Abstract
Regulatory T cells (Tregs) are potently immunosuppressive cells that accumulate within the glioma microenvironment. The reduction in their function and/or trafficking has been previously shown to enhance survival in preclinical models of glioma. Glucocorticoid-induced TNFR-related protein (GITR) is a tumor necrosis factor superfamily receptor enriched on Tregs that has shown promise as a target for immunotherapy. An agonistic antibody against GITR has been demonstrated to inhibit Tregs in a number of models and has only been recently addressed in glioma. In this study, we examined the modality of the antibody function at the tumor site as opposed to the periphery as the blood–brain barrier prevents efficient antibody delivery to brain tumors. Mice harboring established GL261 tumors were treated with anti-GITR monotherapy and were shown to have a significant increase in overall survival (p < 0.01) when antibodies were injected directly into the glioma core, whereas peripheral antibody treatment only had a modest effect. Peripheral treatment resulted in a significant decrease in granzyme B (GrB) expression by Tregs, whereas intratumoral treatment resulted in both a decrease in GrB expression by Tregs and their selective depletion, which was largely mediated by FcγR-mediated destruction. We also discovered that anti-GITR treatment results in the enhanced survival and functionality of dendritic cells (DCs)—a previously unreported effect of this immunotherapy. In effect, this study demonstrates that the targeting of GITR is a feasible and noteworthy treatment option for glioma, but is largely dependent on the anatomical location in which the antibodies are delivered.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Many studies have demonstrated that the immune system can recognize and destroy glioma tissues [1–5]. Despite this, the tumor microenvironment (TME) of glioblastoma multiforme (GBM) is highly immunosuppressive, potently inhibiting anti-tumor responses from occurring [6, 7]. These immunosuppressive factors include myeloid-derived suppressor cell (MDSC) recruitment [8, 9], metabolic constraints [10], and regulatory T cell (Treg) accumulation [11–13]. Tregs are a specialized subset of CD4+ T cells that express the transcription factor Foxp3 [14] have the ability to potently suppress anti-tumor responses. Of clinical significance, such Tregs are known to be found in most malignancies of the body [15]. The accumulation and functional significance of Tregs have been demonstrated in murine models of GBM [16–20] and have also been found to be enriched within glioma tissues of human patients [21–24]. The role Tregs have in suppressing anti-glioma immune responses has been a topic of debate as conflicting studies both support and refute the notion that Treg accumulation is a negative prognostic factor for patient survival [25–28]. Understanding the functional role that Tregs have in inhibiting anti-glioma immunity is critical to the development of their therapeutic benefit, as it is a central target in a number of immunotherapeutic approaches such as anti-CTLA4, anti-CD25, anti-GITR, and denileukin diftitox therapies [29–32].
Glucocorticoid-induced TNFR-related protein (GITR), a tumor necrosis factor superfamily receptor enriched on Tregs [31], has shown promise as a target for immunotherapy. GITR expression is constitutive on Tregs, due to enhancer binding of both Foxp3 and NFκB [33]. Previous studies have suggested that GITR is a superior target of Treg specificity in both murine and human tissues [31, 34]. Further supporting this notion, T cells sorted using the markers CD4+GITR+ can suppress effector T cell proliferation as efficiently as T cells sorted via CD4+Foxp3+ expression [20]. The administration of agonistic antibodies against GITR has previously been shown to enhance animal survival in a number of malignancy settings [35]. While the mechanisms of action are still unclear, FcR-mediated destruction of Tregs [29], activation-induced cell death [36], inhibition of suppressor functions [37], and loss of Treg stability [38] have all been attributed to the function of GITR antibody therapy. Nevertheless, understanding the mechanism of action is critical to enhancing its efficacy for future use in the clinic.
Targeting of this molecule has been recently addressed in glioma [39], and the results of their work suggest that targeting GITR has therapeutic benefit in the context of irradiation and warrant understanding of the mechanisms behind its anti-tumor activities. As the BBB prevents antibodies from efficiently crossing into central nervous system (CNS) tissues [40], we can uniquely determine whether tumoral or systemic administration of anti-GITR influences the efficacy of treatment. This information will be imperative to the future use in the clinic for both CNS and non-CNS malignancies.
Using an implantable murine model of glioma, we determined that targeting Tregs within the tumor after tumor establishment significantly enhances survival in mice with glioma. This survival benefit is dependent on the location of antibody administration and is directly related to the FcR-mediated destruction of Tregs in the TME. We also demonstrate that anti-GITR antibody treatment also has effects on dendritic cell (DC) maturation and function, illuminating another axis by which targeting GITR can have therapeutic efficacy for the treatment of GBM. All of these novel observations are of utmost importance to the future use of GITR-targeting antibodies in the clinic.
Materials and methods
Animal models
OT-1 (Cat# 003831), Foxp3-IRES-GFP B6 (Cat# 006769), perforin knockout (Cat# 002407), and wild-type (Cat# 000664) mice were purchased from Jackson Laboratory (Bar Harbor, ME). FcRγIII knockout mice (Cat# 009637) were obtained from Anne Sperling’s Lab, and OTII-B6 mice were obtained from Qi-Quan Huang at Northwestern University. All animals were maintained by breeding homozygous breeders and verified by genotyping via PCR. All animal protocols were approved by University of Chicago’s Institutional Animal Care and Use Committee. Mice were euthanized by CO2 and cervical dislocation for flow cytometric analysis.
Cell culture and tumor implantation
GL261 cells were obtained from NCI Frederick National Tumor Repository Laboratory and were cultured in DMEM, streptomycin (100 mg/ml) and penicillin (100 U/ml) (Corning, Corning, NY), 10 % Fetal calf serum (GE, Chicago, IL), and incubated at 37 °C with 5 % CO2. Mice were injected with 4 × 105 GL-261 cells in 2.5 μl PBS via intracranial injection, at a 3-mm depth using stereotactic apparatus at 6–8 weeks of age. All surgical procedures were completed in accordance with NIH guidelines on the care and use of laboratory animals for research purposes.
Antibody injections
At 7 and 12 days after GL261 tumor implantation, 10 µg of DTA-1 (BioXcell, Lebanon, NH) was injected via intracranial injection into the same burr-hole as the tumor was initially implanted. Mice were monitored for endpoint analysis, or flow cytometry was performed at the times indicated. For peripheral injections, 500 µg of anti DTA-1 was injected intraperitoneally, at both 7 and 12 days after intracranial implantation of GL-261 tumor.
Bone marrow dendritic cell (BMDC) cultures
Bone marrow was obtained from the long bones of FcRγ−/− B6 mice and plated at a density of 106 cells per 6-well dishes in RPMI (Corning), 10 % FCS, 1 %l-Glutamine, streptomycin (100 mg/ml), and penicillin (100 U/ml). For the maturation of DCs, 40 ng/ml of both GM-CSF and IL-4 (PeproTech, Rocky Hill, NJ) was added to the media. Media were replaced after 3 days, and on the 6th day mature DCs were replated at a density of 5 × 104 per well in U-bottom 96 wells. Two µM of DTA-1 was administered to DCs for all in vitro experiments. For DC-pulsed T cell proliferation assays, BMDC was pulsed with OVA peptides for MHC-class I (SIINFEKL—1 µg/ml) or MHC-class II (ISQAVHAAHAEINEAGR—10 µg/ml) (Sigma) and 2 µM DTA-1. After 24 h, CD8 T cells from OTI mice (CD4+ from OTII mice) were obtained from splenocytes using a CD8 or CD4 T cell isolation kit, respectively (StemCell Technologies, Vancouver, CA), and stained with Cell Trace Violet (Life Technologies, Carlsbad, CA) as previously described [41]. After 72 h, cells were transferred to V-bottom 96 wells and stained for via flow cytometry.
Flow cytometric analyses
For in vitro flow cytometry analysis, cells were harvested and incubated with Fixable Viability dye APC-eFluor 780 (1:1000 dilution, Ebioscience, San Diego, CA) for 20 min. For DC cultures, cells were then stained using directly conjugated antibodies against murine surface antigens, as follows: anti-CD40-PE, anti-CD80-FITC, anti-CD3-PE-Cy7, anti-CD11b-PE, anti-CD11c-APC, anti-CD86-Alexa Fluor 700, anti-CD8-BV605, and anti-MHCII-Percp-Cy5.5 (1:400 dilution, Biolegend, San Diego, CA) and analyzed with a BD LSR-Fortessa Flow Cytometer (Becton–Dickinson, Franklin Lakes, NJ). For OTI T cell analysis, flow cytometry panel was performed as described: anti-CD4-PE, anti-CD8-APC, anti-CD44-Percp-Cy5.5, and anti-CD3 PE/CY7 (1:200, Biolegend). For ex vivo flow cytometry analysis, the directly conjugated antibodies against murine surface antigens are as follows: anti-CD3-Alexa Fluor 700 (1:50), anti-CD4-PE-Cy7 (1:100), anti-CD8-BV605 (1:100), anti-CD44-PE (1:200), and anti-CD62L-APC-Cy7 (1:100) for surface staining (Biolegend). For intracellular staining, cells were fixed/permeabilized stained using the Foxp3 staining kit (Ebioscience) using Foxp3-eFluor 450 (1:100, Ebioscience), granzyme B Alexa Fluor 647 (1:50, Biolegend) as described in the product specification sheet.
Regulatory T cell isolation and cultures
Spleen, inguinal lymph nodes, and mesenteric lymph nodes were dissected from Foxp3-IRES-GFP mice. CD4 positive cells were enriched using StemCell technologies CD4 isolation kit. CD4-enriched cells were then sorted for endogenous GFP+ using FACS (AriaII, BD). Sorted Tregs were plated in anti-CD3-Coated U-bottom 96 wells, supplemented with anti-CD28, IL-2 (100 ng/ml) and treated as outlined in the figures. Heat-killed GL-261 cells were obtained after 3 cycles of incubation at 37 °C then in liquid nitrogen. After 48 h, Tregs have been transferred to V-bottom 96 wells (Corning) and have been stained for flow cytometry analysis.
Statistical analysis
For individual comparisons, Student’s t test was performed. Kaplan–Meier curves were generated to determine relative survival of glioma bearing animals under different courses of treatment, and log-rank tests were performed to address significance between groups. One-way ANOVA was used for comparisons between multiple groups, and Tukey’s post hoc test was performed to obtain appropriate p values. Longitudinal data from multiple groups were analyzed with two-way ANOVA followed with Bonferroni’s multiple sample post hoc test. p ≤ 0.05 was considered significant. *p < 0.05; **p < 0.01; ***p < 0.001; ns not significant.
Results
Regulatory T cells accumulate in glioma tissues and express increasing amounts of granzyme B
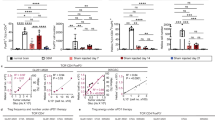
To assess the phenotype of Tregs within glioma tissues [6], we implanted a syngeneic astrocytoma line, GL261, into Foxp3-IRES-GFP mice [42]. These mice stably expressed GFP at the same time as Foxp3, the master regulator of Treg function. After 1, 2, and 3 weeks post-tumor implantation, lymphocytes were harvested from the brain and lymphoid tissues and flow cytometric analyses were performed as noted in Fig. 1a (Fig. 1b–e were analyzed at week 3). The expression of granzyme B (GrB) in Tregs significantly increases over time, from 10 % of Tregs expressing GrB at week one, 25 % at week two, and 60 % at week three after tumor establishment (p ≤ 0.001), consistent with a recent study showing that Tregs express a large amount of GrB in glioma tissues [43] (Fig. 1a). Accumulation of granzyme is restricted to Foxp3+ T cells of the CD4+ lineage as shown in the representative scatter plot (Fig. 1b), suggesting a potential role for GrB in the immunosuppressive activities of Tregs in the TME. GrB expression is only found within T cells that have increased expression of CD44, a well-established marker of T cell activation. To determine whether progressive accumulation of GrB within Tregs occurs specifically within the TME, we measured the GrB expression of Tregs in the draining lymph nodes (CLN), non-draining lymph nodes, (ILN) and the spleen (Fig. 1c), and found a significant increase (p ≤ 0.001) in Treg GrB expression within the brain. We next measured recruitment of GrB-expressing Tregs across tissues in tumor-bearing versus PBS-injected animals (Fig. 1d). While PBS-injected mice have no GrB Tregs in the brain, tumor-bearing mice strongly recruit this subset (1000-fold increase, p ≤ 0.01). To better understand the contribution of the Foxp3+GrB+ cellular subset to the entire CD4+ compartment, we compared percentages of GrB Tregs to the total CD4+ subset (Fig. 1e) and determined that they represent a large component (10 %, p ≤ 0.01) of CD4+ T cell within the brain (Fig. 1d) with a significant increase in the draining lymph nodes. The accumulation of GrB+ Tregs to tumor tissues suggests that either the local TME is recruiting Tregs with this phenotype or they are being programmed within the tumor to begin expressing cytolytic enzymes.
Regulatory T cells accumulating in glioma express large amounts of granzyme B. Foxp3-IRES-GFP mice implanted i.c., had lymphocytic infiltration phenotyped via flow cytometry at 3 weeks post-tumor implantation (1, 2, and 3 weeks post-implantation in a). a The accumulation of GrB expression by Tregs in the tumor over time. b Representative flow plot of CD4+ T cell infiltrates in the tumor tissues of mice. c The percentage of Tregs expressing granzyme in the brain, draining lymph nodes (CLN), non-draining lymph nodes (ILN), and spleens of GBM tumor-bearing mice. d The % contribution of GrB+ Treg+ to the total CD4+ compartment in different tissues. e The total numbers of GrB+ Tregs+ in different tissues of glioma bearing mice. All data in a–d are representative of two independent experiments with 4–5 mice per group/per experiment, and e is compiled from two experiments per time point with a total n = 9–10 per group. *p < 0.05; **p < 0.01; ***p < 0.001
Granzyme expression by Tregs is induced by the TME and can be inhibited with anti-GITR antibodies
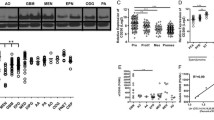
To test whether granzyme expression in Tregs is affected by tumor byproducts, as a previous study demonstrated [44], we harvested supernatant from GL261 cultures, and from heat-killed GL261 tumor lysates and co-cultured them with FACS sorted Foxp3+GFP+ Tregs for 48 h (Fig. 2a). GL261 supernatant and heat-killed GL261 lysates resulted in significantly increased GrB expression (35 and 70 %, respectively) by Tregs compared to Tregs in culture alone (2 %) (Fig. 2c). To address if the production of GrB by Tregs has any functional outcome on the number of antitumor effectors within the tumor, we implanted GL261 into perforin knockout mice (Prf1−/−), which abrogates the release of granzymes in vivo (Fig. 2a, b). The lack of a conditional-knockout model makes it difficult to address how perforin/granzyme expression by Tregs influences tumor growth, as these molecules are also centrally important for effector immune responses. Indeed, the tumor growth of Prf1−/− mice compared to wild-type mice was not significantly different as measured by histological examination (Supplementary Figure S1). However, this model can help us understand whether Tregs are using this pathway to kill effectors within the tumor, and importantly, the percentage of CD4 T cells expressing Foxp3 was significantly reduced within tumors of Prf1−/− mice as compared to wild-type mice (from 50 ± 5 to 40 ± 3 %, respectively) but remained unchanged in peripheral tissue as determined through flow cytometric analysis (Fig. 2b). Furthermore, the ratio of Tregs to CD4+ effectors, CD8+ CTLs, and NKs was significantly reduced (p ≤ 0.05) within the tumors of Prf1−/−mice as compared to controls (Fig. 2c). These data were suggestive of the possibility that Tregs might be using GrB to suppress the proliferation of anti-tumor effectors within the glioma microenvironment. To test this, we used the agonistic antibody against GITR (DTA-1) which has been proposed as an inhibitor of Treg GrB expression and their ability to suppress T cell proliferation [45, 46].
Granzyme expression by Tregs has functional relevance and is regulated by GITR expression. a, b Perforin (which is required for granzyme release) knockout B6 mice were implanted with GL261, and lymphocytic infiltration was assessed via flow cytometry. c Tregs were sorted from Foxp3-GFP mice and cultured in the presence of 10 % GL261 supernatant, heat-killed Gl261 cells or in d, the assay was performed in the presence of DTA-1 antibody. e Mice were injected intracranially with 10 μg of anti-GITR antibody (DTA-1) 7 days post-tumor implantation, and flow cytometry was performed 48 h later to address granzyme expression by different T cell subsets. Data in a, b are representative of two independent experiments with 4 mice per group/per experiment, significance calculated using unpaired Student’s t test. Data in c, d are compiled from two experiments each groups was performed in quintuplicate, significance calculated using a one-way ANOVA followed by a Tukey’s post hoc test for individual comparison. Data in e are shown as mean ± SEM aggregated from two independent experiments with n = 8 mice analyzed. Error bars for all groups calculated as mean ± SEM. *p < 0.05; **p < 0.01; ***p < 0.001
To determine whether DTA-1 treatment inhibits tumor-driven GrB expression by Tregs, we sorted Tregs from Foxp3-GFP mice and cultured with GL261 supernatant for 48 h in the presence of DTA-1 before running flow cytometric analysis (Fig. 2d). DTA-1 treatment alone has no influence on GrB expression by Tregs, while DTA-1 treatment with GL261 supernatant returned GrB levels to those in culture alone. To address if DTA-1 can inhibit GrB expression by Tregs in the tumor, we intracranially injected anti-GITR antibodies directly to the tumor site in Foxp3-GFP mice (Fig. 2e). Intracranial treatment of DTA-1 into GL261 tumor-bearing mice caused a twofold decrease in the percentage of Tregs expressing GrB (Fig. 2e). The reduction in GrB on CD4+Foxp3− T cells was also reduced with DTA-1; however, the expression of GrB by this subset is less than 1 %. No change was seen in CD8+ granzyme expression, which indicates that anti-GITR antibody treatment might be a way to preferentially target Tregs in the glioma tissue without perturbing the effector arm of the anti-tumor response.
Systemic treatment with GITR antibodies has minimal influence on animal survival
To test the influence of anti-GITR therapy on established gliomas, we implanted mice with the murine GL261 line and treated them with 500 µg of DTA-1 at 7 and 12 days post-implantation via i.p. injection, at which time these tumors have already established (Fig. 3). Peripheral treatment of DTA-1 provided no benefit to median survival as determined by log-rank test analysis although 10 % of mice were long-term survivors (Fig. 3a). This is in accordance with a recent study, showing that peripheral treatment with DTA-1 alone does not provide significant benefit to glioma bearing mice [39]. To determine whether the T cell phenotype is changed with peripheral DTA-1 treatment, mice that were treated with DTA-1 (or isotype control) had their brains, draining lymph nodes, and spleen harvested 48 h post-antibody injection to perform flow cytometric analyses (Fig. 3b–e). Analysis revealed that the percentage of Tregs expressing GrB in the brains of tumor-bearing mice was significantly reduced with DTA-1 treatment (26 ± 1 % of Tregs in controls, compared to 14.9 ± 2 % with DTA-1 treatment, p ≤ 0.001) (Fig. 3b); however, the total number of GrB+Foxp3+ T cells was not significantly reduced (Fig. 3c). The percentage of CD4+ that were Tregs in the brain (Fig. 3d) and the total number of Tregs with systemic DTA-1 treatment remained unchanged within the tumor (Fig. 3e), suggesting that systemic treatment has a role in inhibiting Treg functionality in the periphery but does not influence their recruitment to the tissues. Due to the location of the glioma within the CNS, the blood–brain barrier prevents many antibodies from crossing, potentially inhibiting the ability of the antibody to provide efficacy at the tumor site. Furthermore, even though GITR expression is found predominantly on Tregs, its expression is increased almost fivefold on Tregs within the TME (Supplementary Figure. S2), suggesting that the effects of DTA-1 treatment might differ if the antibody is directly injected to the tumor site. To address this limitation due to the blood–brain barrier, we tested to see whether intracranial treatment with DTA-1 had a different effect on animal survival as compared to peripheral administration.
Peripheral anti-GITR treatment does not confer survival benefit in glioma bearing mice. a–e Foxp3-GFP mice were implanted i.c. with 4×105 GL261, followed by two 500 µg i.p. injections of anti-GITR antibody. Endpoint analysis was performed in a. b–e Lymphocytic infiltration was assessed via flow cytometry 48 h after first antibody treatments. Data in a are compiled from two independent experiments with n = 6–7 mice per group, significance was calculated using the log-rank test. b–e Data are representative of two independent experiments with 4–5 mice per group/per experiment, significance calculated using unpaired Student’s t test. Error bars calculated as mean ± SEM. *p < 0.05; **p < 0.01; ***p < 0.001
Intracranial anti-GITR treatment causes significantly increased animal survival in glioma bearing animals
After 1 week of GL261 establishment, we injected anti-GITR antibodies directly to the tumor site. Interestingly, intracranial treatment of DTA-1 caused a significant median survival benefit in mice (30 days for DTA-1 as compared to 19 for control, p ≤ 0.01), with 10 % of mice being long-term survivors (Fig. 4a). While peripheral administration of DTA-1 caused only a decrease in the percentage of Tregs expressing GrB, intracranial treatment caused a significant decrease in both the percentage (p ≤ 0.01) and total number (p ≤ 0.001) of Tregs expressing GrB (Fig. 4b, c). Furthermore, the percentage of intracranial CD4 that are Foxp3+ (46 ± 1 % isotype versus 25 ± 1.3 % for DTA-1, p ≤ 0.001) and their total numbers within the tumor was significantly reduced (1 × 105 ± 2 × 104 isotype versus 3 × 104 ± 4 × 103 for DTA-1, p ≤ 0.05) (Fig. 4d–e). It is important to note that these changes are located solely within the tumor as no significant difference was observed in the DLN or the spleens of DTA-1-treated animals. Highlighting the specificity of the treatment, intracranial DTA-1 administration does not inhibit CD4+Foxp3− (Fig. 4f) or CD8+ subsets (Fig. 4g, h) within the TME, DLN, or spleens. To determine whether the decrease in Tregs is sustained with DTA-1 therapy, we addressed the T cell infiltrates of mice 1 week after DTA-1 administration (Supplementary Figure. S3). The Tregs in the tumors of these mice were not significantly different in the percentage of CD4+ that were Foxp3+ and also in the percentage of Tregs that were GrB+. This suggests that the effects of the antibody treatment are only temporary and could benefit from osmotic pump administration or other long-term infusion strategies.
Intracranial anti-GITR treatment after tumor establishment significantly enhances survival in glioma bearing mice. In a–h Foxp3-GFP mice were implanted i.c. with 4×105 GL261, followed by two 10 µg i.c. injections of anti-GITR antibody directly into the tumor. Endpoint analysis was performed in a. b–h Lymphocytic infiltration was assessed via flow cytometry 48 h after first antibody treatments. Data in a are compiled from three independent experiments with n = 10–11 mice per group, significance was calculated using the log-rank test. b–h Data are representative of two independent experiments with 4–5 mice per group/per experiment, significance calculated using unpaired Student’s t test. Error bars calculated as mean ± SEM. *p < 0.05; **p < 0.01; ***p < 0.001
Intracranial anti-GITR treatment functions via FcγR-mediated elimination of Tregs
Fc-Receptor gamma-mediated destruction of Tregs has been recently described for a number of therapeutic antibody treatments including the action of DTA-1 in a murine model of colon tumors [47]. To address the role of Fc-receptor-mediated Treg depletion, we implanted GL261 into FcγIII receptor knockout mice (FcRγ-) (Fig. 5). This high affinity receptor is thought to be responsible for Fc-mediated destruction/phagocytosis of antibody-bound cells [29]. Interestingly, while implantation into these mice abrogated the survival benefit seen in control mice, there still was significant increase in median animal survival (21 days for DTA-1 as compared to 17 for control, p ≤ 0.05) (Fig. 5a). Intracranial DTA-1 treatments caused no observable changes in percentages of CD4 Foxp3+ or their total numbers in FcRγ- mice (Fig. 5b, c). These data correlate with in vitro assays we performed in which CD3/CD28 stimulated whole splenocytes with DTA-1 for 72 h caused nearly a threefold decrease in Treg/effector T cell ratios, which was significantly abrogated (p ≤ 0.001) when the assay was performed with splenocytes from FcRγ-knockout mice (Fig. 5d). Such findings allow us to contend that the reduction in Tregs that occurs with intracranial DTA-1 treatment occurs via Fc-mediated phagocytosis and not by intrinsic effects on Treg function.
FcR-mediated depletion of Tregs is a major contributing factor to anti-GITR efficacy in glioma. FcRγ−/− B6 mice were implanted i.c. with 4×105 GL261, and then mice were injected intracranially with 10 µg of anti-GITR antibody (DTA-1) at both 7 and 12 days post-tumor implantation, endpoint analysis was performed in a, and flow cytometry was performed 48 h later in b, c. d Splenocytes isolated from either WT or FcRγ−/− mice were stimulated with anti-CD3/CD28 (+IL-2) and treated with anti-GITR or isotype control antibodies, after 3 days, flow cytometric analysis was performed on the cultures. Data in a are compiled from two independent experiments with 4 mice per group/per experiment, significance calculated using the log-rank test. Data in b–d are representative of two experiments with n = 4–5 per group, significance calculated using an unpaired Student’s t test. Error bars for all groups calculated as mean ± SEM. *p < 0.05; **p < 0.01; ***p < 0.001
DTA-1 administration enhances dendritic cell viability and function
Considering that intracranial DTA-1 treatment elicited a significant survival benefit without any overt changes in Treg numbers in FcRγ-mice (Fig. 5), suggests that anti-GITR antibody treatment is exerting its effects in a way that has not yet been determined. Our in vitro analyses revealed that DCs might be influenced by DTA-1 treatment (Fig. 6). To investigate this phenomenon, we determined the DC phenotype of splenocyte cultures with DTA-1 treatment (Fig. 6a–c). Interestingly, in these culture conditions, DCs appeared to be more activated as their expression of MHC-II was upregulated after DTA-1 treatment (Fig. 6a, b). As expected, DTA-1 treatment resulted in the reduction in Tregs in vitro (Fig. 6c). This increase in DC activation suggests that the influence of DTA-1 on Tregs has downstream effects on DC licensing [48], or DTA-1 treatment is having a direct effect on DC function/phenotype. To address this, we generated bone marrow dendritic cells (BMDC) [49] and treated them with DTA-1 or its isotype control (Fig. 6d–f). Treatment of BMDCs with DTA-1 significantly increased the total number of viable DCs in culture by approximately 20 % (p ≤ 0.001) (Fig. 6d), and significant increases in DCs expressing the activation markers MHC-II (p ≤ 0.05) and CD86 (p ≤ 0.01) were also observed (Fig. 6e, f). To determine whether DCs treated with DTA-1 are functionally enhanced, DTA-1 pre-treated BDMCs were pulsed separately with ovalbumin peptides corresponding to either MHC-class I (SIINFEKL—1 µg/ml) or MHC-class II (ISQAVHAAHAEINEAGR—10 µg/ml) and proliferation of ovalbumin-specific T cells was assessed (OTI CD8+ and OTII CD4+, respectively). DTA-1 pre-treatment on BMDCs significantly enhanced proliferation of labeled OTI CD8 T cells, as OTI CD8+ had increased numbers of cells in 3 and 4 divisions compared to isotype control (Fig. 6g, h). DTA-1 pre-treatment on BMDCs also enhanced proliferation of labeled OTII CD4+ T cells by increasing total percentages of antigen-specific cells undergoing division, as well as those in 2, 3, and 4 divisions compared to isotype control (Fig. 6i). The results of this experiment show that anti-GITR has effects directly on DCs and can enhance antigen-specific T cell responses.
Anti-GITR antibodies directly influence DC maturation and licensing by T cells. a–c Splenocyte cultures from FcRγ−/− mice were treated under conditions of T cell stimulating conditions and incubated with 2 μM anti-GITR (or isotype control) antibodies 24 h after plating. 48 h later cells were harvested and analyzed via flow cytometry. d–f BMDC cultures were treated with anti-GITR antibodies (or isotype controls), and 48 h later cells were harvested and analyzed via flow cytometry. g, h BMDCs from FcRγ−/− mice were generated and incubated with anti-GITR and SIINFEKL 24 h before co-culture with CD8 isolated from OTI splenocytes. i The same BMDC experiment was performed using a class II-restricted peptide followed by co-culture of CD4 isolated from OTII splenocytes. 72 h later proliferation was assessed by CTV label retention. Significance calculated using an unpaired Student’s t test. Data shown are representative of two independent experiments with n = 4–5 per group. Error bars for all groups calculated as mean ± SEM. *p < 0.05; **p < 0.01; ***p < 0.001
Discussion
In this study, we demonstrated that targeting the molecule GITR is a potentially advantageous therapeutic strategy for the treatment of glioma. As this therapeutic strategy targets the inhibitory component of T cell responses, an important consideration is to determine its contribution to checkpoint blockade immunotherapy. [50]. Anti-PD-1 therapy, which has recently attracted attention due to its strong benefits for many melanoma patients, reverses T cell exhaustion that is triggered within tumors [51]. However, Tregs are still likely capable of suppressing these aggressive T cells, which is why targeting them may be of critical importance for the efficacy of PD1 immunotherapy [50]. There is currently a humanized anti-GITR antibody being tested in a phase 1 clinical trial for patients with solid growth tumors (NC T01239134). Perhaps in the future, we can extend this therapy to patients with glioma and combine it with checkpoint therapies that target the effector compartment.
Previous studies suggest that DTA-1 treatment exerts its effects via converting Tregs to effectors to drive the anti-tumor response [38]. Our data suggest that both Fc-mediated destruction of Tregs and the direct role of DTA-1 on DC survival and functionality represent a major set of mechanisms of targeting GITR in glioma, and demonstrate the promise of targeting Tregs for immunotherapy. In addition, as the effect of DTA-1 after tumor implantation compared to other Treg-targeted has yet to be elucidated, future studies will aim at understanding how DCs are influenced by GITR modulation and how that may represent a different way to influence DC-centered therapies in the clinic [52]. Furthermore, the results of this study give rise to the discussion as to whether or not the accumulation of Tregs is suppressing anti-tumor immunity in glioma patients [26].
Due the tumors unique location within the CNS, this study allowed us to address whether Treg-targeted therapy systemically or within the tumor elicits different efficacy. Our study demonstrates that the route of administration is critical to the efficacy of the antibody treatment, as GITR expression is dramatically upregulated on Tregs within the tumor, suggesting that targeting Tregs at the tumor site is more efficacious. Our group had previously demonstrated that GITR expression by Tregs alone increases over time within the tumor [6] and suggests that this increase in GITR expression makes them more efficiently targeted with intracranial antibody treatment. It is interesting that the location of antibody administration makes a pronounced difference in efficacy as checkpoint immunotherapies for GBM in the same animal model can efficiently exert their effects when administered systemically [5]. As mechanistic explanations of GITR therapeutic efficacy differ from group to group, these data suggest that it might be influenced by the ability of the antibody to reach the site of the tumor. This paper provides strong evidence that not only does the type of immunotherapy matter for the treatment of glioma, but also the anatomical location of administration may be critical to its effectiveness.
We demonstrate that anti-GITR has an intrinsic effect on Tregs by reducing their GrB expression and thus potentially preventing their cytotoxic capabilities. The targeting of this pathway alone did not elicit significant survival benefit; rather, it was the Fc-mediated destruction of Tregs by which this antibody exerts its effect. This has been proposed as a major mechanism for DTA-1 effectiveness in other murine tumor models [47], and can now be extended to our murine model of glioma. This suggests that targeting Tregs as a whole versus any particular suppressive mechanism is the most effective way to enhance anti-tumor immunity. Considering the myriad of ways in which Tregs can suppress immune responses, this is perhaps not surprising [53]. This study also has shown that there is direct influence of DTA-1 on both DC proliferation and antigen presentation. Previous studies have demonstrated that GITR ligation can signal through the pro-survival NF-kB pathway in T cells and may explain why DTA-1 treatment enhances survival and functionality of DC [54]; however, the reasons why the effects of signaling through DTA-1 have diverging roles on DCs versus Tregs are unknown. Summarily, our data show that targeting the GITR pathway in glioma is an important target for immunotherapy and warrants further studying into the use of this antibody for the treatment of glioma.
Abbreviations
- APC:
-
Allophycocyanin
- BMDC:
-
Bone marrow dendritic cell
- CNS:
-
Central nervous system
- FcγR:
-
Fc gamma receptor
- GBM:
-
Glioblastoma multiforme
- GITR:
-
Glucocorticoid-induced TNFR-related protein
- GrB:
-
Granzyme B
- Prf1:
-
Perforin 1
- TME:
-
Tumor microenvironment
- Treg:
-
Regulatory T cell
References
Heimberger AB, Sampson JH (2011) Immunotherapy coming of age: What will it take to make it standard of care for glioblastoma? Neuro-Oncology 13(1):3–13. doi:10.1093/neuonc/noq169
Vom Berg J, Vrohlings M, Haller S, Haimovici A, Kulig P, Sledzinska A, Weller M, Becher B (2013) Intratumoral IL-12 combined with CTLA-4 blockade elicits T cell-mediated glioma rejection. J Exp Med 210(13):2803–2811. doi:10.1084/jem.20130678
Aldrich WA, Ren C, White AF, Zhou SZ, Kumar S, Jenkins CB, Shaw DR, Strong TV, Triozzi PL, Ponnazhagan S (2006) Enhanced transduction of mouse bone marrow-derived dendritic cells by repetitive infection with self-complementary adeno-associated virus 6 combined with immunostimulatory ligands. Gene Ther 13(1):29–39. doi:10.1038/sj.gt.3302601
Zeng J, See AP, Phallen J, Jackson CM, Belcaid Z, Ruzevick J, Durham N, Meyer C, Harris TJ, Albesiano E, Pradilla G, Ford E, Wong J, Hammers HJ, Mathios D, Tyler B, Brem H, Tran PT, Pardoll D, Drake CG, Lim M (2013) Anti-PD-1 blockade and stereotactic radiation produce long-term survival in mice with intracranial gliomas. Int J Radiat Oncol Biol Phys 86(2):343–349. doi:10.1016/j.ijrobp.2012.12.025
Wainwright DA, Chang AL, Dey M, Balyasnikova IV, Kim C, Tobias AL, Cheng Y, Kim J, Zhang L, Qiao J, Han Y, Lesniak MS (2014) Durable therapeutic efficacy utilizing combinatorial blockade against IDO, CTLA-4 and PD-L1 in mice with brain tumors. Clin Cancer Res 20(20):5290–5301. doi:10.1158/1078-0432.CCR-14-0514
Wainwright DA, Balyasnikova IV, Chang AL, Ahmed AU, Moon KS, Auffinger B, Tobias AL, Han Y, Lesniak MS (2012) IDO expression in brain tumors increases the recruitment of regulatory T cells and negatively impacts survival. Clin Cancer Res 18(22):6110–6121. doi:10.1158/1078-0432.CCR-12-2130
Vega EA, Graner MW, Sampson JH (2008) Combating immunosuppression in glioma. Future Oncol 4(3):433–442. doi:10.2217/14796694.4.3.433
Lindau D, Gielen P, Kroesen M, Wesseling P, Adema GJ (2013) The immunosuppressive tumour network: myeloid-derived suppressor cells, regulatory T cells and natural killer T cells. Immunology 138(2):105–115. doi:10.1111/imm.12036
Ostrand-Rosenberg S, Sinha P (2009) Myeloid-derived suppressor cells: linking inflammation and cancer. J Immunol 182(8):4499–4506. doi:10.4049/jimmunol.0802740
Chang CH, Qiu J, O’Sullivan D, Buck MD, Noguchi T, Curtis JD, Chen QY, Gindin M, Gubin MM, van der Windt GJW, Tonc E, Schreiber RD, Pearce EJ, Pearce EL (2015) Metabolic competition in the tumor microenvironment is a driver of cancer progression. Cell 162(6):1229–1241. doi:10.1016/j.cell.2015.08.016
Maes W, Verschuere T, Van Hoylandt A, Boon L, Van Gool S (2013) Depletion of regulatory T cells in a mouse experimental glioma model through anti-CD25 treatment results in the infiltration of non-immunosuppressive myeloid cells in the brain. Clin Dev Immunol 2013:952469. doi:10.1155/2013/952469
Wainwright DA, Dey M, Chang A, Lesniak MS (2013) Targeting tregs in malignant brain cancer: overcoming IDO. Front Immunol 4:116. doi:10.3389/fimmu.2013.00116
Jordan JT, Sun W, Hussain SF, DeAngulo G, Prabhu SS, Heimberger AB (2008) Preferential migration of regulatory T cells mediated by glioma-secreted chemokines can be blocked with chemotherapy. Cancer Immunol Immunother 57(1):123–131. doi:10.1007/s00262-007-0336-x
Hori S, Nomura T, Sakaguchi S (2003) Control of regulatory T cell development by the transcription factor Foxp3. Science 299(5609):1057–1061. doi:10.1126/science.1079490
Mellman I, Coukos G, Dranoff G (2011) Cancer immunotherapy comes of age. Nature 480(7378):480–489. doi:10.1038/nature10673
Wainwright DA, Sengupta S, Han Y, Lesniak MS (2011) Thymus-derived rather than tumor-induced regulatory T cells predominate in brain tumors. Neuro Oncol 13(12):1308–1323. doi:10.1093/neuonc/nor134
Prins RM, Graf MR, Merchant RE, Black KL, Wheeler CJ (2003) Thymic function and output of recent thymic emigrant T cells during intracranial glioma progression. J Neurooncol 64(1–2):45–54. doi:10.1007/BF02700019
Dey M, Chang AL, Wainwright DA, Ahmed AU, Han Y, Balyasnikova IV, Lesniak MS (2014) Heme oxygenase-1 protects regulatory T cells from hypoxia-induced cellular stress in an experimental mouse brain tumor model. J Neuroimmunol 266(1–2):33–42. doi:10.1016/j.jneuroim.2013.10.012
El Andaloussi A, Han Y, Lesniak MS (2006) Prolongation of survival following depletion of CD4+CD25+ regulatory T cells in mice with experimental brain tumors. J Neurosurg 105(3):430–437. doi:10.3171/jns.2006.105.3.430
Fecci PE, Sweeney AE, Grossi PM, Nair SK, Learn CA, Mitchell DA, Cui XY, Cummings TJ, Bigner DD, Gilboa E, Sampson JH (2006) Systemic anti-CD25 monoclonal antibody administration safely enhances immunity in murine glioma without eliminating regulatory T cells. Clin Cancer Res 12(14):4294–4305. doi:10.1158/1078-0432.Ccr-06-0053
Whiteside TL (2014) Regulatory T cell subsets in human cancer: Are they regulating for or against tumor progression? Cancer Immunol Immun 63(1):67–72. doi:10.1007/s00262-013-1490-y
Heimberger AB, Kong LY, Abou-Ghazal M, Reina-Ortiz C, Yang DS, Wei J, Qiao W, Schmittling RJ, Archer GE, Sampson JH, Hiraoka N, Priebe W, Fuller GN, Sawaya R (2009) The role of Tregs in human glioma patients and their inhibition with a novel STAT-3 inhibitor. Clin Neurosurg 56:98–106. doi:10.1227/01.neu.0000333524.54656.42
Sonabend AM, Rolle CE, Lesniak MS (2008) The role of regulatory T cells in malignant glioma. Anticancer Res 28(2B):1143–1150
El Andaloussi A, Lesniak MS (2007) CD4(+)CD25(+)FoxP3(+) T-cell infiltration and heme oxygenase-1 expression correlate with tumor grade in human gliomas. J Neuro-Oncol 83(2):145–152. doi:10.1007/s11060-006-9314-y
Thomas AA, Fisher JL, Rahme GJ, Hampton TH, Baron U, Olek S, Schwachula T, Rhodes CH, Gui J, Tafe LJ, Tsongalis GJ, Lefferts JA, Wishart H, Kleen J, Miller M, Whipple CA, de Abreu FB, Ernstoff MS, Fadul CE (2015) Regulatory T cells are not a strong predictor of survival for patients with glioblastoma. Neuro Oncol 17(6):801–809. doi:10.1093/neuonc/nou363
Sayour EJ, McLendon P, McLendon R, De Leon G, Reynolds R, Kresak J, Sampson JH, Mitchell DA (2015) Increased proportion of FoxP3+ regulatory T cells in tumor infiltrating lymphocytes is associated with tumor recurrence and reduced survival in patients with glioblastoma. Cancer Immunol Immun 64(4):419–427. doi:10.1007/s00262-014-1651-7
Yue Q, Zhang X, Ye HX, Wang Y, Du ZG, Yao Y, Mao Y (2014) The prognostic value of Foxp3+ tumor-infiltrating lymphocytes in patients with glioblastoma. J Neuro-Oncol 116(2):251–259. doi:10.1007/s11060-013-1314-0
Jacobs JFM, Idema AJ, Bol KF, Grotenhuis JA, de Vries IJM, Wesseling P, Adema GJ (2010) Prognostic significance and mechanism of Treg infiltration in human brain tumors. J Neuroimmunol 225(1–2):195–199. doi:10.1016/j.jneuroim.2010.05.020
Smyth MJ, Ngiow SF, Teng MWL (2014) Targeting regulatory T cells in tumor immunotherapy. Immunol Cell Biol 92(6):473–474. doi:10.1038/icb.2014.33
Litzinger MT, Fernando R, Curiel TJ, Grosenbach DW, Schlom J, Palena C (2007) IL-2 immunotoxin denileukin diftitox reduces regulatory T cells and enhances vaccine-mediated T-cell immunity. Blood 110(9):3192–3201. doi:10.1182/blood-2007-06-094615
Ko K, Yamazaki S, Nakamura K, Nishioka T, Hirota K, Yamaguchi T, Shimizu J, Nomura T, Chiba T, Sakaguchi S (2005) Treatment of advanced tumors with agonistic anti-GITR mAb and its effects on tumor-infiltrating Foxp3+CD25+CD4+ regulatory T cells. J Exp Med 202(7):885–891. doi:10.1084/jem.20050940
Hodi FS, O’Day SJ, McDermott DF, Weber RW, Sosman JA, Haanen JB, Gonzalez R, Robert C, Schadendorf D, Hassel JC, Akerley W, van den Eertwegh AJ, Lutzky J, Lorigan P, Vaubel JM, Linette GP, Hogg D, Ottensmeier CH, Lebbe C, Peschel C, Quirt I, Clark JI, Wolchok JD, Weber JS, Tian J, Yellin MJ, Nichol GM, Hoos A, Urba WJ (2010) Improved survival with ipilimumab in patients with metastatic melanoma. N Engl J Med 363(8):711–723. doi:10.1056/NEJMoa1003466
Tone Y, Kidani Y, Ogawa C, Yamamoto K, Tsuda M, Peter C, Waldmann H, Tone M (2014) Gene expression in the gitr locus is regulated by NF-kappa B and Foxp3 through an enhancer. J Immunol 192(8):3915–3924. doi:10.4049/jimmunol.1302174
Fecci PE, Ochiai H, Mitchell DA, Grossi PM, Sweeney AE, Archer GE, Cummings T, Allison JP, Bigner DD, Sampson JH (2007) Systemic CTLA-4 blockade ameliorates glioma-induced changes to the CD4(+) T cell compartment without affecting regulatory T-cell function. Clin Cancer Res 13(7):2158–2167. doi:10.1158/1078-0432.Ccr-06-2070
Naidoo J, Page DB, Wolchok JD (2014) Immune modulation for cancer therapy. Br J Cancer 111(12):2214–2219. doi:10.1038/bjc.2014.348
Ronchetti S, Zollo O, Bruscoli S, Agostini M, Bianchini R, Nocentini G, Ayroldi E, Riccardi C (2004) GITR, a member of the TNF receptor superfamily, is costimulatory to mouse T lymphocyte subpopulations. Eur J Immunol 34(3):613–622. doi:10.1002/eji.200324804
Lahey TP, Loisel SD, Wieland-Alter W (2007) Glucocorticoid-induced tumor necrosis factor receptor family-related protein triggering enhances HIV-specific CD4(+) T cell cytokine secretion and protects HIV-specific CD4(+) T cells from apoptosis. J Infect Dis 196(1):43–49. doi:10.1086/518613
Schaer DA, Budhu S, Liu CL, Bryson C, Malandro N, Cohen A, Zhong H, Yang X, Houghton AN, Merghoub T, Wolchok JD (2013) GITR pathway activation abrogates tumor immune suppression through loss of regulatory T-cell lineage stability. Cancer Immunol Res 1(5):320–331. doi:10.1158/2326-6066.Cir-13-0086
Patel MA, Kim JE, Theodros D, Tam A, Velarde E, Kochel CM, Francica B, Nirschl TR, Ghasemzadeh A, Mathios D, Harris-Bookman S, Jackson CC, Jackson C, Ye X, Tran PT, Tyler B, Coric V, Selby M, Brem H, Drake CG, Pardoll DM, Lim M (2016) Agonist anti-GITR monoclonal antibody and stereotactic radiation induce immune-mediated survival advantage in murine intracranial glioma. J Immunother Cancer 4:28. doi:10.1186/s40425-016-0132-2
Lampson LA (2011) Monoclonal antibodies in neuro-oncology: getting past the blood–brain barrier. MAbs 3(2):153–160. doi:10.4161/mabs.3.2.14239
Tario JD, Humphrey K, Bantly AD, Muirhead KA, Moore JS, Wallace PK (2012) Optimized staining and proliferation modeling methods for cell division monitoring using cell tracking dyes. J Vis Exp 70:e4287. doi:10.3791/4287
Bettelli E, Carrier Y, Gao W, Korn T, Strom TB, Oukka M, Weiner HL, Kuchroo VK (2006) Reciprocal developmental pathways for the generation of pathogenic effector TH17 and regulatory T cells. Nature 441(7090):235–238. doi:10.1038/nature04753
Choi BD, Gedeon PC, Sanchez-Perez L, Bigner DD, Sampson JH (2013) Regulatory T cells are redirected to kill glioblastoma by an EGFRvIII-targeted bispecific antibody. Oncoimmunology 2(12):e26757. doi:10.4161/onci.26757
Szajnik M, Czystowska M, Szczepanski MJ, Mandapathil M, Whiteside TL (2010) Tumor-derived microvesicles induce, expand and up-regulate biological activities of human regulatory T cells (Treg). PLoS One 5(7):e11469. doi:10.1371/journal.pone.0011469
Gondek DC, Lu LF, Quezada SA, Sakaguchi S, Noelle RJ (2005) Cutting edge: contact-mediated suppression by CD4+CD25+ regulatory cells involves a granzyme B-dependent, perforin-independent mechanism. J Immunol 174(4):1783–1786. doi:10.4049/jimmunol.174.4.1783
Ji HB, Liao G, Faubion WA, Abadia-Molina AC, Cozzo C, Laroux FS, Caton A, Terhorst C (2004) Cutting edge: the natural ligand for glucocorticoid-induced TNF receptor-related protein abrogates regulatory T cell suppression. J Immunol 172(10):5823–5827. doi:10.4049/jimmunol.172.10.5823
Bulliard Y, Jolicoeur R, Windman M, Rue SM, Ettenberg S, Knee DA, Wilson NS, Dranoff G, Brogdon JL (2013) Activating Fc gamma receptors contribute to the antitumor activities of immunoregulatory receptor-targeting antibodies. J Exp Med 210(9):1685–1693. doi:10.1084/jem.20130573
Vecchiarelli A, Pericolini E, Gabrielli E, Agostini M, Bistoni F, Nocentini G, Cenci E, Riccardi C (2009) The GITRL-GITR system alters TLR-4 expression on DC during fungal infection. Cell Immunol 257(1–2):13–22. doi:10.1016/j.cellimm.2009.02.001
Sallusto F, Lanzavecchia A (1994) Efficient presentation of soluble-antigen by cultured human dendritic cells is maintained by granulocyte-macrophage colony-stimulating factor plus interleukin-4 and down-regulated by tumor-necrosis-factor-alpha. J Exp Med 179(4):1109–1118. doi:10.1084/jem.179.4.1109
Lu L, Xu X, Zhang B, Zhang R, Ji H, Wang X (2014) Combined PD-1 blockade and GITR triggering induce a potent antitumor immunity in murine cancer models and synergizes with chemotherapeutic drugs. J Transl Med 12:36. doi:10.1186/1479-5876-12-36
Sakuishi K, Apetoh L, Sullivan JM, Blazar BR, Kuchroo VK, Anderson AC (2010) Targeting Tim-3 and PD-1 pathways to reverse T cell exhaustion and restore anti-tumor immunity. J Exp Med 207(10):2187–2194. doi:10.1084/jem.20100643
Yang L, Guo G, Niu XY, Liu J (2015) Dendritic cell-based immunotherapy treatment for glioblastoma multiforme. Biomed Res Int 2015:717530. doi:10.1155/2015/717530
Sakaguchi S, Wing K, Onishi Y, Prieto-Martin P, Yamaguchi T (2009) Regulatory T cells: How do they suppress immune responses? Int Immunol 21(10):1105–1111. doi:10.1093/intimm/dxp095
Esparza EM, Arch RH (2005) Glucocorticoid-induced TNF receptor functions as a costimulatory receptor that promotes survival in early phases of T cell activation. J Immunol 174(12):7869–7874. doi:10.4049/jimmunol.174.12.7869
Acknowledgments
The authors wish to thank Dr. Atique Ahmed and Joshua Robert Kane for critical review of the manuscript. The work was supported by NIH National Cancer Institute R01CA122930, R01 NS093903 Grants to Maciej S. Lesniak and NIH/National Cancer Institute T32 CA080621-12 to Jason Miska.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Jason Miska and Aida Rashidi have contributed equally to this work.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Miska, J., Rashidi, A., Chang, A.L. et al. Anti-GITR therapy promotes immunity against malignant glioma in a murine model. Cancer Immunol Immunother 65, 1555–1567 (2016). https://doi.org/10.1007/s00262-016-1912-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00262-016-1912-8