Abstract
Chimeric antigen receptor-modulated T lymphocytes (CAR-T) have emerged as a powerful tool for arousing anticancer immunity. Endogenous ligands for tumor antigen may outperform single-chain variable fragments to serve as a component of CARs with high cancer recognition efficacy and minimized immunogenicity. As heterodimerization and signaling partners for human epidermal growth factor receptor 2 (HER2), HER3/HER4 has been implicated in tumorigenic signaling and therapeutic resistance of breast cancer. In this study, we engineered T cells with a CAR consisting of the extracellular domain of heregulin-1β (HRG1β) that is a natural ligand for HER3/HER4, and evaluated the specific cytotoxicity of these CAR-T cells in cultured HER3 positive breast cancer cells and xenograft tumors. Our results showed that HRG1β-CAR was successfully constructed, and T cells were transduced at a rate of 50%. The CAR-T cells specifically recognized and killed HER3-overexpressing breast cancer cells SK-BR-3 and BT-474 in vitro, and displayed potent tumoricidal effect on SK-BR-3 xenograft tumor models. Our results suggest that HRG1β-based CAR-T cells effectively suppress breast cancer driven by HER family receptors, and may provide a novel strategy to overcome cancer resistance to HER2-targeted therapy.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Adoptive immunotherapy has showed broad potential in cancer treatment in the past 30 years. In particular, T lymphocytes genetically engineered to express chimeric antigen receptors (CARs) specific for a tumor associated antigen (TAA) have demonstrated spectacular antitumor activity in clinical studies, especially for hematological malignancies, such as chronic lymphocytic leukemia (CLL) and acute lymphoblastic leukemia (ALL) [1,2,3,4]. Although the findings for CAR-T therapy in solid tumors are less persuasive, new targets for solid tumors are being tested for CAR-T therapy in pre-clinical and clinical trials [5,6,7]. The basic design of CARs is composed of an extracellular antigen recognition domain, typically a single-chain variable fragment (scFv), linked to an intracellular signaling module that includes CD3ζ chain to induce T-cell activation upon antigen binding. To enhance efficacy and longevity of CAR-T cells, the construct can be further modified. Second-generation and third-generation CARs are engineered to additionally express one or two costimulatory signaling domains, such as CD28, 4-1BB or OX40. Fourth-generation CARs have incorporated cytokines or costimulatory ligands to further improve their antitumor capability [8,9,10]. As the basic component of CARs, scFv is constructed by cloning the variable heavy and variable light chains of an antigen-specific monoclonal antibody to enable antigen recognition [11]. However, scFvs do not represent the sole option for antigen binding of CARs.
Heregulin (HRG) is a soluble secreted growth factor, also known as neuregulin (NRG), sensory and motor neurons induced factor (SMDF), Neu differentiation factor (NDF), glial growth factor (GGF), and acetylcholine receptor-inducing activity (ARIA) [12]. The HRG gene family consists of four members, HRG-1, HRG-2, HRG-3 and HRG-4. At least 15 different HRG isoforms are encoded due to alternative splicing. HRGs contain an epidermal growth factor (EGF)-like sub-domain, which binds to the HER3/4 receptors and induces heterodimerization between members of the HER tyrosine kinase receptor family. The follow-up signaling leads to cell proliferation, invasion, survival and differentiation of normal and malignant tissues. Due to its higher receptor affinity, the β isoform of HRG1 is considered to be a potential therapeutic target in HER3/4 positive tumors. In this study, we construct a second-generation CAR containing the extracellular domain of HRG1β and the fused 4-1BB/CD3ζ endodomains to achieve full T-cell activation. The antitumor activity of HRG1β-CAR-T cells was further validated in HER3-overexpressing breast cancer cell lines and xenograft models.
Materials and methods
Cell culture
The human breast cancer cell lines MDA-MB-468, SK-BR-3 and BT-474, an immortalized mammary epithelial cell line, MCF-10A, a normal liver cell line, L02 (hepatocyte), and human embryonic kidney (HEK) 293T cells were obtained from the Type Culture Collection of the Chinese Academy of Sciences (Shanghai, China). Human umbilical vein endothelial cells (HUVECs) were isolated from the umbilical cord and cultured in EBM-2 media (Lonza, Basel, Switzerland). MCF-10A cells were grown in MEGM media (Lonza) with 100 ng/mL cholera toxin (Sigma-Aldrich, St. Louis, MO, USA). MDA-MB-468 cells were cultured in complete medium composed of Leibovitz’s L-15 (Life Technologies, Carlsbad, CA, USA) and 10% FBS (Life Technologies). L-02, SK-BR-3 and BT-474 cells were cultured in RPMI 1640 medium (Life Technologies) supplemented with 10% FBS. SK-BR-3-luciferase cells were generated by infection of cells with luciferase-expressing recombinant lentiviruses (GeneChem, Shanghai, China). HEK-293T and SK-BR-3-luciferase cells were maintained in DMEM (Life Technologies) supplemented with 10% FBS.
PBMC isolation and T-cell magnetic bead separation
Blood samples were obtained from healthy volunteers under an institutional review board-approved protocol. PBMCs were isolated by low-density centrifugation on Lymphoprep (Dakewei, Beijing, China), and cultured with LEAF™ Purified anti-human CD3/CD28 (BioLegend, San Diego, CA, USA) in SuperCulture™ L500 (Dakewei), 100 IU/mL IL-2 (PeproTech, Rocky Hill, NJ, USA) for 3 days. T cells were negatively purified by MojoSort™ Human CD3 T-Cell Isolation Kit (BioLegend). Purified T cells were maintained in the above medium, with the addition of 100 IU/mL IL-2 every other day.
Construction of a plasmid carrying the HRG1β-CAR
The sequence of HRG1β-CAR was synthesized by Sangon Biotech (Shanghai, China). It consisted of a kozak sequence, human CD8α signal peptide, human HRG1β extracellular domain, human CD8α hinge and transmembrane domains, and 4-1BB and CD3ζ cytoplasmic domains. Primers used for the PCR amplification were as follows: Forward-BamH1: 5′-TTTGGATCCCGCCACCATGGCCTTACCAGTGAC-3′, reverse-EcoR1: 5′-TTTGAATTCTTAGCGAGGGGGCAGGGCCTGCAT-3′. The full-length DNA was ligated into the pENTR3C plasmid (Invitrogen, Carlsbad, CA, USA) and recombined into the lentiviral vector pLenti6.3/V5-DEST (Invitrogen) by Gateway® LR Clonase® Enzyme Mix (Invitrogen).
Lentivirus production and cell infection
Lentiviral particles were produced by transfecting HEK-293T cells with the lentiviral expression plasmid and the packaging plasmids. Briefly, HEK-293T cells were seeded into a 100 mm dish, and Lipofectamine 2000 (Invitrogen) was used according to the manufacturer’s instructions. The following amounts of DNA per 100 mm dish were used: 12 μg of HRG1β-CAR transgene plasmid, 3 μg of pMD2.G plasmid (Invitrogen), and 9 μg of packaging psPAX2 plasmid (Invitrogen). Two collections of viral supernatant were made 48 and 72 h after transfection. After filtering the collections through a 0.45-μm filter, virus-containing supernatant was concentrated by PEG-8000 (Sigma-Aldrich) and frozen at − 80 °C for later use.
HEK-293T cells were seeded in a 6-well plate and incubated with lentiviral supernatant at the confluence of 50%. Cells were collected after 48, 72 h for flow cytometry analysis and western blotting to examine the efficiency of infection. Purified and activated CD3-positive T cells were incubated with lentiviral supernatant in a 12-well plate, and centrifuged at 2000 rpm for 2 h. Cells were collected and subjected to further expansion and functional assays.
Western blot analysis
Cells were washed in phosphate buffered saline (PBS) twice before proteins were extracted. Proteins were separated on a SDS/PAGE gel, transferred onto a polyvinylidene fluoride (PVDF) membrane and subjected to immunoblot analysis. Blotting was performed using antibodies targeting CD3ζ (Sigma-Aldrich), HER3 (Cell Signaling Technology, Danvers, MA, USA), β-actin (Sigma-Aldrich). Goat anti-rabbit and goat anti-mouse immunoglobulin horseradish peroxidase-linked F(ab)2 fragments (JingCai, Xi’an, China) were used as secondary antibodies.
Flow cytometry
HRG1β-CAR-T cells were detected by staining with rabbit anti-human HRG1 antibody (Abnova, Taibei, China) and PE-conjugated donkey anti-rabbit IgG (BioLegend) as a secondary antibody. Cell lines were labeled with anti-CD3-PE, anti-CD69-PE, anti-CD4-FITC, anti-CD8-FITC, anti-HER2-PE, anti-HER3-PE (BioLegend), and anti-HER4-PE (R&D Systems, Minneapolis, MN, USA) mAbs and examined using a FACS scan flow cytometer (Beckman Coulter, Brea, CA, USA). Matched isotype control antibodies were used in all analyses. For assays of T-cell cytotoxicity, target cells were subjected to carboxyfluorescein succinimidyl ester (CFSE) and propidium iodide (PI, BioLegend) double staining and flow cytometry analysis. Briefly, tumor cells were collected and incubated with 5 µM/L CFSE at 37 °C, keeping protected from light for 20 min. RPMI 1640 medium was then added to the cells and incubated at 37 °C for 10 min followed by centrifugation at 1000 rpm for 5 min. Labeled target cells were mixed with effector cells at indicated E:T ratios. After 4-h incubation, cells were stained with PI and analyzed by flow cytometry. The mean percentage of specific lysis of triplicate replicates was calculated via the following formula: Cytotoxicity (%) = 100 × [Dead target cells in the sample (%) − Spontaneous dead target cells (%)]/[100 − Spontaneous dead target cells (%)].
Enzyme-linked immunosorbent assay (ELISA)
1 × 105 HRG1β-CAR-T cells were co-incubated with MDA-MB-468, SK-BR-3, BT-474 cells in 96-well flat-bottom for 24 h. IFN-γ and IL-2 in culture supernatants were measured using an ELISA kit (Dakewei). The optical absorbance of the samples was measured at 450 nm using a microplate reader (Bio-Rad, Hercules, CA, USA).
Small interfering RNA (siRNA) transfection and quantitative RT-PCR
HER3-targeting and negative control siRNAs were obtained from GenePharma (Shanghai, China): negative control sense 5′-UUCUCCGAACGUGUCACGUTT-3′ and antisense 5′-ACGUGACACGUUCGGAGAATT-3′, si-508 sense 5′-GUGGAUUCGAGAAGUGACATT-3′ and antisense 5′-UGUCACUUCUCGAAUCCACTT-3′, si-1311 sense 5′-GCAACAUUGAUGGAUUUGUTT-3′ and antisense 5′-ACAAAUCCAUCAAUGUUGCTT-3′, si-1825 sense 5′-CUUGUCCUGUCGAAAUUAUTT-3′ and antisense 5′-AUAAUUUCGACAGGACAAGTT-3′. Each siRNA was used for transfection of cells at a concentration of 50 nM.
Total RNA was extracted from cells using TRIzol reagent (Sigma-Aldrich) according to the manufacturer’s protocol. Reverse transcription was performed using PrimeScript™ RT Master Mix (Takara, Shiga, Japan), and cDNAs were amplified and detected using FastStart Universal SYBR Green Master (Roche, Basel, Switzerland). β-actin was used to normalize the quantitative real-time polymerase chain reaction (qRT-PCR) data. The following primers were used for qRT-PCR amplification: 5′-TGCAGTGGATTCGAGAAGTG-3′ and 5′-GGCAAACTTCCCATCGTAGA-3′ for HER3, 5′-TGGCATCCACGAAACTACC-3′ and 5′-GTGTTGGCGTACAGGTCTT-3′ for β-actin.
Xenograft tumor models and treatment
The protocol for animal study was approved by the Ethics Committee of the Fourth Military Medical University (Xi’an, China). Athymic Balb/c nude mice (5–6-weeks-old, female) were randomly assigned into two groups; ten million SK-BR-3 tumor cells with matrix were injected subcutaneously into the right back near the thigh of nude mice, followed by tail intravenous treatment with 1 × 107 control or HRG1β-CAR-T cells on days 7, 14, 21. Tumor diameter was measured with a Vernier caliper 7 days after injection and recorded every other week. Tumor volume was calculated as the 1/2 × (length × width2), where the length and width are the longest and shortest axes in millimeters. Animal survival was recorded and plotted, or alternatively, mice were killed and tumors were excised for further analysis. For bioluminescent imaging of in vivo tumors, athymic Balb/c nude mice (5–6-weeks-old, female) received injection of 6 × 106 luciferase-expressing SK-BR-3 cells with matrix as aforementioned, followed by tail intravenous treatment with PBS, 1 × 107 control T cells or HRG1β-CAR-T cells on day 14. Mice were injected intraperitoneally (i.p.) with 150 mg/kg d-luciferin (Gold Biotechnology, St. Louis, MO, USA) and images were acquired 10 min later using Xenogen IVIS® Lumina II and Living Image 4.3.1 software (Caliper Life Sciences, Hopkinton, MA, USA). At the 35th day, all mice were killed, and tumors were excised for further analysis.
Immunohistochemistry (IHC)
The streptavidin-peroxidase (SP) method was used to detect the infiltration of T cells in breast cancer samples by immunohistochemistry. Briefly, tissues were sectioned, treated with 3% H2O2, and then incubated in 5% rabbit serum. Primary rabbit anti-human CD3 (Maixin Biotech, Fuzhou, China) was added to serial tissue sections and incubated at room temperature (RT) for 60 min, followed by incubation with a biotin-labeled secondary antibody at RT for 30 min. SP complex was added and then DAB-H2O2 was used for color development before microscopy.
Statistical analysis
All data are expressed as mean ± standard error of mean (SEM) (n ≥ 3). Data were analyzed using GraphPad Prism version 5.00 for Windows. Student’s t test was used to evaluate differences between paired groups, Differences at P values < 0.05 were considered statistically significant.
Results
Construction and transient expression of HRG1β-CAR in HEK-293T cells
The HRG1β-CAR consists of a signal peptide leader sequence of CD8α (SP), the extracellular binding domain of HRG1β, the hinge spacer and the transmembrane region of CD8α, the costimulatory molecule 4-1BB intracellular domain and the CD3ζ signaling moieties (Fig. 1a). The full-length DNA was ligated into pENTR3C, and recombined with pLenti6.3/V5-DEST. The resultant DNA constructs were verified by restriction digest and agarose gel electrophoresis (Fig. 1b). HEK-293T cells were infected with the lentiviral supernatant and the expression of HRG1β-CAR was examined. Approximately 90% of HEK-293T cells expressed HRG1β-CAR after infection (Fig. 1c). Western blotting using an anti-CD3ζ antibody detected an approximately 47 kDa protein (Fig. 1d), suggesting the successful expression of the chimeric protein in HEK-293T cells transduced with HRG1β-CAR constructs.
Construction and transient expression of HRG1β-CAR in HEK-293T cells. a Lentiviral vector construct of HRG1β-CAR. SP signal peptide, ECD extracellular domain, TM transmembrane portion, ICP intracellular portion. b Agarose gel electrophoresis of PCR and restricted DNA products. Left M, 1 kb DNA marker, right M, DL5000 DNA marker, lane 1, two restricted DNA products: pLenti6.3/V5-DEST plasmid (9387 bp) and HRG1β-CAR gene(1312 bp), lane 3, two restricted DNA products: pENTR3C plasmid (2723 bp) and HRG1β-CAR gene(1312 bp), Lane 2 and 4, PCR product: HRG1β-CAR gene (1312 bp). c HRG1 was detected by flow cytometry in HEK-293T cells transduced with HRG1β-CAR. d CD3ζ was detected by western blotting in HEK-293T cells transduced with HRG1β-CAR. Data are representative images of 3 independent experiments
Expression of HRG1β-CAR in activated T lymphocytes
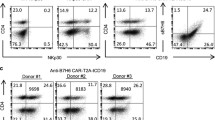
PBMCs were isolated from healthy volunteers’ blood, and analyzed via flow cytometry. The percentage of CD3-positive cells was 56.4%, while CD4 and CD8-positive T cells were 29.1 and 27.5%, respectively. After 1-week culture, the percent of CD3-positive cells was increased to about 74% (Fig. 2a). T cells were obtained from PBMC by magnetic bead separation. One week after culture in the presence of anti-CD3/CD28 antibodies, 90% of the sorted cells are CD3-positive, while 35% cells expressed the antigen of activated T cells, CD69 (Fig. 2b). T cells were infected with the lentiviral vector containing the HRG1β-CAR construct. As a result, approximately 50% of T cells expressed HRG1β-CAR as determined by fluorescence-activated cell sorting (FACS) analysis (Fig. 2c), suggesting the successful generation of HRG1β-CAR-modulated T cells.
Expression of HRG1β-CAR in activated T lymphocytes. a PBMCs were isolated from healthy volunteers’ blood. After isolation, cells were labeled with anti-CD3-PE, anti-CD4-FITC, anti-CD8-FITC, and detected by flow cytometry. b CD3-positive T cells were obtained from PBMC by magnetic bead separation, 1 week after anti-CD3/CD28 activated culture, and were subjected to flow cytometry analysis. c Expression of HRG1β was detected by flow cytometry in activated T-lymphocytes transduced with HRG1β-CAR. Data are representative of PBMCs from five donors
Specific lysis of HER3-positive breast cancer cells by HRG1β-CAR-T cells in vitro
HRG1β is an endogenous ligand for HER3 and HER4 receptors. The expression of different HER family of receptors in breast cancer cell lines was examined by flow cytometry. The results show that SK-BR-3 and BT-474 cell lines expressed high level of HER2 and HER3, but low level of HER4, while none of these HER family members was detected on the surface of MDA-MB-468 cells or 3 non-malignant cell lines (Fig. 3a and Supplementary Fig. 1). Thus, these cells can be used for the examination of specific targeting and cytolysis by HRG1β-CAR-T cells. To determine whether T cells expressing HRG1β-CAR were activated by and cytotoxic to breast cancer cells expressing HER3, we co-incubated CAR-T cells with cells overnight and measured the secretion of cytokines IFN-γ and IL-2. CAR-T cells co-incubated with SK-BR-3 and BT-474 cells, but not HER3-negative MDA-MB-468 or MCF-10A cells produced high levels of IFN-γ and IL-2 when compared with control T cells (Fig. 3b). Next, we evaluated the cytotoxicity of HRG1β-CAR-T cells against HER3-positive tumor cells using CFSE/PI double staining. As a result, these CAR-T cells specifically lysed HER3-positive SK-BR-3 and BT-474 cells, but not HER3-negative cells (Fig. 3c and Supplementary Fig. 1).
Specific lysis by HRG1β-CAR-T cells in vitro. a Breast cancer cell lines and an immortalized mammary epithelial cell line, MCF-10A, were stained, respectively, with monoclonal antibodies specific for the HER2/HER3/HER4 antigen and analyzed by flow cytometry. b ELISA for cytokine production by HRG1β-CAR and control T cells after overnight incubation with the indicated target cells. c Antigen-specific killing of target cells by control T or HRG1β-CAR-T cells was determined using CFSE/PI double staining at the indicated E:T ratio. Data are represented as the mean ± SEM of n = 3 replicates or representative of three independent experiments from three donors. **P < 0.01, ***P < 0.001 (Student’s t test)
To further verify the specific HER3 targeting by HRG1β-CAR-T cells, we knocked down HER3 in SK-BR-3 and BT-474 cell lines. Both mRNA and protein expression levels of HER3 were significantly downregulated by synthesized siRNAs (Fig. 4a–c). The cytotoxicity of CAR-T cells to HER3-positive target cells was improved with the increasing E:T ratios (Fig. 4d). HER3 silencing reduced the killing of these target cells by HRG1β-CAR-T cells to a level comparable to the non-specific cytolysis by control T cells (Fig. 4d). These data suggest that HRG1β-CAR-T cells are a potent killer for HER3-overexpressing breast cancer cells.
HER3 knockdown confers resistance of breast cancer cells to cytolysis by HRG1β-CAR-T cells. a–c SK-BR-3 cells were transfected with indicated siRNAs, and subject to qRT-PCR (a), western blotting (b) and flow cytometry (c) analysis. d Lysis of target cells by control T or HRG1β-CAR-T cells after 4-h incubation at increasing E:T ratios. Data are represented as the mean ± SEM of n = 3 replicates or representative of three independent experiments. *P < 0.05, **P < 0.01, ***P < 0.001 (Student’s t test)
HRG1β-CAR-T cells exert antitumor activity in vivo
To evaluate the antitumor ability of HRG1β-CAR-T cells in vivo, we generated a xenograft tumor model via inoculation of nude mice with SK-BR-3 cells. The intravenous infusion of CAR-T but not control T lymphocytes significantly suppressed tumor growth (Fig. 5a–c), and prolonged the survival of tumor-bearing animals (Fig. 5d). In parallel, bioluminescent imaging of xenograft breast carcinoma derived from luciferase-expressing SK-BR-3 cells showed shrinkage of tumors upon intravenous administration of HRG1β-CAR-T cells but not control T cells or PBS (Fig. 5e, f). Immunohistochemical analysis revealed apparent infiltration of CD3-positive T cells in the tumor tissues in mice receiving treatment with CAR-T cells (Fig. 5g). These results indicated that HRG1β-CAR-T cells specifically target and significantly repress HER3-positive tumors in vivo.
T-lymphocytes expressing HRG1β-CAR exert antitumor activity in vivo. a–c Nude mice (n = 10) were injected subcutaneously with SK-BR-3 cells in the right back to form xenograft tumors, followed by random grouping and weekly tail vein administration of control or HRG1β-CAR-T cells. Tumor volume was monitored and plotted (a). Mice were killed on day 42, and tumors were excised and weighed (b, c). d Xenograft tumors were established and mice were treated as described in (a–c, n = 6 for each group). The survival of mice was monitored and plotted. e–g Nude mice were inoculated with SK-BR-3 cells expressing luciferase (n = 3 for each group), followed by tail intravenous treatment with PBS, control T and HRG1β-CAR-T cells on day 14. Bioluminescent imaging was performed on indicated days (e), and region-of-interest (ROI) bioluminescence emission measurement was calculated for each group (f). Mice were killed on day 35, and tumors were excised, sectioned and subject to immunohistochemical staining for CD3 (g). The numbers of CD3-positive cells in 9 independent microscope fields were plotted. Magnification, ×400. Scale bar = 100 μm. *P < 0.05, **P < 0.01 (Student’s t test)
Discussion
CAR-T cells recognize the target antigen through the antigen binding domain and are activated independently of major histocompatibility complex (MHC) presentation, thereby overcoming the immune escape of tumor cells caused by declined expression of HLA [13, 14]. Despite the accumulating reports on CAR-T-cell therapy targeting various antigens, e.g., CD19 and CD20, the inadequate tumor specificity of targeted antigens has frequently led to the immune toxicity and off-target effects of CAR-T cells [15, 16]. Here, we generated HER3/HER4-targeting CAR-T cells using HRG1β, an endogenous ligand for these tumorigenic receptors. As expected, the resulting CAR-T cells effectively recognized and lysed HER3-positive breast cancer cells in vitro and in a xenograft tumor model. Unlike a majority of CARs based on antibody recognition of tumor cells, we utilized a natural ligand for tumor antigens, which warrants a high affinity to malignant cells in the tumor environment, as well as minimized immunogenicity of the chimeric proteins compared with humanized scFvs [17, 18].
HER2 represents the most important biomarker for approximately 25% of breast cancers. HER2 forms heterodimers with HER3 or HER4 to activate downstream signaling that drives carcinogenesis and maintains the malignant phenotypes of cells [19, 20]. The past decades has witnessed the development and successful application of HER2-targeted therapeutics, e.g., the humanized monoclonal antibody, trastuzumab, and tyrosine kinase inhibitors exemplified by lapatinib. Unfortunately, cancer resistance to these drugs almost inevitably arises due to mutation of this oncogenic protein or activation of alternative growth factor receptor pathways, thereby becoming the major limitations for HER2-based treatment. To this end, targeting the mandatory partners for HER2 signaling may provide effective approaches to circumventing cancer resistance to the aforementioned therapeutics. HER3 expression was found in 20% breast cancers and is highly relevant to HER2, qualifying HER3 as alternative therapeutic targets for HER2-positive neoplasm [21]. Thus, we generated CAR-T cells to target HER3 and demonstrated the efficacy of these cells to fight HER2-positive breast malignancies. Nevertheless, further studies are required to determine whether these CAR-T cells are cytotoxic to breast cancers refractory to HER2-targeted therapy and whether they display synergistic anticancer capabilities.
HRGs are well-characterized endogenous ligands for HER3 and HER4 in various tissues. There are four structurally related genes encoding HRGs such as HRG1, HRG2, HRG3, and HRG4, of which HRG1 has been intensively studied [22]. Of the HRG-1 isoforms which are subdivided into three groups (types I, II, and III), the α and β isoforms both bind to the ErbB3 and ErbB4 receptors, while the γ variant does not bind or activate the receptors. HRG1β shows higher receptor affinity than HRG1α [23]. In the present study, the extracellular domain of HRG1β was used as the binding domain of CAR targeting HER3. Compared with previous reports of HRG-based first generation CARs [24, 25], we created here CARs containing 4-1BB in the cytoplasmic domain, which is capable of delivering a costimulatory signal and hopefully improving the in vivo expansion and persistence of the transfected T cells. In addition, we transduced T cells with both cytokine-secreting CD4 and the cytotoxic CD8 populations, which will synergistically achieve full T-cell activation and exhibit persistent suppression on HER3-positive breast carcinoma.
The appropriate balance of efficacy and safety profiles is the main concern of CAR-T immunotherapy [9]. While the expression of HER3 in a variety of malignancies may warrant a wide applicability of heregulin-based CAR-T cells, further investigations are needed to define whether these CAR-T cells exhibit a common cytolytic activity on cancers overexpressing different homodimers or heterodimers formed by HER3 or HER4 [12, 26]. To this end, Davies et al. achieved flexible targeting of ErbB dimers that drive tumorigenesis using a promiscuous ErbB ligand, T1E [27]. The expression of HER3 in some normal tissues also raised the safety issue of CAR-T cells using heregulin as a recognition ligand [26]. Although our study using xenograft breast cancer models detected no obvious cytotoxicity on normal tissues probably due to a relatively low expression of HER3/HER4 or the lack of cross-binding of murine receptors by human heregulin, it is still possible that these CAR-T cells are also detrimental to normal tissues. Strategies including the generation of switchable dual-receptor CAR-T cells or induced homing of CAR-T cells to the tumor loci are beneficial to improving the specificity of these engineered T cells [28, 29]. Nevertheless, the present study provides novel strategies to suppress breast cancers driven by HER family receptors.
Abbreviations
- ALL:
-
Acute lymphoblastic leukemia
- ARIA:
-
Acetylcholine receptor-inducing activity
- CFSE:
-
Carboxyfluorescein succinimidyl ester
- CLL:
-
Chronic lymphocytic leukemia
- EGF:
-
Epidermal growth factor
- HRG:
-
Heregulin
- HUVEC:
-
Human umbilical vein endothelial cell
- NDF:
-
Neu differentiation factor
- NRG:
-
Neuregulin
- PI:
-
Propidium iodide
- PVDF:
-
Polyvinylidene fluoride
- qRT-PCR:
-
Quantitative real-time polymerase chain reaction
- RT:
-
Room temperature
- SEM:
-
Standard error of mean
- SiRNA:
-
Small interfering RNA
- SMDF:
-
Sensory and motor neurons induced factor
References
Kochenderfer JN, Rosenberg SA (2013) Treating B-cell cancer with T cells expressing anti-CD19 chimeric antigen receptors. Nat Rev Clin Oncol 10(5):267–276. https://doi.org/10.1038/nrclinonc.2013.46
Grupp SA, Kalos M, Barrett D, Aplenc R, Porter DL, Rheingold SR, Teachey DT, Chew A, Hauck B, Wright JF, Milone MC, Levine BL, June CH (2013) Chimeric antigen receptor-modified T cells for acute lymphoid leukemia. N Engl J Med 368(16):1509–1518. https://doi.org/10.1056/NEJMoa1215134
Maude SL, Frey N, Shaw PA, Aplenc R, Barrett DM, Bunin NJ, Chew A, Gonzalez VE, Zheng Z, Lacey SF, Mahnke YD, Melenhorst JJ, Rheingold SR, Shen A, Teachey DT, Levine BL, June CH, Porter DL, Grupp SA (2014) Chimeric antigen receptor T cells for sustained remissions in leukemia. N Engl J Med 371(16):1507–1517. https://doi.org/10.1056/NEJMoa1407222
Lee DW, Kochenderfer JN, Stetler-Stevenson M, Cui YK, Delbrook C, Feldman SA, Fry TJ, Orentas R, Sabatino M, Shah NN, Steinberg SM, Stroncek D, Tschernia N, Yuan C, Zhang H, Zhang L, Rosenberg SA, Wayne AS, Mackall CL (2015) T cells expressing CD19 chimeric antigen receptors for acute lymphoblastic leukaemia in children and young adults: a phase 1 dose-escalation trial. Lancet 385(9967):517–528. https://doi.org/10.1016/s0140-6736(14)61403-3
Gilham DE, Debets R, Pule M, Hawkins RE, Abken H (2012) CAR-T cells and solid tumors: tuning T cells to challenge an inveterate foe. Trends Mol Med 18(7):377–384. https://doi.org/10.1016/j.molmed.2012.04.009
Posey AD Jr, Schwab RD, Boesteanu AC, Steentoft C, Mandel U, Engels B, Stone JD, Madsen TD, Schreiber K, Haines KM, Cogdill AP, Chen TJ, Song D, Scholler J, Kranz DM, Feldman MD, Young R, Keith B, Schreiber H, Clausen H, Johnson LA, June CH (2016) Engineered CAR T cells targeting the cancer-associated Tn-glycoform of the membrane mucin MUC1 control adenocarcinoma. Immunity 44(6):1444–1454. https://doi.org/10.1016/j.immuni.2016.05.014
Burga RA, Thorn M, Point GR, Guha P, Nguyen CT, Licata LA, DeMatteo RP, Ayala A, Espat NJ, Junghans RP, Katz SC (2015) Liver myeloid-derived suppressor cells expand in response to liver metastases in mice and inhibit the anti-tumor efficacy of anti-CEA CAR-T. Cancer Immunol Immunother 64(7):817–829. https://doi.org/10.1007/s00262-015-1692-6
Dai H, Wang Y, Lu X, Han W (2016) Chimeric antigen receptors modified T-cells for cancer therapy. J Natl Cancer Inst 108(7). https://doi.org/10.1093/jnci/djv439
Fesnak AD, June CH, Levine BL (2016) Engineered T cells: the promise and challenges of cancer immunotherapy. Nat Rev Cancer 16(9):566–581. https://doi.org/10.1038/nrc.2016.97
Srivastava S, Riddell SR (2015) Engineering CAR-T cells: design concepts. Trends Immunol 36(8):494–502. https://doi.org/10.1016/j.it.2015.06.004
Hammill JA, VanSeggelen H, Helsen CW, Denisova GF, Evelegh C, Tantalo DG, Bassett JD, Bramson JL (2015) Designed ankyrin repeat proteins are effective targeting elements for chimeric antigen receptors. J Immunother Cancer 3:55. https://doi.org/10.1186/s40425-015-0099-4
Breuleux M (2007) Role of heregulin in human cancer. Cell Mol Life Sci 64(18):2358–2377. https://doi.org/10.1007/s00018-007-7120-0
Batlevi CL, Matsuki E, Brentjens RJ, Younes A (2016) Novel immunotherapies in lymphoid malignancies. Nat Rev Clin Oncol 13(1):25–40. https://doi.org/10.1038/nrclinonc.2015.187
Khalil DN, Smith EL, Brentjens RJ, Wolchok JD (2016) The future of cancer treatment: immunomodulation, CARs and combination immunotherapy. Nat Rev Clin Oncol 13(5):273–290. https://doi.org/10.1038/nrclinonc.2016.25
Bachireddy P, Burkhardt UE, Rajasagi M, Wu CJ (2015) Haematological malignancies: at the forefront of immunotherapeutic innovation. Nat Rev Cancer 15(4):201–215. https://doi.org/10.1038/nrc3907
Kudo K, Imai C, Lorenzini P, Kamiya T, Kono K, Davidoff AM, Chng WJ, Campana D (2014) T lymphocytes expressing a CD16 signaling receptor exert antibody-dependent cancer cell killing. Cancer Res 74(1):93–103. https://doi.org/10.1158/0008-5472.CAN-13-1365
Scott AM, Wolchok JD, Old LJ (2012) Antibody therapy of cancer. Nat Rev Cancer 12(4):278–287. https://doi.org/10.1038/nrc3236
Vanneman M, Dranoff G (2012) Combining immunotherapy and targeted therapies in cancer treatment. Nat Rev Cancer 12(4):237–251. https://doi.org/10.1038/nrc3237
De Keulenaer GW, Doggen K, Lemmens K (2010) The vulnerability of the heart as a pluricellular paracrine organ: lessons from unexpected triggers of heart failure in targeted ErbB2 anticancer therapy. Circ Res 106(1):35–46. https://doi.org/10.1161/CIRCRESAHA.109.205906
Hegde M, Mukherjee M, Grada Z, Pignata A, Landi D, Navai SA, Wakefield A, Fousek K, Bielamowicz K, Chow KK, Brawley VS, Byrd TT, Krebs S, Gottschalk S, Wels WS, Baker ML, Dotti G, Mamonkin M, Brenner MK, Orange JS, Ahmed N (2016) Tandem CAR T cells targeting HER2 and IL13Ralpha2 mitigate tumor antigen escape. J Clin Invest 126(8):3036–3052. https://doi.org/10.1172/JCI83416
Wilson TR, Lee DY, Berry L, Shames DS, Settleman J (2011) Neuregulin-1-mediated autocrine signaling underlies sensitivity to HER2 kinase inhibitors in a subset of human cancers. Cancer Cell 20(2):158–172. https://doi.org/10.1016/j.ccr.2011.07.011
Odiete O, Hill MF, Sawyer DB (2012) Neuregulin in cardiovascular development and disease. Circ Res 111(10):1376–1385. https://doi.org/10.1161/CIRCRESAHA.112.267286
Willem M (2016) Proteolytic processing of neuregulin-1. Brain Res Bull 126(Pt 2):178–182. https://doi.org/10.1016/j.brainresbull.2016.07.003
Altenschmidt U, Kahl R, Moritz D, Schnierle BS, Gerstmayer B, Wels W, Groner B (1996) Cytolysis of tumor cells expressing the Neu/erbB-2, erbB-3, and erbB-4 receptors by genetically targeted naive T lymphocytes. Clin Cancer Res 2(6):11
Muniappan ABB, Lebkowski J, Talib S (2000) Ligand-mediated cytolysis of tumor cells: use of heregulin-zeta chimeras to redirect cytotoxic T lymphocytes. Cancer Gene Ther 7(1):128–134
Amin DN, Campbell MR, Moasser MM (2010) The role of HER3, the unpretentious member of the HER family, in cancer biology and cancer therapeutics. Semin Cell Dev Biol 21(9):944–950. https://doi.org/10.1016/j.semcdb.2010.08.007
Davies DM, Foster J, Van Der Stegen SJ, Parente-Pereira AC, Chiapero-Stanke L, Delinassios GJ, Burbridge SE, Kao V, Liu Z, Bosshard-Carter L, Van Schalkwyk MC, Box C, Eccles SA, Mather SJ, Wilkie S, Maher J (2012) Flexible targeting of ErbB dimers that drive tumorigenesis by using genetically engineered T cells. Mol Med 18:565–576. https://doi.org/10.2119/molmed.2011.00493
Roybal KT, Williams JZ, Morsut L, Rupp LJ, Kolinko I, Choe JH, Walker WJ, McNally KA, Lim WA (2016) Engineering T cells with customized therapeutic response programs using synthetic notch receptors. Cell 167(2):419–432.e416. https://doi.org/10.1016/j.cell.2016.09.011
Sackstein R, Schatton T, Barthel SR (2017) T-lymphocyte homing: an underappreciated yet critical hurdle for successful cancer immunotherapy. Lab Invest 97(6):669–697. https://doi.org/10.1038/labinvest.2017.25
Acknowledgements
This work was supported by the National Natural Sciences Foundation of China (No. 81630069 and 81272646).
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
The authors have no conflict of interest to disclose.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Zuo, BL., Yan, B., Zheng, GX. et al. Targeting and suppression of HER3-positive breast cancer by T lymphocytes expressing a heregulin chimeric antigen receptor. Cancer Immunol Immunother 67, 393–401 (2018). https://doi.org/10.1007/s00262-017-2089-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00262-017-2089-5