Abstract
Background
Adoptive tumor-infiltrating lymphocytes (TIL) therapy and interleukin-2 (IL-2) have been investigated in melanoma.
Aim
To confirm previously observed preventive effects of TIL + IL2 in a subgroup of patients with relapsing metastatic stage III melanoma.
Methodology
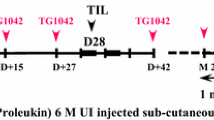
Open-label, randomized two-group, multicenter five-year trial in adult stage III melanoma patients with only one invaded lymph node after complete resection. Patients received TIL + IL2 or abstention. TIL + IL2 was administered within 8 weeks after lymph node resection and 4 weeks after. Disease-free survival was assessed every 2 months up to month 18, every 3 months up to month 36 and every 4 months up to 5 years. A once-a-year follow-up was scheduled beyond the five-year follow-up. Safety was assessed throughout the trial.
Results
Overall, 49 patients accounted for the modified intent-to-treat and 47 for the PP. Slightly more male than female patients participated; mean age was 57.7 ± 11.4 years in the TIL + IL2 group and 53.5 ± 13.0 years in the abstention group. After 5 years of follow-up, 11/26 patients in the TIL + IL2 group and 13/23 in the abstention group had relapsed. There was no statistical difference between the groups (HR: 0.63 CI 95% [0.28–1.41], p = 0.258), nine patients in the TIL + IL2 and 11 in the abstention group died with no significant difference between the two groups (HR: 0.65 CI95% [0.27 − 1.59], p = 0.34). Safety was good.
Conclusion
We did not confirm results of a previous trial. However, ulceration of the primary melanoma may be considered predictive of the efficacy of TIL in melanoma in adjuvant setting, in a manner similar to interferon α.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Over the last decades, the occurrence of malignant melanoma (MM) has strongly increased. With only very few treatment options available for patients with advanced MM during the last decade, the five-year overall survival rate (OS) ranged between 9 and 28% [1, 2]. Nowadays, immunotherapies and targeted therapies allow OS to increase. For example, in a clinical trial in patients with advanced melanoma receiving a combination of nivolumab and ipilimumab, the observed OS was 58% [3]. Despite this encouraging result, a large group of patients does still not respond to treatment after an initial response, thus requiring adjuvant treatments. Adoptive cell therapy (ACT) with tumor-infiltrating lymphocytes (TIL) is one of these adjuvant treatment options. It consists of the outgrowth of tumor resident T cells from tumor material, their expansion ex vivo and their transfer back into the same patient. TIL may be combined with prior lymphodepletion regimen or administered alone. ACT generates large numbers of tumor-reactive TIL, activates cells ex vivo and allows the patient’s immune system to prepare for the invasion of effector cells [4,5,6]. The efficacy and safety of adoptive TIL therapies have been intensively investigated in melanoma patients. Results are promising and TIL may be considered as an alternative treatment approach for stage IV melanoma patients. A certain number of clinical trials show response rates of up to 72%, 10 to 20% of treated patients showed complete remission (CR) and 40% durable clinical responses [7,8,9,10]. However, results may have to be tempered as they were generated, especially for complete remission, during a pre-immune checkpoint period. Moreover, as this was a non-randomized study that subsequently showed a response rate lower than that of previously published studies and, finally, these data were collected using lymphodepletion, which was not used in the present study.
Until today, only a few studies in patients with stage III melanoma have investigated TIL treatment in adjuvant settings. In 2002, we published results from a clinical phase I/II trial about ACT of melanoma TIL as adjuvant therapy for stage III melanoma [11]. Results for this subgroup of patients allowed us to hypothesize that the efficacy of TIL in stage III melanoma may be directly related to the number of invaded lymph nodes and that tumor burden may impact the efficacy and/or in vitro expansion of tumor-specific T cells [12]. Thus, we assumed that TIL would be more efficacious in a setting with minimal tumor burden. Importantly, TIL infusions were not preceded by NMA lymphodepletion and the number of cells infused was about tenfold lower. In 2007, we published results from a ten-year follow-up, confirming results previously obtained [13]. Finally, in 2014, we published results from a 17-year follow-up, confirming the association between the efficacy of TIL and the number of invaded lymph nodes [14]. These latter results strongly suggested that a low tumor burden allows the curative effect of TIL to increase in possible microscopic remaining melanoma. Moreover, our results showed that prolonged survival was associated with the presence of melanoma-specific TIL.
The aim of the present clinical phase III trial was to confirm the previously observed preventive effects of TIL combined with IL2 as adjuvant treatment in a subgroup of patients with relapsing metastatic stage III melanoma after complete lymph node resection with only one invaded lymph node [15, 16].
Material and methods
-
Study design
This was an open-label, randomized two-arm, multicenter five-year clinical trial, conducted between June 2005 and March 2018 in France at three trial sites.
The trial planned for 70 patients to be included. Suitable male or female patients between 18 and 75 years of age had to have a clinically diagnosed stage III melanoma according to the 8th American Joint Committee on Cancer (AJJC) staging with only one invaded lymph node after complete resection confirmed by histology. They also had to have an Eastern Cooperative Oncology Group (ECOG) index of 0–2 and a Karnofsky score of ˃ 80% at baseline [17,18,19]. Patients were randomized to TIL + IL2 or to abstention. Abstention was defined as patients who did not receive any other melanoma treatments prior to inclusion. Patients randomized to TIL + Interleukin-2 (IL2) received an injection within 8 weeks after lymph node dissection and 4 weeks later. TIL expansion was produced according to good manufacturing practices at the cellular and genetic therapy unit of the University Hospital of Nantes, France, following a procedure previously described [13, 14, 20, 21].
Patients were not allowed to receive another immunotherapy than that administered during the trial or anti-tumor chemotherapies, hematological growth factors or long-term systemic corticosteroids. No lymphodepletion was performed before TIL injection.
The primary endpoint was the disease-free survival (DFS); secondary endpoints were OS, tolerance to treatment with TIL + IL2, immunological response, analysis of clinical, biological and histological factors on survival: age, gender, localization of the melanoma lesion, Breslow thickness, Clark score and capsular breaking, ulceration of the primitive lesion and lactate dehydrogenase (LDH) level.
DFS was assessed through a clinical examination every 2 months up to month 18, every 3 months up to month 36 and every 4 months up to 5 years. A once-a-year follow-up was scheduled beyond the five-year follow-up. Clinical examinations were completed by radiological examinations including echography of the abdomen at inclusion, month 4, 8 and 12, and then every 6 months up to 5 years. After that period, patients were followed by clinical examinations only.
OS/death was assessed at each visit; tolerance was evaluated at day 1, 14, 28 and 42, as well as every 2 months during clinical examinations of long-term tolerance, and all adverse events were recorded by the investigators.
Figure 1 provides the therapeutic schedule of the trial.
-
Selected lymph nodes
Only single invaded macroscopic lymph nodes were selected for this trial. The size of each lymph node had to be sufficient to obtain expansion.
From each invaded lymph node (LN), 50% of the tissue was used for TIL expansion, 30% for obtaining the melanoma cell line and 20% for immunochemistry purposes of the LN tissue from which TIL were extracted.
-
TIL production procedure
TIL were produced respecting “Good Manufacturing Practices” conditions in the Cell and Gene Therapy manufacturing facility of the Nantes University Hospital and according to a previously described procedure [20,21,22].
TIL extraction and expansion were obtained from the invaded lymph node alone from all patients participating in this trial. TIL were cultured minimally for a short-term period with low dose recombinant interleukin-2 (rIL2) [20, 21]. Short-term cultured TIL were isolated through culturing fragments of metastatic LN into 12-well tissue culture plates with X-VIVO 15 serum-free medium (BioWhittaker, Walkersville, MD, USA) containing low doses of rIL2 (150 U/ml) (Eurocetus, RueilMalmaison, France) and glutamine (1 mM, BioWhittaker) for 10–14 days. Ex vivo expanded TIL were derived as follows: 1.8 × 106 short-term cultured TIL were plated at 300 viable lymphocytes/well with irradiated feeder cells (allogeneic peripheral blood leukocytes (PBL) and B-EBV cells: Epstein-Barr virus infected B-cells) into U-bottom microplates in always low doses of rIL2 medium (150 µl). PHA-L (phytohemagglutinin-L or leucoagglutinin) (Difco, Detroit, ML, USA) was added on day 0 (1 µg/ml). Ten days later, lymphocytes were recovered from the culture plates, adjusted to 1 × 106 cells/ml in rIL2 medium and transferred into culture cell bags for an additional 10 days. The final TIL harvest was obtained by centrifuging, washing and suspending the TIL in 4% human serum albumin (LFB, Les Ulis, France). A second TIL expansion was performed within one month of the first, starting from cryopreserved short-term cultured TIL. Aliquots of TIL suspensions injected into the patients were cryopreserved to study their tumor specificity, which was carried out later once the autologous tumor cell line had been established in culture.
In 2007, following a request from the French Health Agency, the original culture medium used was replaced by X-VIVO™ Media (LonzaLevollois-Perret, France). Each patient received at least 1 billion TIL at each injection. The total quantity of cells was administered. TIL were administered intravenously at 3 mL/minute for a total duration of 30 to 65 min, corresponding to a total volume of 100 to 200 mL. IL2 was injected subcutaneously at 6 million UI between day 1 and day 5 and between day 8 and day 12. In the event of adverse effects (AEs), IL2 doses were interrupted and restarted after AEs had disappeared; if AEs re-occurred, IL2 treatment was definitively stopped.
-
Establishment of melanoma cell lines
Melanoma cell lines were established as previously described and obtained for 16/24 patients [23, 24]. Briefly, fresh LNs with metastasis were minced into small tumor pieces and plated in a 24-well plate with 1.5 mL RPMI (Roswell Park Memorial Institute) medium per well supplemented with 10% fetal calf serum. Plates were placed at 37 °C in a humidified incubator with 5% CO2 and observed under a light microscope every week and sub-cultured when necessary.
-
Cytokine production
The proportion of tumor-reactive TIL was determined through the measurement of the proportion of interferon-gamma (IFN-γ-)-secreting T cells within the TIL stimulated with the autologous melanoma cell line, as described previously [21]. Succinctly, for each cell line, a total of 500,000 cells per well of a 6-well plate were seeded in 3 mL of culture medium with or without 500 U/mL recombinant IFN-γ (Tebu, Le Perrayen Yvelines, France), in duplicate. After 48 h of incubation, cells were washed, detached from the wells using PBS-EDTA (Lonza, Levallois, France) and processed for flow cytometry.
-
Immunohistochemistry (IHC)
IHC was performed using the streptavidin/peroxidase technique [25] in the 47 lymph nodes of the PP protocol population excluding three patients. Deep-frozen sections were incubated for 30 min at room temperature with the primary antibody. Different monoclonal antibodies including CMH I and II, HLA-A2, CD54, Melan A, gp 100, pan-MAGE, BTLA, PD-1, PD-L-1, IDO, NY-ESO-1, CD3, CD8, CD4, TIM-3 and tyrosinase were used to explore marker expression markers. Negative control assays were performed using a mouse monoclonal immunoglobulin G1 (IgG1) isotype control or a monoclonal immunoglobulin G2a (IgG2a) isotype control (DakoCytomation, DakoCytomation Denmark A/S ProdGBtionsvej 42, Glostrup, Denmark).
Slides were read with a Leica microscope (magnification 25 ×). In each immune-stained serial section, the entire tumor area was evaluated. Each score was evaluated on a five-point scale: absence of expression, weak expression (1–25% of positive cells), moderate expression, (26–50%), intermediate expression (51–75%) and strong expression (> 75%), corresponding, respectively, to 0, 1, 2, 3 and 4. To avoid subjectivity of the reading, all slides were read by two independent blinded evaluators.
-
Statistical Analysis
To confirm a difference of event-free survival equal to or greater than 35% (66% vs 31%) with a beta risk of 20% and an alpha risk of 5% in a bilateral situation, 37 patients per group were required for a conventional binomial procedure with only one final analysis. Kaplan–Meier estimates and log-rank tests were used to analyze the main efficacy analysis. The log-likelihood ratio test was used to assess different factors. The Cox model was used to adjust treatment comparison on baseline characteristics known to have a prognostic significance including Breslow thickness (< 1.5 mm; > 1.5 mm), capsular breaking, number of detectable regional nodes (1, > 1), gender and age (< 50 years, 50 years). Comparison of means and frequencies were made using Mann–Whitney and Fisher tests. The bilateral significance level was set at 5%.
Assumption of proportional hazards was checked for all factors. All interactions were tested. Analyses were performed using SAS and R 3.11 statistical software.
Results
-
General information
The first patient was randomized to treatment or abstention on June 15, 2005, and the last on January 8, 2013. Overall, 81, instead of the 70 initially planned patients, were selected and randomized. In the first version of the protocol, IL2 injections were performed one day after the TIL to understand the AEs related to TIL and those related to IL2 better. However, as survival and proliferation of TIL are dependent on IL2, injections were considered to be more effective when administered immediately after adoptive TIL transfer. Thus, a total of 11 patients having received TIL injections prior to the protocol amendment in 2007 were excluded from the statistical analysis. In total, 49 patients accounted for the modified intent-to-treat (mITT) population (23 in the control and 26 in the TIL + IL group) and 47 for the per protocol (PP) population (23 in the control and 24 in the TIL + IL2 group).
-
Patient characteristics
Overall, 27 men (11 and 16 in the TIL and the abstention group, respectively) and 22 women (15 and seven in the TIL and abstention group, respectively) participated in this trial. Mean age was 57.7 ± 11.4 years in the TIL + IL2 group and 53.5 ± 13.0 years in the abstention group. The mean Breslow index was 2.8 ± 2.4 mm in the abstention and 3.7 ± 0.8 mm in the TIL + IL2 group. The lactate dehydrogenase (LDH) level was normal for 26 patients (14 and 12, respectively, in the TIL and abstention groups) and abnormal for 22 patients (12 and 10, respectively, in the TIL and abstention groups) with Mean ± SD 3.8 ± 1.1 in the abstention group and 3. ± 1.3 in the TIL group. Twenty patients had an ulceration of the primary tumor (seven and 13 patients for the TIL + IL2 and abstention group, respectively), 26 patients had no ulceration (17 and nine patients for the TIL + IL2 and abstention group, respectively); for three patients, the information was missing. Lymph node capsular breaking was absent in 33 patients (16 and 17 patients for the TIL + IL2 and abstention group, respectively), present in 11 patients (five and six patients for the TIL + IL2 and abstention group, respectively) and missing for five patients. Thirteen patients in the TIL + IL2 and ten in the control group received previous adjuvant treatment with low doses of interferon α after primary excision of melanoma performed several years prior to treatment. None of the LNs were necrosed. Patients did not receive any other treatments prior to inclusion. Demographics and disease baseline data are detailed in Table1.
Table 1 Patient demographic and baseline disease data (modified ITT population, n = 49) -
Efficacy
Disease-free survival
-
Modified intent-to-treat population
After 5 years of follow-up, 11/26 patients in the TIL + IL2 group and 13/23 in the abstention group had relapsed. The log-rank test did not show a statistical difference between the two groups (hazard ratio (HR): 0.63 CI 95% [0.28–1.41], p = 0.25; Fig. 2), nine patients in the TIL + IL2 (9/26) and 11 (11/23) in the abstention group died. The log-rank test did not show a statistical difference between the two groups (HR: 0.65 CI95% [0.27–1.59], p = 0.35; Fig. 3).
Fig. 2 -
Per protocol population
The per protocol analysis did not show a statistical significant difference (HR: 0.549, CI 95% [0.231; 1.307], p = 0.169) after 5 years between the two groups; however, a trend to a higher DFS rate was observed in the TIL + IL2 group with 62.5% compared to 47.8% in the abstention group. The difference between both treatment groups for OS was statistically nonsignificant (HR: 0.5031; CI 95% [0.1947; 1.3], p = 0.148). Despite these results, only a trend to a higher OS rate was observed in the TIL + IL2 group (70.8%) compared to 52.2% for the abstention group (Table 2).
Table 2 Disease-free and overall survival (PP population, n = 47) after 5 years When analyzing clinical, biological and histological factors, no differences for progression-free survival, for age, gender, localization of melanoma lesion, Breslow thickness, Clark score and capsular breaking were observed. For seven patients with primary ulceration at baseline in the TIL group and for six with primary ulceration at baseline in the abstention group, DFS was higher, however not statistically significantly higher, in the TIL + IL2, compared to the abstention group (HR: 0.21 [0.025–1.65], p = 0.14, PP population). No significant difference was found between the groups with primary melanoma without ulceration (HR = 1.06 [0.027–4.1], p = 0.94; Fig. 4a and b).
Fig. 4 Disease-free survival in the TIL + IL2 and abstention group for patients with or without ulceration at baseline (PP population). a Ulceration at baseline (n = 19: no information for 2 patients). p = 0.14, log-rank, HR: 0.21, CI 95% [0.025–1.65]. DFS was significantly higher in the group of patients treated with TIL + IL2 with an ulceration of primary melanoma compared to the abstention group. b No ulceration at baseline (n = 25, no information for one patient). p = 0.94, log-rank, HR = 1.06, CI 95% [0.027–4.1]. No significant difference in the two groups with no ulceration of primary melanoma at baseline
-
Number and Phenotype of injected TIL and specificity
Investigations into the number and phenotypes of injected TIL (n = 21 patients), and specificity of TIL (n = 12 patients) for whom autologous melanoma cell line was obtained were performed in patients from one centre. The immunological analysis showed that the difference between the number, nature and specificity of injected TIL was not significant between patients in remission and patients having relapsed. For the number of TIL, HR was 0.94-with a 95% CI of [0.77–1.15], p = 0.54, and for specific TIL, HR was 0.40- with a 95% CI of [0.0006–NA], p = 0.78). See Supplemental Table 1 for details.
-
Immunohistochemistry analysis of the lymph node microenvironment
No significant differences between relapsing and non-relapsing patients were observed between the two groups for melanoma antigen expression, human leukocyte antigens, the profile of cytokines secreted in the tumor microenvironment and the secretion of indoleamine 2,3-dioxygenase (IDO) groups. Refer to Supplemental Table 2 for detailed results.
-
Safety
In total, 812 adverse events (AE) were recorded; 403 (61.2%) were considered definitely, probably or possibly related to treatment. Eighty-one percent (81%) were considered not severe but related to treatment with TIL + IL2 and mainly to IL2 injection. In the TIL group, patients reported mainly fever, tiredness, flu-like symptoms, frequently associated with myalgia and arthralgia, nausea, vomiting and diarrhea, headache and injection site reactions. Four events of vitiligo (2 in each arm) were reported. Tiredness was the most often reported AE (7.6%). AEs resolved in 91.7% of all patients. All were related to IL2 injections and were expected.
Overall, 31 serious AEs (SAEs) were reported for 20 patients; of those, 13 received TIL + IL2. Of 31 SAEs, eight were considered as being related to TIL + IL2, all were expected with IL2. The only SAE attributed to TIL treatment was the reactivation of the human herpes virus-6 which could also be stimulated by IL2. IL2 was suspected as having caused pulmonary embolism and hypereosinophilia in two patients. The remaining SAEs were considered as being related to the disease or its evolution, as well as to comorbidities and intercurrent circumstances. Adverse Events are detailed in Table 3.
Table 3 Adverse Events in abstention arm and TIL + IL2 arm
Discussion
The present trial did not confirm previously obtained results showing that TIL are able to prevent relapse when used in adjuvant setting in stage III melanoma with only one invaded lymph node [13, 14]. Even though the difference between the two patient groups was statistically not significant, we demonstrated a trend to a higher DSF and OS in patients having received TIL.
One of the explanations for these negative results may be the relatively small number of patients. Reasons are multiple: (1) Four (two in TIL arm and two in control arm) patients were included with CT images considered as non-suspect by the radiologist before inclusion, but validated as metastases within 6 months of inclusion, (2) for six patients in the TIL arm (none in the control arm), lymph node dissection was incomplete (less than three lymph nodes) and these patients very rapidly experienced a relapse at the surgery site, (3) three patients (one in the TIL and two in the control group) withdrew their consent and were therefore excluded from the trial.
In addition, an amendment required injecting IL2 at the same time as TIL infusion, instead of the initially proposed IL2 injection after TIL infusion according to the previous adjuvant trial [26]. The initial setting was justified by the objective to evaluate AEs related to IL2 from those related to TIL infusion separately. This amendment led to the exclusion of 19 patients from the statistical analyses (11 from TIL + IL2 arm and eight from the control arm). In addition, two more patients had to be excluded from the PP statistical analysis because they only received one TIL perfusion instead of two.
Another issue which might have contributed to the lower efficacy of infused TIL may be the difference in the TIL amplification method. The replacement of human pooled serum by a synthetic culture medium following a request from the French Health Agency X-VIVO™ Media (Lonza,Levollois-Perret, France) induced a lower TIL amplification compared to the previous trial, which might have impacted the efficacy of the injected TIL. Moreover, when autologous tumor material from patients was available, the tumor reactivity of the generated TIL (tumor-specific TIL) product could be tested in vitro by co-culture of the TIL with the autologous tumor cell lines or tumor digest, with, as read-out, the production of effector cytokines, such as IFN-γ.
In our previous trial, tumor-reactive lymphocytes between 0.21 and 2.7 × 1010 of TIL and between 3 × 106 and 1.12 × 109 were injected during two infusions [27]. The present trial was performed in 21 patients from one hospital site (Nantes, France) and who received TIL doses of only 3.6 × 109 to 17.4 × 109 with a concentration of tumor-reactive lymphocytes between 0.005 × 109 and 2.5 × 109 with a mean of 0.08 × 109. Despite a significant relationship between the infusion of tumor-reactive TIL and a longer relapse-free survival, no correlation could be established between the amounts of total or tumor-reactive TIL infused and the clinical status of treated patients in this trial. Two previously published articles report a correlation between the amount of reactivity of T cells injected against melanoma antigens and the absence of relapse in patients in stage IV melanoma [28, 29].
Thus, we put forward the hypothesis that the lower number of tumor-specific TIL in this trail compared to previous trials might have played a role. Despite this limitation, Zippe et al. reported that from 196 patients with 242 lesions resected to obtain TIL, 121 patients had evaluable biopsies with no effect of metastatic site biopsied on the mean fold TIL expansion [30].
Another reason that could explain why only a nonsignificant difference in the TIL group compared to the control group was observed might be related to the level of immunosuppression of the microenvironment around the lymph node metastasis. Our immunochemistry study focused on the markers used on molecules known to induce an immunosuppressive state of the tumor environment including the programmed cell death protein (PD-1), programmed death ligand (PD-L-1), T cell immunoglobulin and mucin domain-containing protein 3 (TIM-3) as well as IDO expression. In addition, we determined the level of expression of melanoma antigens by the tumor cells and the expression of human leukocyte antigen (HLA) that plays a crucial role in the interaction between tumor cells and T lymphocytes by inducing an inhibition of the T lymphocyte functions as PD1. However, we were unable to show any significant correlation between relapse and a higher expression of inhibitor molecules PD-1, TIM-3 by T lymphocytes. Moreover, there was no correlation between the relapse and PDL-1/IDO expression by the melanoma cells in the either arm.
Our study showed that adjuvant treatment with TIL + IL2 in patients with ulcerated lesions resulted in a higher but nonsignificant DFS than in those having been treated with IL2 alone. This result is of particular interest as it confirms observations made by Eggermont et al. showing that ulceration may be associated with a better efficacy of INFα in adjuvant setting [31]. The hypothesis was that infiltration of ulcerated tumors with a higher number of macrophages and with an up-regulation of pro-inflammatory cytokines is modulated by INFα. Thus, TIL may act with a similar pathway [32]
As for the initial trial, we did not precede TIL infusion by lymphodepletion [13]. Results from this first trial showed, by means of a subgroup analysis, the efficacy of TIL in the prevention of relapse and OS in patients with only one invaded lymph nodes. Therefore, we considered that expected AE and SAE were only related to TIL and/or IL2 injections. The only SAE related to TIL treatment was a reactivation of HHV6 that, however, might also have been stimulated by IL2. Results from our study confirm the good safety and tolerability of the adoptive TIL transfer therapy reported previously [11, 13, 14, 33, 34]. Concerning immune-related events, four patients (two in the TIL-IL2 arm and two in the control arm) experienced vitiligo. These events are to be expected considering the fact that vitiligo is a frequently observed immune reaction associated with melanoma [35]. Interestingly, patients treated with TIL are still in CR after 6 and 7 years of follow-up and, for both patients of the abstention arm, only one has recurred after 4 years of follow-up; the second is still in CR after 7 years of follow-up. The observed cases of vitiligo reflect the state of activation of the immune system which, in addition to tumor cells, sometimes eliminates normal melanocytes, causing skin depigmentation [36]. The adoptive transfer of TIL may enhance the immune system activation status of these patients. In patients with spontaneous vitiligo from the control group, activation of the immune system may be reduced or suppressed by the development of the inhibitory tumor microenvironment, which may explain the recurrence in one of the two patients after 4 years of follow-up.
In conclusion, our randomized adjuvant trial testing TIL + IL2 in stage III melanoma patients with only one invaded lymph node did not confirm previously observed results. Despite the negative outcome, we did show that ulceration of the primary melanoma may be predictive of the efficacy of TIL in melanoma in adjuvant setting, similarly to INF-α.
References
Luke JJ, Flaherty KT, Ribas A, Long GV (2017) Targeted agents and immunotherapies: optimizing outcomes in melanoma. Nat Rev Clin Oncol 14(8):463–482
Svedman FC, Pillas D, Taylor A, Kaur M, Linder R, Hansson J (2016) Stage-specific survival and recurrence in patients with cutaneous malignant melanoma in Europe—a systematic review of the literature. Clin Epidemiol 8:109–122
Wolchok JD, Chiarion-Sileni V, Gonzalez R, Rutkowski P, Grob JJ, Cowey CL et al (2017) Overall survival with combined nivolumab and ipilimumab in advanced melanoma. N Engl J Med 377(14):1345–1356
Rosenberg SA, Lotze MT, Muul LM, Leitman S, Chang AE, Ettinghausen SE et al (1985) Observations on the systemic administration of autologous lymphokine-activated killer cells and recombinant interleukin-2 to patients with metastatic cancer. N Engl J Med 313(23):1485–1492
Rosenberg SA, Yannelli JR, Yang JC, Topalian SL, Schwartzentruber DJ, Weber JS et al (1994) Treatment of patients with metastatic melanoma with autologous tumor-infiltrating lymphocytes and interleukin 2. J Natl Cancer Inst 86(15):1159–1166
Dudley ME, Wunderlich JR, Yang JC, Hwu P, Schwartzentruber DJ, Topalian SL et al (2002) A phase I study of nonmyeloablative chemotherapy and adoptive transfer of autologous tumor antigen-specific T lymphocytes in patients with metastatic melanoma. J Immunother 25(3):243–251
Dudley ME, Wunderlich JR, Robbins PF, Yang JC, Hwu P, Schwartzentruber DJ et al (2002) Cancer regression and autoimmunity in patients after clonal repopulation with antitumor lymphocytes. Science 298(5594):850–854
Dudley ME, Gross CA, Langhan MM, Garcia MR, Sherry RM, Yang JC et al (2010) CD8+ enriched "young" tumor infiltrating lymphocytes can mediate regression of metastatic melanoma. Clin Cancer Res 16(24):6122–6131
Wu R, Forget MA, Chacon J, Bernatchez C, Haymaker C, Chen JQ et al (2012) Adoptive T-cell therapy using autologous tumor-infiltrating lymphocytes for metastatic melanoma: current status and future outlook. Cancer J 18(2):160–175
Andersen R, Donia M, Ellebaek E, Borch TH, Kongsted P, Iversen TZ et al (2016) long-lasting complete responses in patients with metastatic melanoma after adoptive cell therapy with tumor-infiltrating lymphocytes and an attenuated IL2 regimen. Clin Cancer Res 22(15):3734–3745
Dreno B, Nguyen JM, Khammari A, Pandolfino MC, Tessier MH, Bercegeay S et al (2002) Randomized trial of adoptive transfer of melanoma tumor-infiltrating lymphocytes as adjuvant therapy for stage III melanoma. Cancer Immunol Immunother 51(10):539–546
Prendergast GC, Smith C, Thomas S, Mandik-Nayak L, Laury-Kleintop L, Metz R et al (2014) Indoleamine 2,3-dioxygenase pathways of pathogenic inflammation and immune escape in cancer. Cancer Immunol Immunother 63(7):721–735
Khammari A, Nguyen JM, Pandolfino MC, Quereux G, Brocard A, Bercegeay S et al (2007) Long-term follow-up of patients treated by adoptive transfer of melanoma tumor-infiltrating lymphocytes as adjuvant therapy for stage III melanoma. Cancer Immunol Immunother 56(11):1853–1860
Khammari A, Knol AC, Nguyen JM, Bossard C, Denis MG, Pandolfino MC et al (2014) Adoptive TIL transfer in the adjuvant setting for melanoma: long-term patient survival. J Immunol Res 2014:186212
Lotze MT, Frana LW, Sharrow SO, Robb RJ, Rosenberg SA (1985) In vivo administration of purified human interleukin 2. I. Half-life and immunologic effects of the Jurkat cell line-derived interleukin 2. J Immunol 134(1):157–66
Lotze MT, Matory YL, Ettinghausen SE, Rayner AA, Sharrow SO, Seipp CA et al (1985) In vivo administration of purified human interleukin 2. II. Half life, immunologic effects, and expansion of peripheral lymphoid cells in vivo with recombinant IL 2. J Immunol 135(4):2865–75
Oken MM, Creech RH, Tormey DC, Horton J, Davis TE, McFadden ET et al (1982) Toxicity and response criteria of the Eastern Cooperative Oncology Group. Am J Clin Oncol 5(6):649–655
Karnofsky D, Burchenal J (1949) Evaluation of chemotherapeutic agents in cancer. MacLoad, editor. Columbia University Press, New York
Keung EZ, Gershenwald JE (2018) The eighth edition American Joint Committee on Cancer (AJCC) melanoma staging system: implications for melanoma treatment and care. Expert Rev Anticancer Ther 18(8):775–784
Jotereau F, Pandolfino MC, Boudart D, Diez E, Dreno B, Douillard JY et al (1991) High-fold expansion of human cytotoxic T-lymphocytes specific for autologous melanoma cells for use in immunotherapy. J Immunother 10(6):405–411
Pandolfino MC, Labarriere N, Tessier MH, Cassidanius A, Bercegeay S, Lemarre P et al (2001) High-scale expansion of melanoma-reactive TIL by a polyclonal stimulus: predictability and relation with disease advancement. Cancer Immunol Immunother 50(3):134–140
Tessier MH, Pandolfino MC, Jotereau F, Boudart D, Litoux P, Dreno B (1996) Home therapy with autologous tumor-infiltrating lymphocytes and subcutaneous interleukin-2 in metastatic melanoma. Eur J Cancer Engl 32a:735–736
Gervois N, Heuze F, Diez E, Jotereau F (1990) Selective expansion of a specific anti-tumor CD8+ cytotoxic T lymphocyte clone in the bulk culture of tumor-infiltrating lymphocytes from a melanoma patient: cytotoxic activity and T cell receptor gene rearrangements. Eur J Immunol 20(4):825–831
Pandolfino MC, Saiagh S, Knol AC, Dreno B (2010) Comparison of three culture media for the establishment of melanoma cell lines. Cytotechnology 62(5):403–412
Chebassier N, El Houssein O, Viegas I, Dreno B (2004) In vitro induction of matrix metalloproteinase-2 and matrix metalloproteinase-9 expression in keratinocytes by boron and manganese. Exp Dermatol 13(8):484–490
Yee C, Thompson JA, Byrd D, Riddell SR, Roche P, Celis E et al (2002) Adoptive T cell therapy using antigen-specific CD8+ T cell clones for the treatment of patients with metastatic melanoma: in vivo persistence, migration, and antitumor effect of transferred T cells. Proc Natl Acad Sci U.S.A 99(25):16168–16173
Labarriere N, Pandolfino MC, Gervois N, Khammari A, Tessier MH, Dreno B et al (2002) Therapeutic efficacy of melanoma-reactive TIL injected in stage III melanoma patients. Cancer Immunol Immunother 51(10):532–538
Benlalam H, Vignard V, Khammari A, Bonnin A, Godet Y, Pandolfino MC et al (2007) Infusion of Melan-A/Mart-1 specific tumor-infiltrating lymphocytes enhanced relapse-free survival of melanoma patients. Cancer Immunol Immunother 56(4):515–526
Vignard V, Lemercier B, Lim A, Pandolfino MC, Guilloux Y, Khammari A et al (2005) Adoptive transfer of tumor-reactive Melan-A-specific CTL clones in melanoma patients is followed by increased frequencies of additional Melan-A-specific T cells. J Immunol 175(7):4797–4805
Zippel D, Friedman-Eldar O, Rayman S, Hazzan D, Nissan A, Schtrechman G et al (2019) Tissue harvesting for adoptive tumor infiltrating lymphocyte therapy in metastatic melanoma. Anticancer Res 39(9):4995–5001
Eggermont AMM, Robert C, Suciu S (2018) Adjuvant pembrolizumab in resected stage III melanoma. N Engl J Med U S 379:593–595
Jewell R, Elliott F, Laye J, Nsengimana J, Davies J, Walker C et al (2015) The clinicopathological and gene expression patterns associated with ulceration of primary melanoma. Pigment Cell Melanoma Res 28(1):94–104
Rohaan MW, van den Berg JH, Kvistborg P, Haanen JBAG (2018) Adoptive transfer of tumor-infiltrating lymphocytes in melanoma: a viable treatment option. J Immunother Cancer 6(1):102
Zhang X, Zhao Y, Ye Y, Li S, Qi S, Yang Y et al (2015) Lesional infiltration of mast cells, Langerhans cells, T cells and local cytokine profiles in alopecia areata. Arch Dermatol Res 307(4):319–331
Gonzalez R, Torres-Lopez E (2014) Immunological basis of melanoma-associated vitiligo-like depigmentation. Actas Dermosifiliogr 105(2):122–127
Boniface K, Seneschal J, Picardo M, Taieb A (2018) Vitiligo: focus on clinical aspects, immunopathogenesis, and therapy. Clin Rev Allergy Immunol 54(1):52–67
Acknowledgements
The authors acknowledge the participation of the patients in this clinical trial and the writing and editing support of Karl Patrick Göritz, SMWS—Scientific and Medical Writing Services, France.
Funding
This study was performed within the LabEX IGO program, supported by the National Research Agency via the investment of the future program ANR-11-LABX-0016-01. Chiron France kindly provided interleukin-2.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
The trial complied with all French (AgenceNationale de Sécurité du Médicament et des Produits de Santé, ANSM,” Loi n° 2012–300, 5 Mars2012 relative aux recherchesimpliquant la personnehumaine” and all its amendments) and European requirements for the conduct of clinical trials (EuroepanMedicinec Agency, EMA, CPMP/ICH/377/95, EMEA) and the principles of Good Clinical Practices, the 1964 Declaration of Helsinki, received approval for all study sites from the ethics committee of Nantes, France in December 2003 (CPP Ouest IV Nantes n° 553/2005) and is registered at ClinicalTrials.gov under the Identifier: NCT00200577. All patients provided written informed consent prior to inclusion.
Conflict of interest
The authors have no conflict of interest to disclose.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
This paper reports results from an open-label, randomized two-group, multicenter five-year trial in adult stage III melanoma patients with only one invaded lymph node after complete resection.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Khammari, A., Nguyen, JM., Leccia, MT. et al. Tumor infiltrating lymphocytes as adjuvant treatment in stage III melanoma patients with only one invaded lymph node after complete resection: results from a multicentre, randomized clinical phase III trial. Cancer Immunol Immunother 69, 1663–1672 (2020). https://doi.org/10.1007/s00262-020-02572-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00262-020-02572-1