPRNQDWL) [18] peptides were purchased from Anaspec and/or CHI Scientific and were ≥ 95% pure. LPS (cat. #L2880), poly (I:C) (cat. #P9582), and gardiquimod (cat. #SML0877) were purchased from Sigma-Aldrich. CpG (cat. #tlrl-1826) was purchased from InvivoGen. All reagent stocks were reconstituted based on solubility properties or according to manufacturer’s recommendations and maintained at − 20 °C.
IL-2-based products
mIL-2/mCD25 was purified from culture supernatants from mIL-2/mCD25-transfected CHO cells by Ni affinity columns, as previously described [16]. Human IL-2 (hIL-2) (Peprotech, cat. #200-02), mouse IL-2 (mIL-2) (Peprotech, cat. #212-12), and anti-mIL-2 mAb JES-6.1A12 (BioXCell, cat. #BE0043) were reconstituted based on manufacturer’s recommendations. Stocks were stored at − 70 °C and administered intraperitoneally (i.p.) in HBSS or PBS.
Adoptive transfer and immunizations
Sex-matched CD8+ OT-I T cells (CD45.2) (2.5 × 105 cells) were adoptively transferred into CD45.1-congenic C57BL/6 mice. Sex-matched CD8+ Pmel-1 T cells (Thy-1.1) (5 × 105 cells unless otherwise noted) were adoptively transferred into C57BL/6 J mice (Thy-1.2). These T cells were injected i.v. through the tail vein. Unless otherwise indicated, mice were immunized 1 day after adoptive transfer with OVA (10 µg) or hgp100 (100 µg) peptides and LPS (10 µg) i.v. Twenty-four h after immunization, mIL-2/mCD25, mIL-2, hIL-2, or mIL-2/JES-6.1A12 were administered i.p. The mIL-2/JES-6.1A12 complex was prepared by mixing mIL-2 (1.5 µg) and mIL-2 mAb (15 µg) for 15 min prior to administration. When indicated, poly (I:C) (50 µg), gardiquimod (100 µg), or CpG (20 µg) were administered i.v.
Flow cytometric analysis
Red blood cells were lysed from PBMCs or from single cell suspensions from lymphoid and non-lymphoid tissues using ACK lysing buffer solution. For cell surface staining, cells were first incubated with 2.4G2 to block Fc receptors for 1 min, washed with staining buffer (HBSS containing 0.2% BSA and 0.1% (w/v) sodium azide), incubated with antibodies for 15 min at 4 °C, and washed again with staining buffer. Fluorochrome-labeled and biotin-labeled antibodies and fluorochrome–streptavidin conjugates used in this study, with sources and staining concentrations, are listed in Supplementary Table 1. Intracellular staining of Foxp3, Ki67 and granzyme B was performed using the eBioscience Foxp3/Transcription Factor Staining Buffer kit by incubating the cells for 30 min according to manufacturer’s instructions. Intracellular cytokine staining for IFN-γ, TNF, and IL-2 was done using the BD Cytofix/Cytoperm Fixation/Permeabilization kit for 30 min according to the manufacturer’s instructions. Samples were run on BD LSR-Fortessa-HTS or BD LSR-II flow cytometers and data were analyzed using FACS DIVA (version 8.1) or FlowJO (version 10.6.1) software. For quantification of PBMCs, exactly 50 µL of blood was collected and all cells were run on the flow cytometers. The total events were used to calculate PBMCs/mL of blood.
Intracellular cytokine assay
To test for production of IFN-γ, TNF, and IL-2, splenocytes (1 × 106/mL/well) were cultured in 24-well plates with hgp100 (10 nM) and BD GolgiStop (1 µL) (cat #554724) in complete media (CM) consisting of RPMI-1640 (VWR) supplemented with 5% FBS, 100 U/mL penicillin, 100 µg/mL streptomycin, 2 mM l-glutamine, and 0.05 mM β-mercaptoethanol at 37 °C for 4 h prior to mAb staining for flow cytometry.
In vivo tumor model
B16-F10 melanoma was purchased from American Type Culture Collection (ATCC) and maintained as recommended. Cells were tested and verified to be negative for mycoplasma and other pathogenic agents. B16-F10 cells were used after thawing frozen aliquots and were maintained in culture for less than 2 weeks. 1 × 105 B16-F10 were inoculated s.c. into the rear flank of C57BL/6 J. Tumor growth was monitored by measuring two opposing diameters with calipers. Results are presented as tumor volume (mm3) where volume was calculated using the formula: (L × W2)/2 where L = length and W = width. In survival studies, mice were euthanized when tumor volume reached approximately 2000 mm3 or one of the diameters measured reached 20 mm.
Analysis of tumor-infiltrating lymphocytes (TILs)
B16-F10 tumors were established for 13 days prior to adoptive transfer of Pmel-1 cells and then mice were immunized and treated with mIL-2/mCD25, as indicated. On day 5 post-antigen-priming (day 19 of tumor growth), tumors were harvested, weighed, and minced into 1–2 mm pieces and incubated at 37 °C with gentle shaking in CM containing collagenase D (1 mg/mL) for 20 min. Single-cell suspensions were then obtained using the gentleMACS Dissociator and stained for flow cytometry.
Statistical analyses
All data are expressed as mean ± SEM. Statistical analyses were conducted using GraphPad Prism 8 software. For two-group analyses, unpaired t test, unpaired Welch’s t test or Mann–Whitney test were used and for three or more groups, One-way ANOVA with Tukey’s test or Welch’s ANOVA with Dunnett’s T3 test was used when appropriate, after testing for normality (Shapiro–Wilk) and equality of variances (F-test for two groups or Bartlett’s test for three or more groups). Mouse survival was illustrated using the Kaplan–Meier method and analyzed using log-rank (Mantel-Cox) test. Significance is indicated by: *p < 0.05, **p < 0.01, ***p < 0.001, ****p < 0.0001.
Results
A single high dose of mIL-2/mCD25 amplifies CD8+ tumor-reactive Teff cells and promotes CD8+ T cell memory
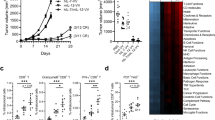
In initial studies, we tested the potential of a single dose of mIL-2/mCD25 to enhance the expansion and persistence of adoptively transferred TCR transgenic CD8+ OT-I T cells, when the FP was administered 1 day after priming with cognate antigen OVA and the TLR4 agonist LPS. At the higher dose (48 µg) of mIL-2/mCD25, the majority of the CD8+ T cell compartment in the PBMCs was composed of OT-I T cells early after priming (Fig. 1a and b). This high dose of mIL-2/mCD25 was also more effective at amplifying OT-I T cells than mIL-2/JES-6.1A12 anti-IL-2 complexes, which also selectively target the high affinity IL-2R (Fig. 1a and b). When an equivalent number of moles of IL-2 (4 μg) and mIL-2/mCD25 (16 µg) were administered, only mIL-2/mCD25 supported large expansion of OT-I T cells, and this expansion was similar to that seen after administering mIL-2/anti-IL-2 complexes (Fig. 1a and b). Besides expansion of the OT-I T cells, high-dose mIL-2/mCD25 also substantially amplified CD8+ memory T cells, as approximately 20% of the T cell compartment was composed of OT-I T cells 256 days after priming (Fig. 1b).
Single administration of mIL-2/mCD25 amplifies antigen-primed T cells. OT-I (2.5 × 105) or Pmel-1 (5 × 105) TCR transgenic T cells were transferred into naϊve C57BL/6 J or CD45.1-congenic C57BL/6 mice and 1 day later were primed with either OVA (10 μg) or hgp100 (100 μg) peptides and LPS (10 μg), respectively. 24 h after antigenic stimulation, mIL-2/mCD25, mIL-2, hIL-2, or mIL-2/JES-6.1A12 complex were administered as indicated. Representative FACS plots of OT-I and Pmel-1 T cells 7 days post priming and quantitative data measured in the blood for CD8+ CD45.2+ OT-I (a and b) (n = 3–4 mice/group) and CD8+ Thy-1.1+ Pmel-1 (c and d) (n = 13–14 mice/group) T cells. e Percent contraction of Pmel-1 CD8+ T cells where peak of response was considered to be on day 7 from data in (d) (n = 7 mice/group). f Transient expansion and rapid contraction of CD4+ Foxp3+ Tregs in the blood of mice receiving Pmel-1 adoptive transferred cells from data in (d) (n = 6–7 mice/group). Data are representative of one experiment (a, b and f) or pooled (c–e) from two independent experiments. Data were analyzed by determining the area under curve (AUC) followed by Welch’s ANOVA and Dunnett’s T3 test for multiple comparisons (b and f) or a Welch’s unpaired t-test (d and e)
Using the same immunization protocol, amplification of the primary response by high-dose mIL-2/mCD25 (100 μg) was also noted for transferred melanoma-reactive CD8+ Thy-1.1+ Pmel-1 T cells (Fig. 1c and d). Furthermore, approximately, 40% of the CD8+ T cell compartment was composed of Pmel-1 T cells 64 days after priming (Fig. 1d). When the maximal response to hgp100 was normalized to 100%, a lower overall contraction of Pmel-1 was noted after administering mIL-2/mCD25 (Fig. 1e), indicating that the higher frequency of memory cells supported by the FP was not simply accounted for by greater expansion of Pmel-1 cells during the primary response. As expected, mIL-2/mCD25 also increased the frequency of Tregs in the PBMCs as seen on day 5; however, the expansion was transient and Tregs rapidly contracted back to baseline levels by day 14 (Fig. 1f).
Using the Pmel-1 system as a model, 50–100 μg of mIL-2/mCD25 led to an over 100-fold amplification of Teff and memory T cells (Supplementary Figure S1a). In this same setting, Tregs also expanded, but these returned to baseline by day 14 post-priming (Supplementary Figure S1b). High-dose mIL-2/mCD25 enhanced the frequency of Pmel-1 cells in a cell dose-dependent manner, where adoptive transfer of higher cell numbers led to higher frequencies of amplified cells in the blood (Supplementary Figure S1c). Collectively, all these experiments demonstrate that high-dose mIL-2/mCD25 leads to amplification of antigen-specific CD8+ effector and memory T cells when the FP is applied shortly after priming, while only transiently increasing Tregs.
A single administration of mIL-2/mCD25 is more effective than IL-2 in expanding adoptively transferred Teff cells
The preceding experiments showed that high-dose mIL-2/mCD25, but not recombinant IL-2, amplified transferred OT-I Teff cells (Fig. 1b). Similarly, a single injection of mIL-2/mCD25 (50 µg), but not IL-2 administered at the same molar amounts (12.5 µg), amplified the responses of Pmel-1 cells (Fig. 2a and b). Based on these results, we tested the extent to which more frequent administrations of IL-2 after antigen-priming might replicate the primary response of Pmel-1 T cells. Even a daily administration of hIL-2 (12.5 μg) did not amplify Pmel-1 cells (Fig. 2a and b). Furthermore, a single high dose of mIL-2/mCD25 (100 µg) was still superior to hIL-2 (50 and 100 μg) administered 5 times over 72 h, which represent maximally tolerable amounts, at expanding the CD8+ Pmel-1 T cells (Fig. 2c and d). Thus, these results show that high doses of mIL-2/mCD25 are more effective than recombinant IL-2 in amplifying antigen-primed CD8+ Teff cells.
A single high-dose administration of mIL-2/mCD25 is more effective than hIL-2 at expanding Pmel-1 T cells. Pmel-1 T cells were transferred and immunized as described in Fig. 1 and then were treated with hIL-2 and mIL-2/mCD25 as indicated. Experimental design (a and c) and quantitative data (b and d) of the frequency of Pmel-1 T cells 7 days post-priming in the blood (b) (n = 5–10 mice/group) or 4 days post-priming in the spleen (d) (n = 4 mice/group). Frequency (e) and CD25 expression (f) of NK cells in the spleen of mice treated as in (c) in the presence or absence of Pmel-1 cells and immunization with hgp100 and LPS (n = 4–5 mice/group). Data were pooled from two independent experiments and analyzed by Welch’s ANOVA and Dunnett’s T3 test for multiple comparisons
We also investigated the relative effects of mIL-2/mCD25 on endogenous NK cells. In the absence of Pmel-1, hIL-2 but not mIL-2/mCD25 increased NK cells. In contrast, in the context of mice adoptively transferred with Pmel-1 T cells, hIL-2 and mIL-2/mCD25 increased NK cells to similar frequencies; these responses were greater than detected in mice without Pmel-1 that only received hIL-2 (Fig. 2e). CD25 expression was enhanced by administration of hIL-2 or the FP on NK cells and this effect was further increased by the adoptively transferred Pmel-1 T cells (Fig. 2f). These results indicate that mIL-2/mCD25-induced IL-2R signaling might synergize with vaccine-induced factors produced by Pmel-1 T cells to promote CD25 expression and proliferation of NK cells.
High-dose mIL-2/mCD25 optimally amplifies memory CD8+ T cells in lymphoid and non-lymphoid tissues, while promoting central (TCM) and effector (TEM) memory-phenotypic cells
At 258 days post-priming, high-dose mIL-2/mCD25-amplified OT-I T cells were readily found in lymphoid tissues, including the spleen, mesenteric lymph nodes (MLN), and bone marrow (BM), and in non-lymphoid tissues such as the lungs and liver (Fig. 3a). High-dose (48 μg) mIL-2/mCD25 supported a higher frequency of persistent OT-I T cells in these tissues than a lower dose of mIL-2/mCD25 (16 μg), IL-2/anti-IL-2 complexes, or IL-2. In addition, on day 21 post-priming, mIL-2/mCD25-amplified CD8+ Pmel-1 T cells were readily detected at high frequencies that correspond to 10–100-fold greater cell numbers in the spleen, inguinal lymph nodes, MLN, and BM when compared to Pmel-1 T cells primed without the FP (Supplementary Figure S2a). These findings indicate that high-dose mIL-2/mCD25 supports the development of a dominant CD8+ T memory population that resides in or circulates through lymphoid and non-lymphoid tissues.
High-dose mIL-2/mCD25 amplifies memory CD8+ T cells in lymphoid and non-lymphoid tissues and supports the generation of TEM and TCM cells. OT-I CD8+ T cells were transferred and treated as described in Fig. 1. a Quantification of OT-I T cells in the spleen, mesenteric lymph nodes (MLN), bone marrow (BM), lung, and liver 258 days post-priming. b Representative FACS plots showing frequencies of TEM and TCM OT-I cells 7 and 256 days post-priming in the blood. c Frequency of OT-I TEM and TCM for mIL-2/mCD25 and mIL-2/JES-6.1A12 complex in the blood. Memory cell distribution was assessed based on expression of CD127 and CD62L on CD44+ OT-I CD8+ T cells. Data are representative of one experiment (n = 3–4 mice/group) and analyzed using ordinary one-way ANOVA and Tukey’s test for multiple comparisons (a)
Longitudinal analysis of OT-I T cells from PBMCs showed that near the peak of the expansion, on day 7, OT-I cells consisted of Teff (CD44+ CD62Llo CD127lo) and TEM (CD44+ CD62Llo CD127hi) cells when using IL-2 or the other IL-2-based molecules (Fig. 3b). However, as early as day 14 post-priming (data not shown) and anytime thereafter, the vast majority of OT-I cells were TEM and TCM (CD44+ CD62Lhi CD127hi) cells, and these were highly abundant only in the groups that received either high-dose mIL-2/mCD25 or mIL-2/JES-6.1A12 complexes (Fig. 3b and c). Similar results were obtained for mIL-2/mCD25-amplified Pmel-1 cells (Supplementary Figure S2b and c). Notably, in this experiment at the peak of expansion, on day 5, the Pmel-1 cells were predominately Teff cells. These data in conjunction with the OT-I results suggest a rapid switch from Teff to TEM cells, which is followed by generating a similar mix of persistent CD8+ TEM and TCM cells.
High-dose mIL-2/mCD25 enhances activation and function of tumor antigen-specific T cells
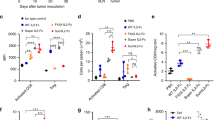
We explored the consequences of high-dose mIL-2/mCD25 on Pmel-1 T cell activation and function when assessed 5 days after priming with the relevant antigenic peptide and LPS. With respect to T cell activation, mIL-2/mCD25 enhanced the frequencies of Pmel-1 cells expressing CD25, CD44, and Ki67 while CD122 remained highly expressed (Fig. 4a and b).
High-dose mIL-2/mCD25 enhances T cell activation and functional activity. Pmel-1 T cells were transferred and immunized as described in Fig. 1 using mIL-2/mCD25 (100 μg). Representative FACS plots (a) and quantitative data (b) of activation and proliferation markers on Pmel-1 T cells 5 days post-priming in the blood. Representative FACS plots with percent (top) and mean fluorescence intensity (bottom) (c and e) and quantitative data (d and f) of the functional activity of Pmel-1 Teff cells 5 days post-priming (c and d), and memory Pmel-1 cells (e and f) 64 days post-priming in the blood after in vitro re-stimulation with hgp100. Data (a–d) (n = 6–13 mice/group) were pooled from two to three independent experiments and (e and f) are representative of one experiment (n = 2 mice/group). Data were analyzed using unpaired t test (d and f) or Welch’s unpaired t test (b)
High-dose mIL-2/mCD25 not only increased the frequencies of Pmel-1 Teff cells (Fig. 1), but also caused the amplified Pmel-1 Teff (Fig. 4c and d) and memory T cells (Fig. 4e and f) to produce IFN-γ, TNF, and granzyme B at a higher frequency and amounts, the latter based on mean fluorescence intensity, consistent with increased Teff function. In contrast, mIL-2/mCD25 led to Pmel-1 Teff, but not memory T cells, that produced IL-2 at a lower frequency. This result might reflect the capacity of Blimp-1 and T-bet to act in an IL-2-dependent negative feedback mechanism to reduce IL-2 production by Teff cells [19,20,21]. Collectively, these data show that high-dose mIL-2/mCD25, when coupled to antigenic stimulation, not only leads to exponential expansion of CD8+ Pmel-1 T cells (Fig. 1), but also enhances their functional activity.
TLR signaling is required for high-dose mIL-2/mCD25-mediated persistence of CD8+ memory T cells
TLR-dependent signaling during T cell priming with antigen has been linked to the enhanced generation of CD8+ T cell memory and protective immunity [22,23,24]. We investigated the requirement for TLR-dependent signaling in high-dose mIL-2/mCD25-dependent Teff expansion and persistent CD8+ T memory development. Administering hgp100 followed by mIL-2/mCD25 without a TLR agonist supported substantial expansion of Pmel-1 Teff cells. Combining poly (I:C), LPS, gardiquimod, or CpG, (TLR3, TLR4, TLR7/8, and TLR9 agonists, respectively) with hgp100 modestly increased mIL-2/mCD25-amplified Pmel-1 Teff cells (Fig. 5a and b). However, Pmel-1 memory T cells only developed in mice immunized with LPS or gardiquimod during antigen-priming and treated with high-dose mIL-2/mCD25 (Fig. 5a and b). Moreover, the frequency of CD25+ Pmel-1 CD8+ T cells was lower in the LPS and gardiquimod groups (Fig. 5c). As CD25 is an IL-2-responsive gene [25], this finding may reflect that these cells received lower IL-2R signaling during priming, a condition that is known to support development of CD8+ T memory formation [6, 26]. Thus, antigen- and mIL-2/mCD25-dependent expansions of CD8+ Teff cells are largely independent of TLR signaling, while the development of CD8+ T memory requires TLR4 or TLR7/8 signaling.
TLR signaling is required for high-dose mIL-2/mCD25-mediated persistent Pmel-1 memory T cells. Pmel-1 T cells were transferred and immunized as described in Fig. 1 in the presence or absence of different TLR agonists and high-dose mIL-2/mCD25 (100 µg). Numbers (a) and frequencies (b) of Pmel-1 T cells in the blood. Frequencies of CD25+ Pmel-1 CD8+ T cells (c) on day 5 post-priming in the blood. All data were pooled from two independent experiments (n = 6–7 mice/group). Frequency data in (b) were analyzed by determining the area under curve (AUC) followed by Welch’s ANOVA and Dunnett’s T3 test for multiple comparisons. Data in (c) were analyzed using an unpaired t test
mIL-2/mCD25-amplified tumor-reactive T cells promote antitumor immunity
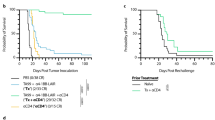
Longitudinal analysis of CD8+ OT-I and Pmel-1 T cells showed that limited application of high-dose mIL-2/mCD25 promotes substantial development of persistent memory. Mice harboring such memory Pmel-1 T cells 65 days post-priming were challenged with B16-F10. When compared to unprimed mice or mice primed 65 days previously with only hgp100/LPS, the persistent mIL-2/mCD25-amplified Pmel-1 memory cells effectively delayed tumor growth and significantly enhanced survival (Fig. 6a). Eventually, all mice with mIL-2/mCD25-dependent Pmel-1 memory T cells developed tumors. These tumors may be antigen-loss variants as they lost the typical dark pigmentation characteristic of B16-F10 melanoma (data not shown), which depends, in part, on gp100 [27].
mIL-2/mCD25-amplified effector and memory tumor-reactive T cells lead to antitumor responses. Pmel-1 T cells were transferred and immunized as described in Fig. 1 using mIL-2/mCD25 (100 μg). a–c Tumor growth (left) and survival curves (right). a Mice were challenged with B16-F10 tumor cells 65 days post-priming (n = 5–7 mice/group). b Mice received B16-F10 tumor cells and 2 days later were transferred with Pmel-1 T cells followed by immunization, as described above (n = 12–13 mice/group). c B16-F10 tumors were pre-established for 3 days prior to vaccination with peptide and LPS, and mIL-2/mCD25 (100 µg) was administered 24 h post-priming (n = 4–6 mice/group). Data are representative of (a) one experiment repeated 3 times, (b) pooled from three independent experiments, or (c) one experiment performed twice and were analyzed using the log-rank (Mantel-Cox) test. Significance values shown correspond to the mIL-2/mCD25-treated group compared to the HBSS group (a and b) or by comparing the untreated group to each of the other groups (c)
Mice with pre-established tumors prior to adoptive transfer of Pmel-1 T cells and immunization also showed a further delay in tumor growth and increased survival when high-dose mIL-2/mCD25 was administered just after priming (Fig. 6b). Adoptive transfer of the TCR transgenic cells was required to obtain significant mIL-2/mCD25-dependent antitumor responses, since vaccination and mIL-2/mCD25 administration did not enhance these antitumor responses in mice that lacked TCR transgenic T cells (Fig. 6c). Collectively, these data indicate that in the context of TCR-specific transferred T cells, a peptide vaccine and a single administration of high-dose mIL-2/mCD25 amplified tumor-specific CD8+ effector or memory T cells that mediated robust antitumor responses.
mIL-2/mCD25 enhances the frequency and functional activity of tumor-reactive T cells infiltrating B16-F10 melanoma tumors
Since tumor-reactive T cells amplified by high-dose mIL-2/mCD25 effectively cleared tumors in 38% of the mice, we assessed the immune composition of the tumor microenvironment (TME). In these experiments, mice were inoculated with B16-F10 tumor cells, and 13 days later, when the tumors were approximately 400 mm3, Pmel-1 T cells were transferred into the mice followed by immunization and application of HBSS or mIL-2/mCD25. Four days later, at the peak of the Teff response in the periphery, tumor-infiltrating lymphocytes were isolated for immunophenotyping (Fig. 7). With respect to CD8+ tumor-reactive Pmel-1 T cells, mIL-2/mCD25 increased their numbers approximately 5.7-fold (Fig. 7a), and that was accompanied by an increase in granzyme B-producing cells (Fig. 7b and c). However, Pmel-1 T cells within the tumors of mIL-2/mCD25-treated mice displayed lower expression of CD25 and Ki67 (Fig. 7b and c). The reason for this finding is not clear since the Pmel-1 T cells readily expanded to the FP in the TME. Expression of checkpoints PD-1 and CTLA-4 on tumor-infiltrating Pmel-1 cells was low; however, mIL-2/mCD25 supported lower frequencies of PD-1+ tumor-reactive T cells within these tumors (Fig. 7b and c).
High-dose mIL-2/mCD25 enhances the frequency and functional activity of tumor-infiltrating Pmel-1 T cells. B16-F10 tumors were pre-established for 13 days prior to transfer of Pmel-1 T cells. A day after transfer, the cells were primed with peptide and LPS; mIL-2/mCD25 (100 µg) was administered 24 h post-priming. Tumors were analyzed 5 days after T cell priming. a Numbers of Pmel-1 T cells per gram of tumor (n = 6–7 mice/group). Representative FACS plots (b) and quantitative data (c) showing expression of the indicated markers on Pmel-1 T cells (n = 6–7 mice/group). All data were pooled from two independent experiments and analyzed using Welch’s unpaired t test
The above data show that mIL-2/mCD25 increased the frequency of tumor-reactive T cells infiltrating B16-F10 tumors. We also investigated the effect of high-dose mIL-2/mCD25 on other immune populations in the TME. Absolute numbers of Tregs (per gram of tumor) were slightly lower in mice that received Pmel-1 T cells and mIL-2/mCD25 (Supplementary Figure S3a). This led to an approximately 11.1-fold increase in the ratio of Pmel-1 T cells to Tregs (Supplementary Figure S3b). With respect to other host-derived immune cells, a trend was noted toward a higher frequency of CD8+ polyclonal T cells, NK cells, CD11b+ cells, and Ly6G+ cells, and lower frequencies of CD19+ B cells within the tumors of mice that received Pmel-1 T cells along with mIL-2/mCD25 (Supplementary Figure S3c). mIL-2/mCD25 also increased the frequency of granzyme B-expressing host-derived T and NK cells while CD25 and Ki67 variably increased among these cell populations (Supplementary Figure S3d). Taken together, these findings show that high-dose mIL-2/mCD25 not only amplifies tumor-reactive T cells within the tumor, but also raises the possibility that other immune cells may contribute to the antitumor response against B16-F10 melanoma.
Discussion
The immunostimulatory activity of IL-2 has been shown to elicit potent anti-cancer responses, thus providing a rationale to develop new strategies that maximize these beneficial features while minimizing shortcomings of this cytokine. Two significant drawbacks of IL-2 are severe non-specific toxicities and broad targeting of IL-2R-expressing cells that may limit the antitumor response. Several new IL-2-based biologics have been developed to increase half-life and/or direct IL-2R signaling selectively toward cells expressing the high versus the intermediate affinity IL-2R [12,13,14,15]. In this regard, we have developed the mIL-2/mCD25 FP with a substantially increased half-life (approximately 16 h) and selectivity for cells expressing the high affinity IL-2R [16].
Five important findings emerge from the current study. First, only a single high dose of mIL-2/mCD25 was required to amplify CD8+ effector and memory T cells after adoptive transfer of TCR transgenic cells. Second, this single high dose of mIL-2/mCD25, which was applied to coincide with the induction of the high affinity IL-2R by vaccination with tumor antigens and TCR stimulation, led to effective antitumor immunity. Third, limited application of mIL-2/mCD25 not only promoted the expansion of tumor-reactive T cells but also enhanced markers of functional activity as assessed by intracellular levels of IFNγ, TNF, and granzyme B. Fourth, by limiting the use of mIL-2/mCD25 to a single application following vaccination, the concurrent stimulation of Tregs was only transient, allowing favorable ratios of antitumor Teff cells over Tregs within the TME. Lastly, when compared with a single equivalent dose of hIL-2, only mIL-2/mCD25 amplified antitumor Teff cells. Even when hIL-2 was frequently administered at maximal tolerable dosing, the magnitude of the antitumor Teff responses was still greater after a single application of high-dose mIL-2/mCD25.
Our strategy was to substantially and selectively increase the overall efficacy of tumor antigen-specific T cells, which are likely the most desirable cells to elicit antitumor immunity. We reasoned that promoting IL-2R signaling in tumor antigen-specific T cells would only require application of mIL-2/mCD25 at a time that coincided with tumor antigen induction of the high affinity IL-2R on T cells [4]. This tactic avoided the broad targeting of lymphoid cells, particularly CD8+ T and NK cells, associated with IL-2. Tregs also express the high affinity IL-2R but using a single application of high-dose mIL-2/mCD25 only led to a transient increase in Tregs, thus avoiding more extensive and persistent expansion of these potential tumor-suppressive cells. The amplification of OT-I T cells by mIL-2/mCD25 (16 μg) was similar to that seen with the mIL-2/anti-IL-2 (JES-6.1A12) complexes that also target the high affinity IL-2R. These complexes also increased the frequency of OVA-specific polyclonal CD8+ T cells which conferred protective immunity to Listeria monocytogenes expressing OVA [28]. Thus, the ability of mIL-2/mCD25 to amplify Pmel-1 Teff and memory cells does not reflect an idiosyncratic effect of this fusion protein on CD8+ T cells. Rather, these findings suggest that increasing the persistence of IL-2R signaling on antigen-activated CD8+ T cells is the critical factor, which is supported by mIL-2/mCD25 or agonist IL-2/anti-IL-2 complexes, as both have much longer half-lives than rIL-2.
Another strategy under development by others uses novel IL-2-based biologics to preferentially target memory-phenotypic CD8+ and NK cells, that express high amounts of the intermediate affinity IL-2R, i.e., cells that express CD122 (IL-2Rβ) and CD132 (γc) with low to no-CD25 (IL-2Rα) [12,13,14,15], while minimizing expansion of Tregs. A potential reservation of targeting the intermediate affinity IL-2R is that tumor antigen-specific CD4+ and CD8+ Teff cells express low amounts of the IL-2Rβ subunit compared to memory-phenotypic CD8+ T and NK cells and are less reactive to these types of IL-2 variants. Additionally, the expansion of tumor antigen-specific CD8+ Teff cells will be in competition with other non-tumor antigen-reactive CD8+ T cells. As these non-specific CD8+ T cells are likely much more numerous, the potential to expand tumor antigen-specific CD8+ T cells becomes unpredictable. Moreover, Tregs express relatively high amounts of CD122 and CD132, albeit at levels lower than CD8+ T and NK cells, and can still respond to IL-2-based biologics that target the intermediate affinity IL-2R, as demonstrated for IL-2/S4B6 anti-IL-2 complexes [15]. Thus, expansion of Tregs might also occur when using such modified IL-2s, depending upon the dose and frequency used. Our data clearly show that a single high dose of mIL-2/mCD25 used after vaccination with a tumor antigen may represent a viable alternative strategy by more effectively maintaining peripheral T cell homeostasis, while favorably increasing tumor-reactive T cells over Tregs in the TME. The associated transient increase in peripheral Tregs with a single high dose of mIL-2/mCD25 might also contribute to limiting tumor immunotherapy-associated autoimmunity while effectively eradicating tumors [29,30,31].
The slow dissociation of non-covalent mIL-2/mCD25 trans-dimers into biologically active mIL-2/mCD25 monomers accounts for its long half-life and selectivity toward cells expressing the high affinity IL-2R [16]. This property leads to a more constant and persistent IL-2R signaling over an extended time and is concordant with the higher expansion of Pmel-1 cells by a single high dose of mIL-2/mCD25 when compared to the same molar amounts or maximally tolerable doses of hIL-2. At the doses used, the concentration of the active monomer remains at a low enough level to exert minimal activity on cells within the TME that primarily express the intermediate affinity IL-2R. Correspondingly, mIL-2/mCD25 was insufficient to enhance the frequencies of non-tumor antigen-specific CD8+ T and NK cells in the B16-F10 TME. Notably, mIL-2/mCD25 FP exhibits this selectivity and persistence without introduction of mutations to the IL-2 sequence, binding IL-2 to a specific anti-IL-2 mAb, or coupling to non-specific immunoglobulin, as done with other approaches to re-engineer IL-2 for use in immunotherapy.
Compared to IL-2, mIL-2/mCD25 FP exhibits more favorable pharmacokinetics and pharmacodynamics [16], which allows for less frequent dosing to promote the expansion of tumor-specific T cells. Our data support a translational approach where limited dosing with high amounts of mIL-2/mCD25 is applied in the context of tumor vaccines that target tumor-associated self- or neoantigens and incorporate TLR agonist(s), such as poly-ICLC and imiquimod, among others [32], to induce antitumor immunity. The development of CD8+ T cell memory after vaccination with mIL-2/mCD25 is an especially attractive feature, as this may control cancer reoccurrence after eliminating the initial tumor burden. These studies lay the groundwork to adapt this approach to enhance endogenous polyclonal tumor-specific or adoptively transferred T cells.
Availability of data and materials
Data are available upon reasonable request. All data relevant to the study are included in the article or uploaded as supplementary information.
Code availability
Not applicable.
References
Rosenberg SA (2014) IL-2: the first effective immunotherapy for human cancer. J Immunol 192(12):5451–5458. https://doi.org/10.4049/jimmunol.1490019
Kohler PC, Sondel PM (1989) The role of interleukin-2 in cancer therapy. Cancer Surv 8(4):861–873
Boyman O, Sprent J (2012) The role of interleukin-2 during homeostasis and activation of the immune system. Nat Rev Immunol 12(3):180–190. https://doi.org/10.1038/nri3156
Malek TR, Castro I (2010) Interleukin-2 receptor signaling: at the interface between tolerance and immunity. Immunity 33(2):153–165. https://doi.org/10.1016/j.immuni.2010.08.004
Jiang T, Zhou C, Ren S (2016) Role of IL-2 in cancer immunotherapy. Oncoimmunology 5(6):e1163462. https://doi.org/10.1080/2162402X.2016.1163462
Kalia V, Sarkar S (2018) Regulation of effector and memory CD8 T cell differentiation by IL-2-A balancing Act. Front Immunol 9:2987. https://doi.org/10.3389/fimmu.2018.02987
Lotze MT, Frana LW, Sharrow SO, Robb RJ, Rosenberg SA (1985) In vivo administration of purified human interleukin 2. I. Half-life and immunologic effects of the Jurkat cell line-derived interleukin 2. J Immunol 134(1):157–166
Atkins MB, Lotze MT, Dutcher JP, Fisher RI, Weiss G, Margolin K, Abrams J, Sznol M, Parkinson D, Hawkins M, Paradise C, Kunkel L, Rosenberg SA (1999) High-dose recombinant interleukin 2 therapy for patients with metastatic melanoma: analysis of 270 patients treated between 1985 and 1993. J Clin Oncol 17(7):2105–2116. https://doi.org/10.1200/JCO.1999.17.7.2105
Kammula US, White DE, Rosenberg SA (1998) Trends in the safety of high dose bolus interleukin-2 administration in patients with metastatic cancer. Cancer 83(4):797–805
Shang B, Liu Y, Jiang SJ, Liu Y (2015) Prognostic value of tumor-infiltrating FoxP3+ regulatory T cells in cancers: a systematic review and meta-analysis. Sci Rep 5:15179. https://doi.org/10.1038/srep15179
Sim GC, Martin-Orozco N, Jin L, Yang Y, Wu S, Washington E, Sanders D, Lacey C, Wang Y, Vence L, Hwu P, Radvanyi L (2014) IL-2 therapy promotes suppressive ICOS+ Treg expansion in melanoma patients. J Clin Invest 124(1):99–110. https://doi.org/10.1172/JCI46266
Boyman O, Arenas-Ramirez N (2019) Development of a novel class of interleukin-2 immunotherapies for metastatic cancer. Swiss Med Wkly 149:w14697. https://doi.org/10.4414/smw.2019.14697
Charych DH, Hoch U, Langowski JL, Lee SR, Addepalli MK, Kirk PB, Sheng D, Liu X, Sims PW, VanderVeen LA, Ali CF, Chang TK, Konakova M, Pena RL, Kanhere RS, Kirksey YM, Ji C, Wang Y, Huang J, Sweeney TD, Kantak SS, Doberstein SK (2016) NKTR-214, an engineered cytokine with biased IL2 receptor binding, increased tumor exposure, and marked efficacy in mouse tumor models. Clin Cancer Res 22(3):680–690. https://doi.org/10.1158/1078-0432.CCR-15-1631
Carmenate T, Pacios A, Enamorado M, Moreno E, Garcia-Martinez K, Fuente D, Leon K (2013) Human IL-2 mutein with higher antitumor efficacy than wild type IL-2. J Immunol 190(12):6230–6238. https://doi.org/10.4049/jimmunol.1201895
Boyman O, Kovar M, Rubinstein MP, Surh CD, Sprent J (2006) Selective stimulation of T cell subsets with antibody-cytokine immune complexes. Science 311(5769):1924–1927. https://doi.org/10.1126/science.1122927
Ward NC, Yu A, Moro A, Ban Y, Chen X, Hsiung S, Keegan J, Arbanas JM, Loubeau M, Thankappan A, Yamniuk AP, Davis JH, Struthers M, Malek TR (2018) IL-2/CD25: a long-acting fusion protein that promotes immune tolerance by selectively targeting the IL-2 receptor on regulatory T cells. J Immunol 201(9):2579–2592. https://doi.org/10.4049/jimmunol.1800907
Hogquist KA, Jameson SC, Heath WR, Howard JL, Bevan MJ, Carbone FR (1994) T cell receptor antagonist peptides induce positive selection. Cell 76(1):17–27. https://doi.org/10.1016/0092-8674(94)90169-4
Overwijk WW, Theoret MR, Finkelstein SE, Surman DR, de Jong LA, Vyth-Dreese FA, Dellemijn TA, Antony PA, Spiess PJ, Palmer DC, Heimann DM, Klebanoff CA, Yu Z, Hwang LN, Feigenbaum L, Kruisbeek AM, Rosenberg SA, Restifo NP (2003) Tumor regression and autoimmunity after reversal of a functionally tolerant state of self-reactive CD8+ T cells. J Exp Med 198(4):569–580. https://doi.org/10.1084/jem.20030590
Martins GA, Cimmino L, Liao J, Magnusdottir E, Calame K (2008) Blimp-1 directly represses Il2 and the Il2 activator Fos, attenuating T cell proliferation and survival. J Exp Med 205(9):1959–1965. https://doi.org/10.1084/jem.20080526
Oh S, Hwang ES (2014) The role of protein modifications of T-bet in cytokine production and differentiation of T helper cells. J Immunol Res 2014:589672. https://doi.org/10.1155/2014/589672
Hwang ES, Hong JH, Glimcher LH (2005) IL-2 production in developing Th1 cells is regulated by heterodimerization of RelA and T-bet and requires T-bet serine residue 508. J Exp Med 202(9):1289–1300. https://doi.org/10.1084/jem.20051044
Cui W, Joshi NS, Liu Y, Meng H, Kleinstein SH, Kaech SM (2014) TLR4 ligands lipopolysaccharide and monophosphoryl lipid a differentially regulate effector and memory CD8+ T Cell differentiation. J Immunol 192(9):4221–4232. https://doi.org/10.4049/jimmunol.1302569
Salem ML, Kadima AN, Cole DJ, Gillanders WE (2005) Defining the antigen-specific T-cell response to vaccination and poly(I:C)/TLR3 signaling: evidence of enhanced primary and memory CD8 T-cell responses and antitumor immunity. J Immunother 28(3):220–228. https://doi.org/10.1097/01.cji.0000156828.75196.0d
Rudra JS, Banasik BN, Milligan GN (2018) A combined carrier-adjuvant system of peptide nanofibers and toll-like receptor agonists potentiates robust CD8+ T cell responses. Vaccine 36(4):438–441. https://doi.org/10.1016/j.vaccine.2017.12.017
Kim HP, Kelly J, Leonard WJ (2001) The basis for IL-2-induced IL-2 receptor alpha chain gene regulation: importance of two widely separated IL-2 response elements. Immunity 15(1):159–172. https://doi.org/10.1016/s1074-7613(01)00167-4
Kalia V, Sarkar S, Subramaniam S, Haining WN, Smith KA, Ahmed R (2010) Prolonged interleukin-2Ralpha expression on virus-specific CD8+ T cells favors terminal-effector differentiation in vivo. Immunity 32(1):91–103. https://doi.org/10.1016/j.immuni.2009.11.010
McGlinchey RP, Shewmaker F, McPhie P, Monterroso B, Thurber K, Wickner RB (2009) The repeat domain of the melanosome fibril protein Pmel17 forms the amyloid core promoting melanin synthesis. Proc Natl Acad Sci U S A 106(33):13731–13736. https://doi.org/10.1073/pnas.0906509106
Castro I, Dee MJ, Malek TR (2012) Transient enhanced IL-2R signaling early during priming rapidly amplifies development of functional CD8+ T effector-memory cells. J Immunol 189(9):4321–4330. https://doi.org/10.4049/jimmunol.1202067
Overacre-Delgoffe AE, Vignali DAA (2018) Treg fragility: a prerequisite for effective antitumor immunity? Cancer Immunol Res 6(8):882–887. https://doi.org/10.1158/2326-6066.CIR-18-0066
Jacob JB, Kong YC, Nalbantoglu I, Snower DP, Wei WZ (2009) Tumor regression following DNA vaccination and regulatory T cell depletion in neu transgenic mice leads to an increased risk for autoimmunity. J Immunol 182(9):5873–5881. https://doi.org/10.4049/jimmunol.0804074
Tanaka A, Sakaguchi S (2017) Regulatory T cells in cancer immunotherapy. Cell Res 27(1):109–118. https://doi.org/10.1038/cr.2016.151
Smith M, Garcia-Martinez E, Pitter MR, Fucikova J, Spisek R, Zitvogel L, Kroemer G, Galluzzi L (2018) Trial Watch: toll-like receptor agonists in cancer immunotherapy. Oncoimmunology 7(12):e1526250. https://doi.org/10.1080/2162402X.2018.1526250
Acknowledgements
We thank Michael Dee and Aixin Yu for technical assistance, Mary Struthers and Francisco Ramirez-Valle at Bristol Myers Squibb for critically reading the manuscript, and Patricia Guevara, Jay Enten, and Shannon Saigh from the Flow Cytometry Core of the Sylvester Comprehensive Cancer Center (supported by NIH P30CA240139).
Funding
This research was supported by funding to T.R.M. from the NIH (R21CA195334) and Sylvester Comprehensive Cancer Center at the University of Miami.
Author information
Authors and Affiliations
Contributions
Conception and design: RH and TRM. Production and validation of mIL-2/mCD25: ASS. Acquisition of data: RH, KHT, JP, SH. Analysis and interpretation of data: RH and TRM. Manuscript Writing: RH and TRM. Revisions: All authors edited and approved the manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The University of Miami, T.R.M. and R.H. have patents pending on IL-2/CD25 fusion proteins (Wo2016022671A1;T.R.M) and their use (PCT/US20/13152; T.R.M., R.H) that have been licensed exclusively to Bristol Myers Squibb, and some research on IL-2/CD25 fusion proteins has been supported in part by a collaboration and sponsored research and licensing agreement with Bristol Myers Squibb. The other authors have no financial conflicts of interest.
Ethics approval
Animal studies were approved by the Institutional Animal Care and Use Committee at the University of Miami (Protocol 18-147).
Consent to participate
Not applicable.
Consent for publication
Not applicable.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Hernandez, R., Toomer, K.H., Põder, J. et al. Sustained IL-2R signaling of limited duration by high-dose mIL-2/mCD25 fusion protein amplifies tumor-reactive CD8+ T cells to enhance antitumor immunity. Cancer Immunol Immunother 70, 909–921 (2021). https://doi.org/10.1007/s00262-020-02722-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00262-020-02722-5