Abstract
Peptide vaccines represent an attractive alternative to conventional anti-tumor therapies, but have not yet achieved significant clinical efficacy with commonly used formulations. Combination of short antigenic peptides, synthetic melanin and TLR9 agonist (Toll-like receptor 9, CpG-28) was reported as highly efficient to trigger strong CD8 + T-cell responses. We compared this vaccine approach to the standard adjuvant formulation that combines the incomplete Freund’s adjuvant (IFA) and CpG-28, using either an ovalbumin epitope (pOVA30) or a spontaneously occurring tumor neoepitope (mAdpgk).
Melanin-based vaccine induced significantly higher cytotoxic T lymphocytes (CTL) responses than IFA-based vaccine in both pOVA30- and mAdpgk-targeted vaccines. The anti-tumor efficacy of melanin-based vaccine was further assessed in mice, grafted either with E.G7-OVA cells (E.G7 cells transfected with ovalbumin) or with MC38 cells that spontaneously express the mAdpgk neoepitope. Melanin-based vaccine induced a major inhibition of E.G7-OVA tumor growth when compared to IFA-based vaccine (p < 0.001), but tumors eventually relapsed from day 24. In the MC38 tumor model, no significant inhibition of tumor growth was observed. In both cases, tumor escape appeared related to the loss of antigen presentation by tumor cells (loss of ovalbumin expression in E.G7-OVA model; poor presentation of mAdpgk in MC38 model), although the CTL responses displayed an effector memory phenotype, a high cytolytic potential and low programmed cell death-1 (PD1) expression.
In conclusion, synthetic melanin can be efficiently used as an adjuvant to enhance T-cells response against subunit vaccine antigens and compared favorably to the classic combination of IFA and TLR9 agonist in mice.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Our perception of cancer immunotherapy has dramatically changed during the past decade. The recent clinical successes of immune check-point inhibitors (ICIs), such as anti-PD1 (programmed cell death-1) and anti-CTLA4 (cytotoxic T-lymphocyte-associated protein 4) antibodies, have indeed proven that the immune system can eradicate cancer cells in subsets of patients. Yet, most patients do not develop a specific cytotoxic CD8 + T-lymphocytes (CTLs) response able to recognize tumor-associated antigens and subsequently eradicate cancer cells. Cancer vaccines that trigger or stimulate a specific anti-tumor CTL response in patients thus represent an attractive alternative to conventional anticancer treatment, in particular in combination with ICIs.
The development of cancer vaccines has been sparked by the discovery and characterization of several tumor antigens found in various cancers. Cancer-associated antigens have been classified into two broad categories: tumor-associated antigens (TAAs) that are preferentially expressed by tumor cells but also in lower amounts in normal tissues, and antigens derived from tumor-specific mutant proteins (neo-antigens). These tumor-specific mutations are ideal targets for cancer immunotherapy since vaccination against a neo-antigen minimizes the potential induction of central and peripheral tolerance as well as the risk of autoimmunity [2].
Besides the identification of relevant antigens, cancer vaccines are facing the problem of efficacy. Most currently available vaccines usually trigger a strong humoral response (antibodies-mediated) but a weak CTL response, insufficient to achieve anti-tumor effects in humans. Part of the limitation might be related to the use of a small part of the antigenic protein within so-called subunit vaccines. Indeed, peptides are typically poorly immunogenic, and both high antigenic doses and strong adjuvants are needed to elicit an effective immune response in an immune-resistant tumor environment [3].
Generally, peptide-based cancer vaccines are using water-in-oil emulsions as adjuvants (e.g., incomplete Freund’s adjuvant—IFA, Montanide) [4,5,6,7]. After subcutaneous administration, peptides are gradually released from the emulsion and bound to major histocompatibility complex (MHC) molecules on antigen-presenting cells (APCs) such as dendritic cells, macrophages and B lymphocytes. Nevertheless, this emulsion remains at the injection site, leading to the local accumulation of CTLs and limited migration to the tumor site, consequently producing poor anti-tumor effects [8, 9].
We have recently shown that the attachment of short peptidic epitopes to synthetic melanin allows these antigens to reach the lymph nodes and consequently activate CD8+ T cells [10]. These melanin–peptide particles have diameters between 10 and 20 nm, a suitable size for rapid penetration of tissue barrier and draining to lymph nodes [11]. Melanin–peptide particles specifically reach the paracortical area of lymph nodes, which is a T-cell zone, where they are phagocytized by macrophagic cells and efficiently presented to CD8+ T cells. Melanin-based immunizations indeed proved to trigger strong CD8+ immune responses in vivo [10].
To support the relevance of this vaccine approach in cancer, we compared the efficacy of melanin-based vaccines to formulations using IFA combined with a TRL9 agonist that are widely used to trigger CD8+ lymphocytes [4, 7]. These formulations were tested against class I MHC epitopes reported in the literature: the well-characterized ovalbumin epitope, which is routinely used to test new vaccine adjuvants [12, 13], and a neoepitope recently described within the Adpgk protein [14].
We here show that melanin-based immunizations were highly efficient against both epitopes. We further studied the efficacy of the immune response in two syngeneic tumor models: E.G7-OVA (G7 cells transfected with a plasmid expressing chicken ovalbumin) and MC38 line that spontaneously expresses the Adpgk neoepitope [14].
Materials and methods
Peptides and melanin
Two endotoxin-free synthetic peptides: one long (SMLVLLPKKVSGLKQLESIINFEKLTKWTS; pOVA30) and one short (KKASMTNMELM; mAdpgk), were purchased from Genosphere Biotechnologies (Paris, France). These peptides contain the published H-2b epitopes (underlined) of the ovalbumin protein [13] and of the mutated Adpgk protein [14], respectively. To prepare the vaccine formulations, peptides (3.6 µg/mouse for pOVA30 and 50 µg/mouse for mAdpgk) were mixed with L-Dopa (40 µg/mouse for pOVA30 and 200 µg/mouse for mAdpgk; Sigma-Aldrich, Saint-Quentin-Fallavier, France) in a total volume of 400 μL H2O. The pH was adjusted to 8.5 (with NaOH), and the solutions were then oxidized in aerated conditions under vigorous agitation (Eppendorf Thermomixer, 1000 rpm) as previously described [10], to generate peptide–melanin nanoaggregates (pOVA30-Mel or mAdpgk-Mel, respectively). The binding of peptides to melanin was confirmed by SDS-page (Supplementary data, Fig. 1).
Cell lines
E.G7-OVA, a chicken egg OVA gene-transfected clone of EL4, was obtained from American Type Culture Collection (ATCC, Manassas, VA, US). E.G7-OVA cells were cultured in high glucose RPMI 1640 medium with 2 mM L-glutamine, 0.05 mM 2-mercaptoethanol, 0.4 mg/ml G418; 10% fetal bovine serum (Eurobio, Les Ulis, France); 100 units/ml penicillin and 100 μg/mL streptomycin [15].
The mutated MC38 colon cancer cell line was kindly provided by Laurence Zitvogel, Gustave Roussy, Paris. MC38 cells were cultured in high glucose DMEM medium (Dulbecco modified Eagle medium, Eurobio) with 10% fetal bovine serum, 100 units/ml penicillin and 100 μg/mL streptomycin. All experiments were performed with mycoplasma-free cells.
Immunization protocols
The melanin-based vaccine formulations were composed of peptide–melanin nanoaggregates (pOVA30-Mel or mAdpgk-Mel) in which the phosphorothioate oligonucleotide CpG-28 (5′-TAAACGTTATAACGTTATGACGTCAT) (Oligovax, Paris, France), a B-type CpG-ODN [16, 17], was added (50 µg/mouse) just before the immunizations. Control mice were injected with free peptide and 50 μg CpG-28 emulsified in incomplete Freund’s adjuvant (vol/vol) (Sigma-Aldrich, Saint-Quentin-Fallavier, France) or with 50 μg CpG-28 and melanin without peptide. Vaccine formulations were administered subcutaneously in the flank of the mice (100 µl/injection) on the indicated day.
Tumor models
Under anesthesia with 2.5% isoflurane, mice were injected subcutaneously with 5 × 105 E.G7-OVA cells or 1 × 106 MC38 cells. Mice developed palpable tumors around day 4 in both models. Mice were then immunized subcutaneously on days 4 and 18 after E.G7-OVA tumor graft or on days 9 and 17 after MC38 tumor graft. Tumors were measured every 3 days using calipers, and the tumor volumes were calculated using the following formula: π/6 × length x width2 [18]. Complete tumor regression was defined as a tumor volume below the limit of detection (≤ 10 mm3) lasting more than 2 weeks. Mice were sacrificed by cervical dislocation at the indicated day or when tumors reached a maximal volume of 3000 mm3.
Preparation of splenocytes, TILs and tumor cells
Spleens were filtered through a 70-µm nylon mesh, and splenocytes were collected after erythrocytes lysis using an ammonium–chloride–potassium (ACK) buffer (150 mM NH4Cl, 10 mM KHCO3 and 0.1 mM ethylenediaminetetraacetic acid, EDTA) for 2 min. When necessary, CD8+ splenic T cells were isolated by magnetic beads (Miltenyi Biotec, Bergisch Gladbach, Germany) according to the manufacturer’s recommendations.
For tumor-infiltrating lymphocytes and tumor cells cytometry analysis, tumor tissues were mechanically dissociated using the gentleMACS™ Dissociator (Miltenyi Biotec) and then filtered through a 70-µm cell strainer. For co-culture experiments, MC38 cells were trypsinized (trypsin–EDTA 0.05%/0.02% in phosphate-buffered saline, PBS, Pan Biotech, Aidenbach, Germany) and resuspended in RPMI 1640 medium. For co-culture with ex vivo tumor cells, tumors were harvested 24 days from implantation and dissociated using scalpels, digested with collagenase 3 mg/ml (SIGMA) at 37 °C under agitation for 60 min, filtered through a 70-µm cell strainer and then resuspended in RPMI 1640 medium.
ELISpot
Epitope-specific IFNγ (interferon gamma) production by splenocytes was determined with IFNγ ELISpot kits (Diaclone, Besançon, France) as previously described [10]. Briefly, single-cell suspensions of splenocytes (5 × 105 cells/well) were stimulated at 37 °C in 5% CO2 for 21 h with 10 µg/ml of SIINFEKL or ASMTNMELM epitopes. Positive controls included cells stimulated with 100 ng/mL of phorbolmyristate acetate and 1 ng/mL of ionomycin (Sigma-Aldrich). Negative controls included cells cultured with an irrelevant epitope. IFNγ spot-forming cells (SFCs) were counted on Cellular Technology Ltd. (Ohio, USA) ELISpot reader using 5.0.3 software. The results are presented as the difference of the average spot count of triplicate experimental wells and the average spot count of duplicate negative control wells.
For co-culture experiments, CD8+ splenic T cells isolated from mAdpgk-immunized or control mice were cultured (1 × 105 cells/well) in duplicate with tumor cells (5 × 104 cells/well) loaded or not with the mAdpgk peptide (ASMTNMELM, 10 µg/mL).
Flow cytometry analysis
After incubation with anti-mouse CD16/CD32 (clone 93, Biolegend, San Diego, CA, USA) and Amcyan LIVE/DEAD Fixable Aqua Dead Cell Stain Kit (Invitrogen, Waltham, MA, USA) at room temperature for 15 min, cells were stained with the following anti-mouse-conjugated antibodies for cell surface molecules (1 × 106 cells/well at 4 °C for 30 min): Pacific Blue anti-CD3 (clone 17A2, Biolegend), Alexa Fluor 700 anti-CD45 (clone 30-F11, Biolegend), APC-eFluor 780 anti-CD8a (clone 53–6.7, eBioscience, San Diego, CA, USA), APC-eFluor780 anti-CD4 (clone RM4-5, eBioscience), Alexa Fluor 700 anti-CD62L (clone MEL-14, eBioscience), FITC anti-CD279 (clone J43, eBioscience), PerCP/Cy5.5 anti-CD197 (CCR7) (clone 4B12, Biolegend), APC anti-CD11b (clone M1/70, Biolegend), PE anti-H2 Kb/H2 Db (clone 28–8-6, Biolegend), PE/Cy7 anti-CD366 (clone B8.2C12, Biolegend), APC anti-CD223 (clone C9B7W, Biolegend), APC anti-CD44 (clone IM7, eBioscience) and BV650 anti-NK1.1 (clone PK136, BD Bioscience) antibodies.
For intracellular molecules, cells were fixed and permeabilized using Fix/Perm buffer (eBioscience) according to the manufacture’s protocol and then stained with the following anti-mouse-conjugated antibodies at 4 °C for 60 min: PE anti-FOXP3 (clone FJK-16 s, eBioscience) and PE-Cyanine7 anti-T-bet (clone 4B10, eBioscience).
The PE H2-Kb SIINFEKL and H2-Db ASMTNMELM dextramers were obtained from Immudex (Copenhagen, Denmark). Cells were incubated with PE-labeled dextramer (45 min at 4 °C in the dark), and labeled anti-CD8 antibodies were used to phenotype these positive dextramer CD8+ T cells. An irrelevant dextramer was used in each experiment to take into account the background values. Acquisitions were performed on BD LSRII, and data were analyzed with FlowJo software (Tree Star) [19].
Total RNA extraction and qRT-PCR
Total ribonucleic acid (RNA) was extracted from EL4 and E.G7-OVA cell lines or tumor samples by using RNeasy Mini Kit (Qiagen, Hilden, Germany). Messenger RNA was reverse transcribed by using M-MLV Reverse Transcriptase kit (Invitrogen). Quantitative real-time reverse transcriptase–polymerase chain reaction (qRT-PCR) was performed using LightCycler® FastStart DNA MasterPLUS kit containing the fluorescent dye Sybr Green (Roche, Basel, Switzerland). Forward and reverse primers for OVA and β-actin genes were purchased from Biomers, Ulm, Germany. The conditions of real-time PCR reaction were as follows: 10 s at 95 °C, 4 s at 60 °C and 14 s at 72 °C (45 cycles). Quality and specificity of amplicons in each sample were detected by dissociation curve analysis. Triplicates were performed for each experimental point. For quantization, threshold cycle (CT) values were determined by the Lightcycler 1.5® Roche, and ΔCT was obtained by subtracting CT of reference gene, β-actin, to CT of target gene. Gene expression was presented as relative amount of mRNA normalized to β-actin and was calculated as 2−ΔCt [15, 20].
Statistics
Continuous variables are presented as the mean for normally distributed variables and as the median for nonparametric variables. Mann–Whitney test was used to calculate associations between non-normally distributed variables and paired Student's t test for normally distributed variables. Differences in tumor size among groups were determined using the ANOVA repeated-measures test. All reported p values were based on two-sided tests at a significance level of 0.05. All statistical analyses were performed using Statview 5.0 software® [10].
Results
Melanin vaccine triggers significantly enhanced CTL responses in vivo
The ability of melanin-based vaccine formulation to trigger CTL responses against an ovalbumin epitope was first compared to incomplete Freund’s adjuvant (IFA) formulation, combined with a TLR9 agonist (CpG-28) in both cases. Mice were immunized subcutaneously on days 0 and 14, with peptide–melanin mixed with 50 µg CpG-28 [pOVA30-Mel + CpG], or with free peptide mixed with IFA vol/vol and 50 µg CpG-28 [pOVA30-IFA + CpG]. When the CTL response was assessed by IFNγ ELISpot 7 days after the last immunization, the melanin-based formulation was statistically more efficient than [pOVA30-IFA + CpG] group ( p < 0.01) (Fig. 1b). Multiparametric flow cytometry analysis showed an effector Th1 memory profile of the SIINFEKL-specific T cells (dextramer+ T cells), with low CD62L and CCR7, high CD44 and high T-bet expression, as well as a very low PD1 expression in the [pOVA30-Mel + CpG] group (0.66% ± 0.23, Fig. 1c1. Peripheral amount of NK and NKT cells was very low, and no difference was observed between the different groups of treatment: NK cells rate within total splenocytes of 0.19% ± 0.03 versus 0.21% ± 0.03 in [pOVA30-Mel + CpG] and [Mel + CpG] group, respectively; NKT cells rate within total splenocytes of 0.02% ± 0.00 versus 0.02% ± 0.00 in [pOVA30-Mel + CpG] and [Mel + CpG] group, respectively.
Melanin vaccine significantly inhibits E.G7-OVA tumor growth
The functional efficacy of the immune response was then tested in a tumor model with ovalbumin-transfected cells (E.G7-OVA). E.G7-OVA cells were injected subcutaneously into C57BL/6 mice, and mice were immunized on days 4 and 18 with [pOVA30-Mel + CpG] or [pOVA30-IFA + CpG] as described above. All mice developed measurable tumors. Immunizations with [pOVA30-Mel + CpG] induced a complete tumor regression in 2/8 mice (not shown) and a significant decrease in the tumor growth when compared to [pOVA30-IFA + CpG]-treated mice (p < 0.001) (Fig. 1e).
Escape tumor mechanisms in E.G7-OVA model
As mentioned, two mice had complete tumor regression after immunization with [pOVA30-Mel + CpG], but tumor outgrowth occurred in six vaccinated mice post-day 21 (Fig. 1e). To explore whether this tumor escape was related to immune exhaustion induced by the tumor, the immune response after immunization with [pOVA30-Mel + CpG] was analyzed in tumor-free and tumor-bearing mice. The percentage of OVA-specific T cells was similar between tumor-free and tumor-bearing mice (Fig. 2a), and no significant difference in PD1 expression by SIINFEKL-specific T cells was found between both groups (Fig. 2b).
pOVA30-melanin vaccine is superior to IFA in terms of CTL response and anti-tumor effect. a Outline of the in vivo experiments in E.G7-OVA model. b ELISpot analysis of splenocytes on day 21, after immunizations on days 0 and 14. Each point represents an individual mouse (n = 8 mice/group with pooled data from 2 different experiments). c Expression of CD62L, CD44, CCR7, T-bet and PD1 in the CD8+dextramer+ population of [pOVA30-Mel + CpG] and [pOVA30-IFA + CpG] immunized mice (n = 8 mice/group, with pooled data from two different experiments). d Tumor growth curve of C57BL/6 mice bearing subcutaneous tumors and treated with different protocols. Bars = mean values ± standard error of the mean (SEM). *p < 0.5, **p < 0.01, ***p < 0.001
We then analyzed the tumor-infiltrating lymphocytes (TILs) at day 24 (after immunizations on days 4 and 18). CD8 + TILs were significantly higher in [pOVA30-Mel + CpG]-immunized mice compared to both [pOVA30-IFA + CpG]-immunized mice and control mice (injected with Mel + CpG). CD4 + TILs were similar in the three groups of mice (Fig. 2c). The percentage of regulatory T cells (Treg) FoxP3 + cells/CD4 + TILs was also similar in immunized and control mice (Fig. 2f). Furthermore, the percentage of SIINFEKL-specific CD8+ TILs within total CD8 + TILs was significantly higher in [pOVA30-Mel + CpG]-immunized mice compared to [pOVA30-IFA + CpG]-immunized mice (44.56 ± 4.61 versus 24.83 ± 5.65, p < 0.05) (Fig. 2d). In addition, phenotype analysis revealed a very low expression of exhausted molecules such as LAG3 and TIM3 in SIINFEKL-specific CD8 + as well as CD4 + TILs in [pOVA30-Mel + CpG]-immunized mice. In particular, percent rate of PD1 + LAG3 + cells within SIINFEKL-specific CD8 + TILs was significantly lower in [pOVA30-Mel + CpG]-immunized mice than [pOVA30-IFA + CpG]-immunized mice (respectively, 1.69% ± 1.24 versus 55.33 ± 8.07, p < 0.01) (Fig. 2g).
We then hypothesized that the tumor could escape the immune response by reducing ovalbumin expression. Ovalbumin expression in tumors implanted for 28 days was thus assessed in immunized and non-immunized mice. qRT-PCR analysis revealed a number of ovalbumin copies drastically reduced in [pOVA30-Mel + CpG] mice but not in the control [Mel + CpG] group (p < 0.01, Fig. 2h), suggesting that E.G7-OVA tumor escaped T-lymphocyte attack by tumor editing of the ovalbumin expression.
Melanin-based vaccine is more potent to increase CTL response in MC38 tumor model than IFA vaccine
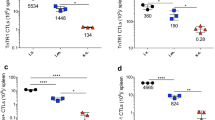
We then extended our analysis to the MC38 carcinoma model that spontaneously expresses a mutated epitope of Adpgk [14]. Mice were injected subcutaneously with MC38 tumor cells and then immunized with [mAdpgk + CpG], [mAdpgk-Mel + CpG] or [mAdpgk-IFA + CpG] on days 9 and 17. All mice developed measurable tumors and were sacrificed on day 24 to assess the immune response (Fig. 3a). CTL response against mAdpgk was first assessed by IFNγ ELISpot in splenocytes. The number of IFNγ-SFCs was significantly higher in [mAdpgk-Mel + CpG]-vaccinated mice compared to other groups, including the [mAdpgk-IFA + CpG] group (p < 0.001, Fig. 33). Flow cytometry analysis showed that the phenotype of the mAdpgk-specific T CD8+ cells (dextramer+ T cells) was similar in all groups with low CD62L and CCR7 (C–C chemokine receptor type 7) expression, high CD44 and T-bet expression, as well as low PD1 expression (Fig. 3c). Peripheral amount of NK and NKT cells was very low, and no difference was observed between the different groups of treatment: percent rate of NK cells within total splenocytes of 0.53% ± 0.04 versus 0.56% ± 0.08 in [mAdpgk-Mel + CpG] and [Mel + CpG] group, respectively; percent rate of NKT cells within total splenocytes of 0.11% ± 0.02 versus 0.09% ± 0.02 in [mAdpgk-Mel + CpG] and [Mel + CpG] group, respectively.
Tumor escape mechanisms in MC38 model
Although melanin-based vaccine formulation induced potent CTL responses against the mAdpgk peptide, tumor growth was not significantly reduced in [mAdpgk-Mel + CpG]-immunized mice when compared to control groups [Mel + CpG], [mAdpgk + CpG] and [mAdpgk-IFA + CpG] (Fig. 3d). Furthermore, no correlation between the CTL response, expressed as number of IFNγ-SFCs, and the tumor volume was observed (Pearson coefficient = 0.68, Fig. 3e).
To investigate the mechanism behind this discrepancy, we first analyzed TILs at day 24 (after tumor implantation and immunizations on days 9 and 17). CD4 + and CD8 + lymphocytes tumor infiltration was similar in both immunized and control mice (injected with Mel + CpG) (Fig. 3f). The percentage of regulatory T cells (Treg) FoxP3 + cells/CD4 + TILs was also similar in immunized and control mice (Fig. 3i). Finally, the expression of PD1, TIM3 (T-cell immunoglobulin mucin-3) and LAG3 (lymphocyte-activation gene 3) was low in both CD4 + and CD8 + TILs in [mAdpgk-Mel + CpG]-immunized mice (Fig. 3g, h). Furthermore, cytometry analysis of dextramer + CD8 + TILs did not show any enrichment in mAdpgk-specific CD8 + lymphocytes within total CD8 + TILs in immunized mice when compared to the control group treated with [Mel + CpG]. Given the exiguous number of mAdpgk-specific T-cell-infiltrating tumors, we did not perform any phenotype analysis on these cells.
We then investigated whether tumor cells escaped T-cell recognition either by downregulation of MHC class I expression or weak presentation of the neoepitope. Cytometry analysis confirmed that MHC class I expression was maintained on both MC38 cell line (97.3%, Fig. 4a) and on tumor cells implanted for 24 days, even in mice immunized with [mAdpgk-Mel + CpG]: average amount of MHC class I expressing cells of 58.7 ± 15.1% and 57.0 ± 3.5% in tumors withdrawn from immunized and control mice, respectively. We further investigated the ability of anti-mAdpgk CD8+ T cells to recognize MC38 cells by a co-culture experiment. Few IFNγ-SFCs were observed in co-culture when MC38 tumor cells were mixed with CD8+ T cells from [mAdpgk-Mel + CpG]-immunized mice, but not from [Mel + CpG] control mice (average number of IFNγ-SFCs ± SEM /1 × 105 CD8+ T cells: 9.5 ± 1.7 versus 0.4 ± 0.2, respectively; p < 0.05; SEM: standard error of the mean). Actually, this immune response appeared very limited as the number of IFNγ-SFCs was dramatically enhanced (p < 0.01) when tumor cells were loaded with the mutated Adpgk peptide by adjunction of the peptide into the culture medium. In this condition, CD8 + T cells from anti-mAdpgk-immunized mice, but not from control mice, were activated in co-culture (number of IFNγ-SFCs: 48.3 versus 0.8, p < 0.001), demonstrating the capacity of tumor cells to efficiently present the epitope to CD8 + T cells when mAdpgk was added to the medium. Supporting the poor presentation of mAdpgk within implanted tumors, when ex vivo tumor cells from established tumors were co-cultured with anti-mAdpgk CD8+ T cells, no immune response was observed, except if these tumor cells were loaded with mAdpgk by adjunction of the peptide into the culture medium (Fig. 4b).
Tumor escape mechanisms in the E.G7-OVA model. Peripheral CTL response in tumor-bearing and tumor-free mice after immunization on days 4 and 18 with [pOVA30-Mel + CpG]: a SIINFELKL-specific T cells within the splenic T CD8+ cells by flow cytometry; b PD1 expression within the CD8+dextramer+ population (SIINFELKL-specific T cells). c–g Cytometry analysis of CD8 + and CD4+ TILs in tumor-bearing mice immunized on days 4 and 18 with [melanin + CpG], [pOVA30-Mel + CpG] or [pOVA30-Mel + CpG] and sacrificed on day 24. h Number of RNA OVA copies (normalized to β actin) by qRT-PCR analysis in tumors that have grown in [melanin + CpG]- and [pOVA30-Mel + CpG]-treated mice, at day 28. Non-transfected-E.G7 and E.G7-OVA tumors cells, cultured in vitro, were used as controls. Each point represents an individual mouse, pooled data from two different experiments. Bars = mean values ± SEM. ns: not statistically significant; *p < 0.5, **p < 0.01, *** p < 0.001
mAdpgk-melanin vaccine significantly increases CTL response in the MC38 tumor model but does not inhibit tumor growth. a Outline of the in vivo experiments in MC38 model. b ELIspot analysis of splenocytes on day 21, after immunizations on days 0 and 14. Each point represents an individual mouse (n = 8 mice/group with pooled data from 2 different experiments). c Immune phenotype of mAdpgk-specific CD8+ T splenic cells (dextramer+ cells) in the different groups of mice. d Tumor growth in MC38-bearing mice, treated with the different vaccine formulations. Results are expressed as the mean ± SEM of tumor volumes. e Correlation between tumor volume at day 24 and number of IFNγ-SFCs (Spot forming cells) after stimulation in vitro of splenocytes with the MHC class I-epitope (CD8). Pearson coefficient = 0.68. f Percentage of CD8+ and CD4+ cells of CD45+ total cells in cytometry analysis of TILs (tumor infiltrating lymphocytes) of the [mAdpgk-Mel + CpG] immunized mice and [Mel + CpG] mice. g, h Phenotype of tumor infiltrating lymphocytes: percentage of PD1+ cells, TIM3+ cells and LAG3+ cells within CD4+ and CD8+ TILs in [mAdpgk-Mel + CpG] immunized mice. i Percentage of FoxP3+ cells in CD4+ TILs in [mAdpgk-Mel + CpG] and [Mel + CpG] group. Each point represents an individual mouse (pooled data from 2 different experiments). Bars = mean values ± SEM. *p < 0.5, **p < 0.01, ***p < 0.001
MC38 tumor cells are poorly recognized by anti-mAdpgk CD8 T cells. a Percentage of MHC class I (H2 Kb/Db) positive cells within MC38 cells in cytometry analysis of in vitro MC38 line cells. b CD8 splenic T cells isolated from mice vaccinated with [mAdpgk-Mel + CpG] were co-cultured for 24 h with medium + mAdpgk, MC38 cells, MC38 cells with mAdpgk peptide, ex vivo tumor cells or ex vivo tumor cells with mAdpgk peptide. Each point represents the mean value of IFNγ-SFC in triplicate wells (pooled data from three different experiments). Bars = mean values ± SEM. *p < 0.05, **p < 0.01
All these results suggest that despite the persistence of MHC class I expression by tumor cells in vivo as well as the high binding affinity of mAdpgk to the MHC class I molecules, tumor cells do not present the mAdpgk epitope sufficiently to be recognized and attacked by CD8+ T specific cells.
Discussion
Next-generation sequencing and bioinformatic analysis are providing an increasing number of tumor-specific epitopes that could be used as target for cancer peptide vaccines [2, 21]. Nevertheless, peptide vaccine formulations with adjuvants commonly used (e.g., IFA, Montanide) have not yet achieved significant clinical efficacy [5, 8, 9]. We here showed that melanin vaccine formulation induced a significantly higher CTL response than with other conventional vaccine adjuvants such as CpG or [IFA + CpG] in two different models. The CD8 + T cells responses were characterized by an effector memory phenotype and high cytolytic potential, with high expression of T-bet and low expression of PD1. Peripheral NK and NKT populations were not modified after melanin-based vaccine and thus are unlikely to contribute to anti-tumor effect.
In line with previous studies, IFA formulation mitigates the CTL response triggered by CpG in our models probably because IFA, as other water-in-oil emulsions, slowly releases antigen at the injection site [9, 22,23,24]. This phenomenon, coupled with the local inflammation induced by the emulsion itself, might result in the sequestration and apoptosis of primed CD8 + T cells [9, 24]. On the contrary, melanin conjugated to peptides is rapidly transferred to lymph nodes [10, 25, 26], thus potentially increasing the optimal uptake of the latter by APCs in draining lymph nodes and therefore efficient presentation to CD8+ cytotoxic cells. When mouse bone marrow-derived dendritic cells or macrophages were cultured in vitro in the presence of melanin, no activation (TNFα, IL6 or IL10 secretion; CD80 and CD86 expression) was seen in both cell types (preliminary data, not shown), suggesting that melanin has no direct immunostimulating effects. The adjuvant effect of melanin nanoparticles thus probably relies on a carrier effect, by driving peptides to the T-cell area of the lymph nodes as reported with several other nanoparticles [27, 28].
To support the potential applications of this vaccine approach in cancer, we then compared the efficacy of melanin-based vaccines to formulations using IFA combined with a TRL9 agonist in two tumor models expressing ovalbumin and mAdpgk (E.G7-ova and MC38, respectively). In the E.G7-OVA tumor model, the melanin-based formulation compared favorably with IFA + CpG in terms of anti-tumor effect, allowing tumor eradication in two mice. Flow cytometry analysis of TILs in [pOVA30-Mel + CpG]-treated mice revealed a high infiltration of anti-SIINFEKL CD8+ cells as along with a very low expression of exhaustion molecules (PD1, LAG3, TIM3) in these cells. On the contrary, anti-SIINFEKL CD8 + TILs in [pOVA30-IFA + CpG]-immunized mice were sparse and characterized by a very high rate of PD1 + LAG3 + . As seen in several human cancers, the co-expression of PD1 and LAG3 is associated with a dysfunctional state of T CD8+ lymphocytes [29,30,31] and can partially explain the reduced efficacy of IFA formulation in this model. At time of tumor relapse in this model, qRT-PCR revealed a drastic reduction of the number of ovalbumin copies within the tumors. This observation suggests that E.G7-OVA tumor escaped T-lymphocyte attack by tumor editing of the ovalbumin expression rather than by blunting of immune response. As reported in earlier studies, especially when the antigen target is not essential to tumor cells survival as in the case of ovalbumin protein, cancer immunoediting is a T-cell-dependent immune selection process that leads to the growth of tumor cells that rejected antigen expression [32, 33].
In contrast to E.G7-OVA model, no significant tumor growth inhibition was observed in [mAdpgk-Mel + CpG]-immunized mice, despite a strong peripheral immune response. Discordant results on tumor growth inhibition after immunization with mAdpgk have been published in the literature, some investigators reporting an anti-tumor effect [14], while others did not [34]. These discrepancies might be explained by minor differences between expression levels of the neoepitope in various sub-clones of the MC38 cell line [34]. In our model, indeed, such limited anti-tumor efficacy in MC38 model was related to an insufficient presentation of mAdpgk neoepitope by tumor cells leading to poor recognition by CD8 + T cells. No other mechanisms such as peripheral or local immunosuppression or immune tolerance have been found to explain the lack of anti-tumor efficacy.
The lack of neoepitope presentation is thus impacting the relevance of this model for comparing melanin and IFA vaccine formulations. Results of this tumor model are, nonetheless, interesting since they are parallel to what is described in clinical studies, in which presentation of neoepitopes by tumor cells represents a critical limitation in vaccination strategy.
In conclusion, melanin-based vaccine provides an efficient method to trigger CTL response against short and long synthetic peptides for tumor immunotherapy and compared favorably to the classic combination of IFA and TLR9 agonist in mice. Melanin formulation represents an interesting approach for anti-tumor vaccination.
Abbreviations
- ACK:
-
Ammonium–chloride–potassium
- APC:
-
Antigen-presenting cell
- CCR7:
-
C–C chemokine receptor type 7
- CTL:
-
Cytotoxic T lymphocyte
- CTLA4:
-
Cytotoxic T-lymphocyte-associated protein 4
- DMEM:
-
Dulbecco modified Eagle medium
- EDTA:
-
Ethylenediaminetetraacetic acid
- ICI:
-
Immune check-point inhibitor
- IFA:
-
Incomplete Freund’s adjuvant
- IFNγ:
-
Interferon gamma
- LAG3:
-
Lymphocyte-activation gene 3
- MHC:
-
Major histocompatibility complex
- PBS:
-
Phosphate-buffered saline
- PD1:
-
Programmed cell death 1
- qRT-PCR:
-
Quantitative real-time reverse transcriptase–polymerase chain reaction
- RNA:
-
Ribonucleic acid
- SEM:
-
Standard error of the mean
- SFC:
-
Spot-forming cell
- TAA:
-
Tumor-associated antigens
- TIL:
-
Tumor-infiltrating lymphocyte
- TIM3:
-
T-cell immunoglobulin mucin-3
- TLR9:
-
Toll-like receptor 9
- Treg:
-
Regulatory T cells
References
Carpentier AF, Tran T, Sejalon F, Geinguenaud F, Tartour E, Motte L, Banissi C. The adjuvant effect of melanin is superior to incomplete Freund adjuvant in a tumor subunit vaccine model, Eur J Cancer, March 2018 Volume 92, Supplement 1, p S2–S3 [Abstract A4]
Schumacher TN, Schreiber RD (2015) Neoantigens in cancer immunotherapy. Science 348(6230):69–74
Kumai T, Kobayashi H, Harabuchi Y, Celis E (2017) Peptide vaccines in cancer-old concept revisited. Curr Opin Immunol 45:1–7
Speiser DE, Liénard D, Rufer N, Rubio-Godoy V, Rimoldi D, Lejeune F, Krieg AM, Cerottini JC, Romero P (2005) Rapid and strong human CD8+ T cell responses to vaccination with peptide, IFA, and CpG oligodeoxynucleotide 7909. J Clin Invest 115(3):739–746
Obeid J, Hu Y, Slingluff CL Jr (2015) Vaccines, adjuvants, and dendritic cell activators–current status and future challenges. Semin Oncol 42:549–561
Melssen MM, Petroni GR, Chianese-Bullock KA, Wages NA, Grosh WW, Varhegyi N, Smolkin ME, Smith KT, Galeassi NV, Deacon DH, Gaughan EM, Slingluff CL Jr (2019) A multipeptide vaccine plus toll-like receptor agonists LPS or polyICLC in combination with incomplete Freund's adjuvant in melanoma patients. J Immunother Cancer 7(1):163
Souleimanian NE, Tosello V, Bhardwaj N, Adams S, O'Neill D, Pavlick A, Escalon JB, Cruz CM, Angiulli A, Angiulli F, Mears G, Vogel SM, Pan L, Jungbluth AA, Hoffmann EW, Venhaus R, Ritter G, Old LJ, Ayyoub M (2007) Vaccination with NY-ESO-1 protein and CpG in Montanide induces integrated antibody/Th1 responses and CD8 T cells through cross-priming. Proc Natl Acad Sci U S A 104(21):8947–8952
Hailemichael Y, Dai Z, Jaffarzad N, Ye Y, Medina MA, Huang XF, Dorta-Estremera SM, Greeley NR, Nitti G, Peng W, Liu C, Lou Y, Wang Z, Ma W, Rabinovicj B, Sowell RT, Schluns KS, Davis RE, Hwu P, Overwijk WW (2013) Persistent antigen at vaccination sites induces tumor-specific CD8+ T cell sequestration, dysfunction and deletion. Nat Med 19(4):465–472
Bijker MS, van den Eeden SJ, Franken KL, Melief CJ, Offringa R, van der Burg SH (2007) CD8+ CTL priming by exact peptide epitopes in incomplete Freund’s adjuvant induces a vanishing CTL response, whereas long peptides induce sustained CTL reactivity. J Immunol 179(8):5033–5040
Carpentier AF, Geinguenaud F, Tran T, Sejalon F, Martin A, Motte L, Tartour E, Banissi C (2017) Synthetic melanin bound to subunit vaccine antigens significantly enhances CD8+ T-cell responses. PLoS ONE 12(7):e0181403
Bachmann MF, Jennings GT (2010) Vaccine delivery: a matter of size, geometry, kinetics and molecular patterns. Nat Rev Immunol 10(11):787–796
Rötzschke O, Falk K, Stevanović JG, Walden P, Rammensee HG (1991) Exact prediction of a natural T cell epitope. Eur J Immunol 21(11):2891–2894
Ahonen CL, Doxsee CL, McGurran SM, Riter TR, Wade WF, Barth RJ, Vasilakos JP, Noelle RJ, Kedl RM (2004) Combined TLR and CD40 triggering induces potent CD8+ T cell expansion with variable dependence on type I IFN. J Exp Med 199:775–784
Yadav M, Jhunjhunwala S, Phung QT, Lupardus P, Tanguay J, Bumbaca S, Franci C, Cheung TK, Fritsche J, Weinschenk T, Modrusan Z, Mellman I, Lill JR, Delamarre L (2014) Predicting immunogenic tumour mutations by combining mass spectrometry and exome sequencing. Nature 515:572–576
Lorenzi S, Mattei F, Sistigu A, Bracci A, Spadaro F, Sanchez M, Belardelli F, Gabriele L, Schiavoni G (2011) Type I IFNs control antigen retention and survival of CD8α+ dendritic cells after uptake of tumor apoptotic cells leading to cross-priming. J Immunol 186(9):5142–5150
Maubant S, Banissi C, Beck S, Chauvat A, Carpentier AF (2011) Adjuvant properties of cytosine-phosphateguanosine oligodeoxynucleotide in combination with various polycations in an ovalbumin-vaccine model. Nucleic Acid Ther 21:231–240
Carpentier A, Metellus P, Ursu R, Zohar S, Lafitte F, Barrie´ M, Meng Y, Richard M, Parizot C, Laigle-Donadey F, Gorochov G, Psimaras D, Sanson M, Tibi A, Chinot O, Carpentier AF (2010) Intracerebral administration of CpG oligonucleotide for patients with recurrent glioblastoma, a phase II study. Neuro Oncol 12:401
Carpentier AF, Chen L, Maltonti F, Delattre JY (1999) Oligodeoxynucleotides containing CpG motifs can induce rejection of a neuroblastoma in mice. Cancer Res 59(21):5429–5432
Tran T, Diniz MO, Dransart E, Gey A, Merillon N, Lone YC, Godefroy S, Sibley C, Ferreira LC, Medioni J, Oudard S, Johannes L, Tartour E (2016) A Therapeutic her2/neu vaccine targeting dendritic cells preferentially inhibits the growth of low her2/neu-expressing tumor in HLA-A2 transgenic mice. Clin Cancer Res 22(16):4133–4144
Schmittgen TD, Livak KJ (2008) Analyzing real-time PCR data by the comparative C(T) method. Nat Protoc 3:1101–1108
Sahin U, Tureci O (2018) Personalized vaccines for cancer immunotherapy. Science 359(6382):1355–1360
Makinen SR, Zhu Q, Davis HL, Weeratna RD (2016) CpG-mediated augmentation of CD8+ T-cell responses in mice is attenuated by a water-in-oil emulsion (Montanide ISA-51) but enhanced by an oil-in-water emulsion (IDRI SE). Int Immunol 28(9):453–461
Kuball J, de Boer K, Wagner E, Wattad M, Antunes E, Weeratna RD, Vicari AP, Lotz C, van Dorp S, Hol S, Greenberg PD, Heit W, Davis HL, Theobald M (2011) Pitfalls of vaccinations with WT1-, proteinase3- and MUC1-derived peptides in combination with montanideisa51 and CpG7909. Cancer Immunol Immunother 60(2):161–172
Hailemichael Y, Dai Z, Jaffarzad N, Ye Y, Medina MA, Huang XF, Dorta-Estremera SM, Greeley NR, Nitti G, Peng W, Liu C, Lou Y, Wang Z, Ma W, Rabinovich B, Sowell RT, Schluns KS, Davis RE, Hwu P, Overwijk WW (2013) Persistent antigen at vaccination sites induces tumor-specific CD8+ T cell sequestration, dysfunction and deletion. Nat Med 19(4):465–472
Hemmi H, Yoshino M, Yamazaki H, Naito M, Iyoda T, Omatsu Y, Shimoyama S, Letterio JJ, Nakabayashi T, Tagaya H, Yamane T, Ogawa M, Nishikawa S, Ryoke K, Inaba K, Hayashi S, Kunisada T (2001) Skin antigens in the steady state are trafficked to regional lymph nodes by transforming growth factor-beta1-dependent cells. Int Immunol 13(5):695–704
Yoshino M, Yamazaki H, Shultz LD, Hayashi S (2006) Constant rate of steady-state self-antigen trafficking from skin to regional lymph nodes. Int Immunol. 18(11):1541–1548
Wang W, Liu Z, Zhou X, Guo Z, Zhang J, Zhu P, Yao S, Zhu M (2019) Ferritin nanoparticle-based SpyTag/SpyCatcher-enabled click vaccine for tumor immunotherapy. Nanomedicine 16:69–78
Heße C, Kollenda S, Rotan O, Pastille E, Adamczyk A, Wenzek C, Hansen W, Epple M, Buer J, Westendorf AM, Knuschke T (2019) A tumor-peptide-based nanoparticle vaccine elicits efficient tumor growth control in antitumor immunotherapy. Mol Cancer Ther 18(6):1069–1080
Andrews LP, Marciscano AE, Drake CG, Vignali DA (2017) LAG3 (CD223) as a cancer immunotherapy target. Immunol Rev 276(1):80–96
Tu L, Guan R, Yang H, Zhou Y, Hong W, Ma L, Zhao G, Yu M (2019) Assessment of the expression of the immune checkpoint molecules PD-1, CTLA4, TIM-3 and LAG-3 across different cancers in relation to treatment response, tumor-infiltrating immune cells and survival. Int J Cancer 147(2):423–439
Zelba H, Bedke J, Hennenlotter J, Mostböck S, Zettl M, Zichner T, Chandran A, Stenzl A, Rammensee HG, Gouttefangeas C (2019) PD-1 and LAG-3 dominate checkpoint receptor-mediated T-cell inhibition in renal cell carcinoma. Cancer Immunol Res 7(11):1891–1899
Matsushita H, Vesely MD, Koboldt DC, Rickert CG, Uppaluri R, Magrini VJ, Arthur CD, White JM, Chen Y-S, Shea LK, Hundal J, Wendl MC, Demeter R, Wylie T, Allison JP, Smyth MJ, Old LJ, Mardis ER, Schreiber RD (2012) Cancer exome analysis reveals a T-cell-dependent mechanism of cancer immunoediting. Nature 482(7385):400–404
Dharmaraj N, Piotrowski SL, Huang C, Newton JM, Golfman LS, Hanoteau A, Koshy ST, Li AW, Pulikkathara MX, Zhang B, Burks JK, Mooney DJ, Lei YL, Sikora AG, Young S (2019) Anti-tumor immunity induced by ectopic expression of viral antigens is transient and limited by immune escape. Oncoimmunology 8(4):e1568809
Hos B, Camps MGM, van den Bulk J, Tondinin E, van den Ende T, Ruano D, Franken K, Janssen GMC, de Ru AH, Filippov DV, Arens R, van Veelen PA, de Miranda NFCC, Ossendorp F (2019) Identification of a neo-epitope dominating endogenous CD8 T cell responses to MC-38 colorectal cancer. Oncoimmunology. https://doi.org/10.1080/2162402X.2019.1673125
Acknowledgments
The authors thank the Association Oligocyte and the Association pour le development des neurosciences a Avicenne (ADNA) for supporting our research.
Funding
No funding sources were used for this report.
Author information
Authors and Affiliations
Contributions
SC, CT, ET and AFC contributed to the study concept and design of experiments. SC, CB, MSR and TT performed the experiments. CB and TT helped in methodology and software analysis. SC and CB were involved in data analysis and interpretation of results. SC and AFC wrote the preliminary version of the paper. All authors participated in critical review and revision of the final manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The AP/HP (Assistance Publique de Hopitaux de Paris) filed a provisional patent application on this method. AF Carpentier & C Banissi are listed as inventors. AF Carpentier holds shares in Altevax inc. and is consultant for BMS. The authors declare that there are no other conflicts of interest.
Ethical approval and ethical standards.
Experiments were conducted on female C57BL/6 mice. All animal experiments were approved by the ethics committee of Paris Descartes University (Project APAFIS #5337 N° 2016021517305775) and performed in accordance with European Union guidelines for animal experiments.
Human and animal rights
Animal source: Female C57BL/6 aged 5 to 6 week old were purchased from Janvier Labs (Le Genest-Saint-Isle, France) and kept under specific-pathogen-free conditions.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Published as a poster abstract at the 5th Immunotherapy of Cancer Conference: [1]
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Cuzzubbo, S., Banissi, C., Rouchon, M.S. et al. The adjuvant effect of melanin is superior to incomplete Freund’s adjuvant in subunit/peptide vaccines in mice. Cancer Immunol Immunother 69, 2501–2512 (2020). https://doi.org/10.1007/s00262-020-02631-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00262-020-02631-7