Abstract
Ocular adverse events (OAEs) including vision-threatening intraocular inflammation after immune checkpoint inhibitor (ICI) treatment have been increasingly reported; however, the risk factors associated with OAEs remain elusive. Here, we determined the factors associated with OAEs after ICI treatment. We analyzed 40 consecutive patients who experienced OAEs after ICI treatments. The OAEs included anterior uveitis, chorioretinitis, papillitis, foveal interdigitation zone thickening/serous retinal detachment (IZT/SRD), retinal vascular occlusion, and strabismus and ptosis. Of 40 patients, 18 (45%) were treated with atezolizumab, 13 (33%) with pembrolizumab, 7 (18%) with nivolumab, 1 (3%) with ipilimumab/nivolumab, and the other 1 (3%) with durvalumab/tremelimumab. BRAF/MEK inhibitors were concurrently used in 19 (48%) patients. Occurrence of intraocular inflammation was significantly associated with previous ocular surgery and trauma history (P = 0.015) and pembrolizumab use (P = 0.031). Neuro-ophthalmic complications and IZT/SRD were associated with brain metastasis (P = 0.005) and treatment with BRAF/MEK inhibitor (P < 0.001), respectively. In extensive literature review for clinical cases, we identified seven cases with intraocular inflammation, which were not observed with ipilimumab treatment, that occurred after a change of the drug to pembrolizumab. Collectively, these findings provide better understandings of OAEs after ICI treatment.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Since the remarkable effects of pembrolizumab on advanced melanoma and non-small cell lung cancer have been published, immune checkpoint inhibitors (ICIs) have become one of the mainstays of cancer treatment [1, 2]. Currently, six FDA-approved ICIs are widely used to treat various cancers. These are monoclonal antibodies that activate the immune system by targeting CTLA-4 [Ipilimumab (Yervoy®)], PD-1 [pembrolizumab (Keytruda®), nivolumab (Opdivo®)], and PD-L1 [avelumab (Bavencio®), durvalumab (Imfinzi®), and atezolizumab (Tecentriq)] [3]. CTLA-4 and PD-1 are receptors on the surface of activated T cells, while PD-L1 resides on cancer cells [4]. PD1/PD-L1 and CTLA4/B7-1 or B7-2 interactions suppress T-cell function, and ICIs counteract this inhibitory process, resulting in T-cell activation [5, 6].
By increasing T-cell activity, systemic inflammatory side effects associated with ICI treatment have been reported [3]. Possible mechanisms underlying immune-related adverse events include increasing T-cell activity against antigens that are present in tumors and healthy tissues, increasing levels of preexisting autoantibodies, increasing levels of inflammatory cytokines, and enhancing complement-mediated inflammation [7]. Since Robinson et al. described the uveitis developed during anti-CTLA-4 therapy in 2004, many ocular adverse events (OAEs) affecting orbit and ocular adnexa, ocular surface, optic nerve, uvea, and retina have also been reported [3, 8]. The clinical features of OAEs have been described in case reports and small case-series studies; however, the factors associated with OAEs remain elusive [9,10,11,12,13,14,15,16,17,18,19,20,21,22,23,24,25,26,27,28,29,30,31,32,33,34,35]. In the present study, we analyzed the characteristics and statistically associated factors of the development of OAEs after ICI treatment, and have comprehensively reviewed the literature reporting intraocular inflammation with posterior segment complications or Vogt–Koyanagi–Harada (VKH)-like features after ICI treatment.
Methods
Study population and approval
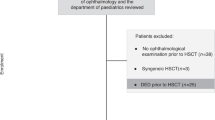
The medical records of consecutive patients treated with FDA-approved ICIs (ipilimumab, pembrolizumab, nivolumab, avelumab, durvalumab, and atezolizumab) and visited the ophthalmology department after ICI treatment between January 2011 and April 2019 were retrospectively reviewed. The patients who met the following criteria were included: (1) completed an ophthalmic examination, and (2) had newly developed ocular signs during ICI treatment. Patients with only mild dry eye/cataract or no newly developed ophthalmic abnormalities were excluded from the study. The recorded parameters included the primary tumor for ICI treatment, the use of other chemotherapeutic agents including BRAF/MEK inhibitors, and tumor metastasis. This research adhered to the tenets of the Declaration of Helsinki. The institutional Review Board/Ethics Committee approved this retrospective study (Severance Hospital, Yonsei University Health System, IRB no. 4-2019-1100).
Ophthalmic examinations
Each patient underwent a comprehensive ophthalmic examination including best-corrected visual acuity, slit-lamp biomicroscopy, tonometry, fundus photography, and a detailed fundus examination. Ancillary testing was performed with spectral-domain optical coherence tomography (Spectralis, Heidelberg Engineering, Heidelberg, Germany), fluorescein angiography, and indocyanine green angiography. Depending on the case, the visual field test, cover-uncover test, prism cover test, or measurement of marginal reflex distance were performed. At the initial visit, the patients were asked for detailed personal history including ocular trauma and surgery.
Statistical analysis
All statistical analyses were performed using SPSS statistical software for Windows, version 25.0 (SPSS, Chicago, IL, USA). The Kruskal–Wallis test and Pearson’s chi-square test were used to study the differences in clinical characteristics and features of OAEs among ICIs. Binomial logistic regression analysis was performed to identify factors associated with different types of OAEs. The odds ratios of the factors detected in the univariate and multivariate analyses were calculated. P < 0.05 was considered to indicate statistical significance.
Literature review
A comprehensive search of the electronic database including PubMed and Embase was performed from inception to July 2019. The searches were conducted using the combination of the following keywords in the title or abstract of the article: “ipilimumab”, “pembrolizumab”, “nivolumab”, “atezolizumab”, “durvalumab”, “avelumab”, “immune checkpoint inhibitor”, “uveitis”, “ocular”, “eye”, “adverse event”, and “ophthalmology”. The case reports, case-series, and review articles addressing intraocular inflammation with posterior segment complications or VKH-like features after ICI treatment were reviewed. Bibliographies of the reviewed articles were checked manually to prevent missing potentially relevant studies.
Results
Study population
Forty patients met the inclusion criteria and were included in the analyses. Of 40 patients, 18 (45%) were treated with atezolizumab, 13 (33%) with pembrolizumab, 7 (18%) with nivolumab, 1 (3%) with ipilimumab/nivolumab, and the other 1 (3%) with durvalumab/tremelimumab. The most common primary tumor for ICI treatment was lung cancer (15 patients), followed by skin melanoma (7 patients). Notably, 19 (48%) patients were treated with BRAF/MEK inhibitors during the ICI treatments. The details of the demographic characteristics are presented and summarized in Table 1.
Ocular adverse events associated with ICIs
The details of the OAEs are presented in Table 1. The median time between the OAE and initial ICI treatment was 42.0 days [interquartile range (IQR), 28.0–111.5 days]. The median observation period after the OAE was 157.0 days (IQR, 47.0–265.5 days). Twenty-nine patients (73%) developed OAEs within 60 days of the initial ICI infusion. Notably, all patients treated with atezolizumab were treated with BRAF/MEK inhibitors.
We divided the OAEs into four categories: (1) intraocular inflammation, (2) neuro-ophthalmic complications without intraocular inflammation, (3) foveal interdigitation zone thickening/serous retinal detachment (IZT/SRD) without visible intraocular inflammation, and (4) others. Intraocular inflammation developed in five patients. One patient who had nivolumab and ipilimumab combination treatment developed bilateral anterior uveitis. Panuveitis or neuroretinitis developed in four patients who had pembrolizumab treatment (Fig. 1a–d). In these four patients, pembrolizumab was discontinued, and periocular/systemic steroid was used to control inflammation. Neuro-ophthalmic complications included one bilateral nonarteritic anterior ischemic optic neuropathy, one ptosis, one papilledema, one internuclear ophthalmoplegia, one third nerve palsy, and four exotropia. Eight (89%) out of nine patients with neuro-ophthalmic complications had brain metastasis of the primary tumor. Brain magnetic resonance imaging (MRI) of one patient (Patient 13) without brain metastasis revealed posterior cerebral artery territory infarction. Foveal IZT/SRDs were observed in 18 patients (Fig. 1e–j). Comprehensive and ancillary ophthalmic examinations did not reveal any intraocular inflammation in most patients; one patient, however, differed markedly from the rest. Patient 15 had unilateral anterior uveitis refractory to steroid treatment, but aqueous humor cytology showed many skin melanoma cells, suggesting metastasis. Other OAEs included central retinal vein occlusion, branch retinal vein occlusion, newly developed ellipsoid zone disruption and cotton wool spots, rhegmatogenous retinal detachment, neurotrophic keratitis, and preseptal cellulitis.
Representative enhanced depth imaging optical coherence tomography images of patients with ellipsoid zone thickening/serous retinal detachment or chorioretinitis after immune checkpoint inhibitor therapy. a, b A 58-year-old female (Patient 2) presented with bilateral chorioretinitis/papillitis 47 days after pembrolizumab therapy. c, d, A 54-year-old female (Patient 3) presented with chorioretinitis/papillitis 51 days after pembrolizumab therapy (c). Undulating retinal pigment epithelium, subretinal fluid, and thickened was resolved after posterior subtenon triamcinolone injection and systemic steroid treatment (d). e–h A 54-year-old man (Patient 30) presented with bilateral ellipsoid zone thickening (e, f) 13 days after atezolizumab/cobimetinib therapy. Serous retinal detachment developed after five cycles of atezolizumab/cobimetinib therapy (g, h). i, j A 66-year-old woman (Patient 15) presented with bilateral ellipsoid zone thickening and serous retinal detachment 4 days after pembrolizumab/trametinib therapy. No abnormality was detected during pembrolizumab monotherapy in this patient
Factors associated with the types of ocular adverse events
The details of factors associated with different types of OAE are presented in Table 2. Using multivariate analysis, ocular trauma/surgery (P = 0.001) and pembrolizumab (P = 0.001) were associated with intraocular inflammation. Only brain metastasis (P = 0.005) and the treatment with BRAF/MEK inhibitor (P = 0.021) were associated with neuro-ophthalmic complications and foveal IZT/SRD, respectively. Atezolizumab was significantly associated with foveal IZT/SRD using univariate binomial logistic regression, but not using multivariate analysis.
Literature review for clinical cases presenting intraocular inflammation with posterior segment complications or VKH-like features associated with immune checkpoint inhibitors
Intraocular inflammation with posterior segment complications or VKH-like features has been considered as immune-related sight-threatening OAEs. We reviewed the literature and found 48 cases from 28 studies, including the present study. The clinical features of the cases are summarized and presented in Table 3. The most common primary tumor was skin melanoma (38 cases, 79%), followed by lung cancer (5 cases, 10%), and choroidal melanoma (3 cases, 6%). Four (8%) cases presented unilaterally. Eighteen (44%), fourteen (23%), eight (17%), six, and one case were treated with pembrolizumab, ipilimumab, nivolumab, nivolumab/ipilimumab, and pembrolizumab/ipilimumab infusion, respectively. Notably, only one case (Case 20) developed unilateral uveal effusion and SRD accompanied by shallow anterior chamber and closed angle after atezolizumab infusion. Nine patients had a history of ocular surgery, and one patient had metastatic choroidal melanoma. Nineteen patients showed co-occurring adverse events such as poliosis/vitiligo, preceding headache, hypophysitis, aseptic meningitis, and hearing abnormalities. ICI treatments were discontinued in 31 (65%) cases. In seven cases (Case 8, 9, 35, 36, 37, 41, and 42), the OAEs were not observed in the previous ipilimumab treatments, which occurred after pembrolizumab infusions. In case 42, bilateral panuveitis developed 1 week after unilateral cataract surgery during pembrolizumab treatment. Panuveitis recurred after reinfusion of pembrolizumab in this case.
Discussion
The use of ICIs has led to great advances in cancer therapy. They reverse the immune evasion of cancer cell by blocking the innate immune inhibitory process of T cells [1, 2, 5, 6]. Enhanced T-cell activity has been known to induce various immune responses in other parts of the body, called immune-related complications [7]. These adverse events have also been found in the eye. Intraocular inflammation (uveitis) and dry eye are common, and Graves’s ophthalmopathy is known to occur [3, 36]. In the present study, intraocular inflammation, foveal IZT/SRD, neuro-ophthalmic complications, and retinal vein occlusion were observed.
Foveal IZT/SRD was associated with the use of atezolizumab, but multivariate analysis suggested that it was due to BRAF/MEK inhibitors that were concurrently used. Serous retinal detachments after ICI therapy have been mostly reported in skin melanomas and lung cancers. In these tumors, gain-of-function mutations in members of the MAPK pathway were common [37, 38]. BRAF/MEK inhibitors have been known to cause foveal IZT/SRD without inflammation; thus, it is likely due to BRAF/MEK inhibitors rather than ICIs in the present study [39,40,41]. Likewise, regarding neuro-ophthalmic complications, all patients presenting with strabismus or ptosis had brain metastases or infarctions in the present study. Foveal IZT/SRD and neuro-ophthalmic complications have been reported after ICI therapy [3]. Our findings suggest that the use of concurrent BRAF/MEK inhibitors and the evaluation of brain lesions should be carefully considered when determining the association between the use of ICIs and OAEs.
Intraocular inflammation, an immune-related OAE, occurs in approximately 1% of patients treated with ICIs [3, 42]. Most intraocular inflammation is known to present as anterior uveitis, but vision-threatening posterior segment complications have been reported [18, 19, 25, 28, 30]. In our study, patients with intraocular inflammation were associated with a history of intraocular trauma/surgery. Furthermore, of 48 cases in our literature review, 27 (56%) cases exhibited a history of ocular surgery or co-occurring systemic immune-related adverse event. These findings suggest that in patients vulnerable to the development of autoimmune disease or sensitized to intraocular antigen, the risk of ICI-related, vision-threatening posterior uveitis is high. Notably, there were several cases to support this possibility. Case 40 had a six-melanoma helper peptide vaccine before developing panuveitis [31]. Case 42 had severe bilateral panuveitis after elective unilateral cataract surgery during pembrolizumab treatment [33].
Distinguishing ICI-related severe uveitis from VKH is often difficult, because the inflammation also exhibit chorioretinitis, exudative retinal detachment, choroidal thickening, poliosis/vitiligo/hypophysitis, and sensorineural hearing loss. The difficulty is aggravated by similarity in responses to steroid treatment. However, in cases of ICI-related complications, the previous reports indicate that discontinuation of the drug often resolves and re-initiation of the drug re-induces the inflammatory response. Thus, the evaluation of underlying medical conditions and evaluation of the prescription will be valuable in distinguishing inflammation from VKH. Considering that VKH is common among Asian population and that it is associated with HLA-DR4, further studies are also needed on racial differences and the role of HLA subtype in intraocular inflammation following ICI treatment.
Recent reports indicate that intravitreous metastasis is an important differential diagnosis to consider in patients suspected to have uveitis following ICI infusion [43, 44]. The reported cases so far are those patients with skin melanoma; similarly, the masquerading uveitis observed in Patient 15 of our study also had underlying skin melanoma. Our study results suggest that in cases of intraocular inflammation after ICI infusion that do not respond to treatment, further investigations such as cytology are needed to verify the presence of vitreoretinal metastasis.
In the present study, all cases involving posterior uveitis occurred after treatment with pembrolizumab, a PD-1 inhibitor. While systemic immune-related adverse events appear to be more common with ipilimumab, a CTLA-4 inhibitor, than with other ICIs, most published cases with serous chorioretinitis/papillitis were associated with the use of PD-1 inhibitors [7]. A recent study involving patients with melanoma reported that PD-1 inhibitors could be safely used after severe ipilimumab-related systemic adverse events [45]. In contrast, we found seven cases with intraocular complications, which were not observed with ipilimumab treatment, that occurred after a change of the drug to pembrolizumab [12, 13, 26,27,28, 32, 33]. Collectively, these findings suggested that the use of PD-1 inhibitors may increase the chance of severe intraocular inflammation when compared with the use of CTLA-4 or PD-L1 inhibitors. Serious posterior uveitis associated with PD-L1 inhibitors have been rarely reported, which is likely to be because PD-L1 is expressed on the surface of cancer cells, unlike PD-1 or CTLA-4. Since PD-1 is expressed on T cells, activated T cells following PD-1 inhibition may theoretically be involved with ‘off-target’ inflammation more frequently. In most cases with intraocular inflammation with posterior segment complication or VKH-like features, ICI was discontinued, but visual sequelae remained. We think that the discontinuation of ICI treatment should be carefully determined over benefits therapeutic responses and costs of systemic immune-related adverse events.
The use of ICIs is already one of the mainstays of cancer treatment, and their use is increasing. Clinicians should be aware that severe intraocular inflammation can developed after ICI treatment, particularly in patients who have undergone ocular surgery/trauma. The use of BRAF/MEK inhibitors and the presence of brain metastasis should be also examined in patients with OAEs.
Abbreviations
- ICI:
-
Immune checkpoint inhibitor
- IQR:
-
Interquartile range
- IZT:
-
Interdigitation zone thickening
- OAE:
-
Ocular adverse event
- SRD:
-
Serous retinal detachment
References
Garon EB, Rizvi NA, Hui R et al (2015) Pembrolizumab for the treatment of non-small-cell lung cancer. N Engl J Med 372:2018–2028. https://doi.org/10.1056/NEJMoa1501824
Robert C, Schachter J, Long GV et al (2015) Pembrolizumab versus ipilimumab in advanced melanoma. N Engl J Med 372:2521–2532. https://doi.org/10.1056/NEJMoa1503093
Dalvin LA, Shields CL, Orloff M, Sato T, Shields JA (2018) Checkpoint inhibitor immune therapy: systemic indications and ophthalmic side effects. Retina 38:1063–1078. https://doi.org/10.1097/IAE.0000000000002181
Wei SC, Duffy CR, Allison JP (2018) Fundamental mechanisms of immune checkpoint blockade therapy. Cancer Discov 8:1069–1086. https://doi.org/10.1158/2159-8290.CD-18-0367
Dong H, Strome SE, Salomao DR, Tamura H, Hirano F, Flies DB, Roche PC, Lu J, Zhu G, Tamada K, Lennon VA, Celis E, Chen L (2002) Tumor-associated B7–H1 promotes T-cell apoptosis: a potential mechanism of immune evasion. Nat Med 8:793–800. https://doi.org/10.1038/nm730
Pardoll DM (2012) The blockade of immune checkpoints in cancer immunotherapy. Nat Rev Cancer 12:252–264. https://doi.org/10.1007/s10067-019-04451-2
Postow MA, Sidlow R, Hellmann MD (2018) Immune-related adverse events associated with immune checkpoint blockade. N Engl J Med 378:158–168. https://doi.org/10.1056/NEJMra1703481
Robinson MR, Chan CC, Yang JC, Rubin BI, Gracia GJ, Sen HN, Csaky KG, Rosenberg SA (2004) Cytotoxic T lymphocyte-associated antigen 4 blockade in patients with metastatic melanoma: a new cause of uveitis. J Immunother 27:478–479. https://doi.org/10.1097/00002371-200411000-00008
Hahn L, Pepple KL (2016) Bilateral neuroretinitis and anterior uveitis following ipilimumab treatment for metastatic melanoma. J Ophthalmic Inflamm Infect 6:14. https://doi.org/10.1186/s12348-016-0082-3
Wilson MA, Guld K, Galetta S, Walsh RD, Kharlip J, Tamhankar M, McGettigan S, Schuchter LM, Fecher LA (2016) Acute visual loss after ipilimumab treatment for metastatic melanoma. J Immunother Cancer 4:66. https://doi.org/10.1186/s40425-016-0170-9
Abu Samra K, Valdes-Navarro M, Lee S, Swan R, Foster CS, Anesi SD (2016) A case of bilateral uveitis and papillitis in a patient treated with pembrolizumab. Eur J Ophthalmol 26:e46–e48. https://doi.org/10.5301/ejo.5000724
Hanna KS (2016) A rare case of pembrolizumab-induced uveitis in a patient with metastatic melanoma. Pharmacotherapy 36:e183–e188. https://doi.org/10.1002/phar.1839
Taylor SC, Hrisomalos F, Linette GP, Rao PK (2016) A case of recurrent bilateral uveitis independently associated with dabrafenib and pembrolizumab therapy. Am J Ophthalmol Case Rep 2:23–25. https://doi.org/10.1016/j.ajoc.2016.04.004
Mantopoulos D, Kendra KL, Letson AD, Cebulla CM (2015) Bilateral choroidopathy and serous retinal detachments during ipilimumab treatment for cutaneous melanoma. JAMA Ophthalmol 133:965–967. https://doi.org/10.1001/jamaophthalmol.2015.1128
Crews J, Agarwal A, Jack L, Xu D, Do DV, Nguyen QD (2015) Ipilimumab-associated retinopathy. Ophthalmic Surg Lasers Imaging Retina 46:658–660. https://doi.org/10.3928/23258160-20150610-10
Tsui E, Madu A, Belinsky I, Yannuzzi LA, Freund KB, Modi YS (2017) Combination ipilimumab and nivolumab for metastatic melanoma associated with ciliochoroidal effusion and exudative retinal detachment. JAMA Ophthalmol 135:1455–1457. https://doi.org/10.1001/jamaophthalmol.2017.4872
Wong RK, Lee JK, Huang JJ (2012) Bilateral drug (ipilimumab)-induced vitritis, choroiditis, and serous retinal detachments suggestive of vogt-koyanagi-harada syndrome. Retin Cases Brief Rep 6:423–426. https://doi.org/10.1097/ICB.0b013e31824f7130
Arai T, Harada K, Usui Y, Irisawa R, Tsuboi R (2017) Case of acute anterior uveitis and Vogt-Koyanagi-Harada syndrome-like eruptions induced by nivolumab in a melanoma patient. J Dermatol 44:975–976. https://doi.org/10.1111/1346-8138.13612
Matsuo T, Yamasaki O (2017) Vogt-Koyanagi-Harada disease-like posterior uveitis in the course of nivolumab (anti-PD-1 antibody), interposed by vemurafenib (BRAF inhibitor), for metastatic cutaneous malignant melanoma. Clin Case Rep 5:694–700. https://doi.org/10.1002/ccr3.911
Noble CW, Gangaputra SS, Thompson IA, Yuan A, Apolo AB, Lee JM, Papaliodis GN, Kodati S, Bishop R, Magone MT, Sobrin L, Sen HN (2019) Ocular adverse events following use of immune checkpoint inhibitors for metastatic malignancies. Ocul Immunol Inflamm 23:1–6. https://doi.org/10.1080/09273948.2019.1583347
Thomas M, Armenti ST, Ayres MB, Demirci H (2018) Uveal effusion after immune checkpoint inhibitor therapy. JAMA Ophthalmol 136:553–556. https://doi.org/10.1001/jamaophthalmol.2018.0920
Sun MN, Levinson RD, Filipowicz A, Anesi S, Kaplan HJ, Wang W, Goldstein DA, Gangaputra S, Swan RT, Sen HN, Gordon LK (2020) Uveitis in patients treated with CTLA-4 and PD-1 checkpoint blockade inhibition. Ocul Immunol Inflamm 28:217–227. https://doi.org/10.1080/09273948.2019.1577978
Wang W, Lam WC, Chen L (2019) Recurrent grade 4 panuveitis with serous retinal detachment related to nivolumab treatment in a patient with metastatic renal cell carcinoma. Cancer Immunol Immunother 68:85–95. https://doi.org/10.1007/s00262-018-2260-7
O'Bryhim BE, Sychev Y, Rao PK (2018) Bilateral choroidal detachments secondary to ipilimumab and pembrolizumab use. Retin Cases Brief Rep. https://doi.org/10.1097/ICB.0000000000000785
Tamura T, Akimoto E, Matsumoto C, Mori S, Nishi T, Kudo K, Kuyama S (2018) Vogt-Koyanagi-Harada syndrome induced by pembrolizumab in a patient with non-small cell lung cancer. J Thorac Oncol 13:1606–1607. https://doi.org/10.1016/j.jtho.2018.04.026
Reid G, Lorigan P, Heimann H, Hovan M (2019) Management of chronic hypotony following bilateral uveitis in a patient treated with pembrolizumab for cutaneous metastatic melanoma. Ocul Immunol Inflamm 27:1012–1015. https://doi.org/10.1080/09273948.2018.1459733
Sandhu HS, Kolomeyer AM, Lau MK, Shields CL, Schuchter LM, Nichols CW, Aleman TS (2019) Acute exudative paraneoplastic polymorphous vitelliform maculopathy during vemurafenib and pembrolizumab treatment for metastatic melanoma. Retin Cases Brief Rep 13:103–107. https://doi.org/10.1097/ICB.0000000000000604
Bricout M, Petre A, Amini-Adle M, Bezza W, Seve P, Kodjikian L, Dalle S, Thomas L (2017) Vogt-Koyanagi-Harada-like syndrome complicating pembrolizumab treatment for metastatic melanoma. J Immunother 40:77–82. https://doi.org/10.1097/CJI.0000000000000154
Elwood KF, Pulido JS, Ghafoori SD, Harper CA, Wong RW (2019) Choroidal neovascularization and chorioretinal atrophy in a patient with melanoma-associated retinopathy after ipilimumab/nivolumab combination therapy. Retin Cases Brief Rep. https://doi.org/10.1097/ICB.0000000000000882
Obata S, Saishin Y, Teramura K, Ohji M (2019) Vogt-Koyanagi-Harada disease-like uveitis during nivolumab (Anti-PD-1 antibody) treatment for metastatic cutaneous malignant melanoma. Case Rep Ophthalmol 10:67–74. https://doi.org/10.1159/000496682
Rodriguez C, Sieburth R, Newman S, Gaughan E, Shildkrot YE (2019) Bilateral mydriasis and serous retinal detachments associated with ipilimumab and 6-melanoma helper peptide vaccine for cutaneous melanoma. JAMA Ophthalmol. https://doi.org/10.1001/jamaophthalmol.2019.2451
Aaberg MT, Aaberg TM Jr (2017) Pembrolizumab administration associated with posterior uveitis. Retin Cases Brief Rep 11:348–351. https://doi.org/10.1097/ICB.0000000000000368
Diem S, Keller F, Rüesch R, Maillard SA, Speiser DE, Dummer R, Siano M, Urner-Bloch U, Goldinger SM, Flatz L (2016) Pembrolizumab-triggered uveitis: an additional surrogate marker for responders in melanoma immunotherapy? J Immunother 39:379–382. https://doi.org/10.1097/CJI.0000000000000143
Acaba-Berrocal LA, Lucio-Alvarez JA, Mashayekhi A, Ho AC, Dunn JP, Shields CL (2018) Birdshot-like chorioretinopathy associated with pembrolizumab treatment. JAMA Ophthalmol 136:1205–1207. https://doi.org/10.1001/jamaophthalmol.2018.1851
Conrady CD, Larochelle M, Pecen P, Palestine A, Shakoor A, Singh A (2018) Checkpoint inhibitor-induced uveitis: a case series. Graefes Arch Clin Exp Ophthalmol 256:187–191. https://doi.org/10.1007/s00417-017-3835-2
Park ESY, Rabinowits G, Hamnvik OR, Dagi LR (2018) A case of Graves' ophthalmopathy associated with pembrolizumab (Keytruda) therapy. J AAPOS 22:310–312. https://doi.org/10.1016/j.jaapos.2018.01.006
Amaral T, Sinnberg T, Meier F, Krepler C, Levesque M, Niessner H, Garbe C (2017) The mitogen-activated protein kinase pathway in melanoma part I—activation and primary resistance mechanisms to BRAF inhibition. Eur J Cancer 73:85–92. https://doi.org/10.1016/j.ejca.2016.12.010
Heigener DF, Gandara DR, Reck M (2015) Targeting of MEK in lung cancer therapeutics. Lancet Respir Med 3:319–327. https://doi.org/10.1016/S2213-2600(15)00026-0
Francis JH, Habib LA, Abramson DH, Yannuzzi LA, Heinemann M, Gounder MM, Grisham RN, Postow MA, Shoushtari AN, Chi P, Segal NH, Yaeger R, Ho AL, Chapman PB, Catalanotti F (2017) Clinical and morphologic characteristics of MEK inhibitor-associated retinopathy: differences from central serous chorioretinopathy. Ophthalmology 124:1788–1798. https://doi.org/10.1016/j.ophtha.2017.05.038
Stjepanovic N, Velazquez-Martin JP, Bedard PL (2016) Ocular toxicities of MEK inhibitors and other targeted therapies. Ann Oncol 27:998–1005. https://doi.org/10.1093/annonc/mdw100
Urner-Bloch U, Urner M, Jaberg-Bentele N, Frauchiger AL, Dummer R, Goldinger SM (2016) MEK inhibitor-associated retinopathy (MEKAR) in metastatic melanoma: long-term ophthalmic effects. Eur J Cancer 65:130–138. https://doi.org/10.1016/j.ejca.2016.06.018
Zimmer L, Goldinger SM, Hofmann L et al (2016) Neurological, respiratory, musculoskeletal, cardiac and ocular side-effects of anti-PD-1 therapy. Eur J Cancer 60:210–225. https://doi.org/10.1016/j.ejca.2016.02.024
Breazzano MP, Barker-Griffith AE (2015) Features of cutaneous malignant melanoma metastatic to the retina and vitreous. Ocul Oncol Pathol 2:80–85. https://doi.org/10.1159/000439259
Francis JH, Berry D, Abramson DH et al (2020) Intravitreous cutaneous metastatic melanoma in the era of checkpoint inhibition. Ophthalmology 127:240–248. https://doi.org/10.1016/j.ophtha.2019.09.018
Menzies AM, Johnson DB, Ramanujam S et al (2017) Anti-PD-1 therapy in patients with advanced melanoma and preexisting autoimmune disorders or major toxicity with ipilimumab. Ann Oncol 28:368–376. https://doi.org/10.1093/annonc/mdw443
Funding
This research was supported by the Basic Science Research Program through the National Research Foundation of Korea (NRF) under grant funded by the Korea government (Ministry of Science and ICT) (NRF-2019R1A2C2002393). The funding organization had no role in the design or conduct of this research.
Author information
Authors and Affiliations
Contributions
YJK, SCL, and CSL conceived the study. Clinical data collection and interpretation were done by YJK, JSL, JL, SCL, TK, SHB, and CSL. YJK and CSL wrote the manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no potential conflicts of interest.
Ethics approval
This study was reviewed and approved by the Institutional Review Board of Severance Hospital, Seoul Korea (approval number: 4-2019-1100).
Informed consent
The requirement for informed consent for the retrospective study was waived by the Institutional Review Board.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Kim, Y.J., Lee, J.S., Lee, J. et al. Factors associated with ocular adverse event after immune checkpoint inhibitor treatment. Cancer Immunol Immunother 69, 2441–2452 (2020). https://doi.org/10.1007/s00262-020-02635-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00262-020-02635-3