Abstract
Introduction
Myelodysplastic syndromes (MDS) are a group of clonal bone marrow disorders, with dysplasia, cytopenias and increased risk of progression to acute myeloid leukemia. A dysregulated immune system precipitates MDS, and to gain insights into the relevance of cytotoxic T lymphocyte (CTL) in this process, we examined the frequency and function of CX3CR1- and CD57-positive T lymphocytes from MDS patients.
Materials and methods
Peripheral blood and/or bone marrow samples from 31 MDS patients and 12 healthy controls were examined by flow cytometry. Expression of cytotoxic granule constituents, immunological co-receptors, adhesion molecules and markers of activation were quantified on unstimulated lymphocytes. Degranulation, cytotoxicity and conjugate formation with target cells following co-culture of CTL with target cell lines or autologous bone marrow-derived CD34+ cells were quantified by flow cytometry.
Results
CX3CR1 expression was increased in bone marrow from high-risk MDS patients compared to healthy controls. Expression of CD57 and CX3CR1 was closely correlated, identifying a CTL subset with high cytotoxic capacity. In vitro, TCR-induced redirected cytotoxicity was markedly decreased for high-risk MDS patients compared to controls. CTL from MDS patients with the lowest target cell cytotoxicity had reduced expression of adhesion molecules and formed fewer conjugates with target cells.
Discussion
Although phenotypically defined CTL numbers were increased in the bone marrow of MDS patients, we found that CTL from high-risk MDS patients exhibited a lower TCR-induced redirected cytotoxic capacity. Thus, decreased T cell cytotoxicity seems related to reduced adhesion to target cells and may contribute to impaired anti-leukemic immune surveillance in MDS.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Myelodysplastic syndromes (MDS) are a group of clonal bone marrow disorders, characterized by dysplastic maturation of myeloid progenitor cells in the bone marrow, cytopenias of one or more myeloid lineage and high risk of transformation to acute myeloid leukemia (AML) [1, 2]. Several aberrations in the cellular immune system have been described in MDS [3–5]. Chronic inflammation dominates low-risk MDS, with increased levels of Th17 cells and low/normal levels of T regulatory cells (Tregs) [6, 7]. In contrast, myeloid-derived suppressor cells (MDSCs) [8] and Tregs [7, 9] are expanded and create an immunosuppressive environment in high-risk MDS. Oligoclonal expansions of cytotoxic T cells have been reported in MDS [10–12], but no clinical or pathophysiological significance of these expansions has been determined [12].
Chemokine receptors are important for lymphocyte trafficking in homeostasis and inflammation. We have previously reported increased levels of T cells expressing the chemokine receptor CX3CR1 in peripheral blood from MDS patients [13]. CX3CR1 defines a subset of cytotoxic lymphocytes with high levels of perforin and granzyme B in healthy individuals [14], and CX3CR1-expressing T cells, as well as NK cells, exert anti-tumor cytotoxicity in several models [15]. CX3CR1 is functionally important for migration of cells into areas of inflammation and has been shown to be important for recruitment of cytotoxic T cells to the bone marrow in immune thrombocytopenia [16] and aplastic anemia [17]. CX3CR1+ T cells have been described to co-express CD57 [14], and increased numbers of CD57+ T cells have been observed in both peripheral blood and bone marrow from MDS patients [11, 12, 18]. Interestingly, hypomethylating agents used in MDS have recently been described to induce cytotoxic T cell immune responses in cancer models including MDS and AML [19–21].
Here, we have examined the prevalence and function of CX3CR1+ and CD57+ lymphocytes in MDS bone marrow at diagnosis and during treatment. To investigate the cytotoxic potential of T cells from MDS patients, peripheral blood lymphocytes were incubated with target cell lines or lymphocyte-depleted, autologous bone marrow mononuclear cells and assessed for degranulation, production of pro-inflammatory cytokines (TNF and IFN-γ), or granzyme B-mediated target cell cytotoxicity. In order to further elucidate how hypomethylating agents may affect CTL immune responses, we investigated the in vivo effects of azacytidine treatment on the levels of CX3CR1+ and CD57+ T cells in bone marrow and performed a matched assessment of degranulation and CD34+BMMC-directed cytotoxicity prior to and post-azacytidine treatment.
Materials and methods
Samples and cell lines
The study was approved by the regional ethical committee (REK Vest), and samples taken after informed consent. Reporting is done in compliance with MIATA guidelines [22]. Peripheral blood and/or bone marrow samples from 31 unselected patients (median age 75 (54–91), male/female ratio 3.4) and 12 healthy volunteers (median age 41 (33–60), male/female ratio 1.4) were investigated. We divided patients in two groups based on IPSS-R, namely low risk (very low risk and low risk, N = 19, median age 78 (59–91), male/female ratio 2.8) and high risk (intermediate, high and very high risk, N = 12, median age 73 (54–83), male/female ratio 5.0). Patients are described in more detail in Table 1.
Peripheral blood samples were obtained by venipuncture using EDTA-containing Vacutainer tubes (BD). Bone marrow samples were obtained from the iliac crest and collected in 50-ml tubes (BD) with 10 ml RPMI-1640 medium with added heparin (5000 IU). Bone marrow and peripheral blood mononuclear cells (BMMCs and PBMCs, respectively) were acquired using gradient separation (Lymphoprep) following manufacturer’s instructions. Median cell yields were 35 × 106 BMMCs from 20 ml bone marrow aspirate and 20 × 106 PBMCs from 30 ml peripheral blood. BMMCs and PBMCs were cryopreserved in cryovials (Thermo Scientific) with 8 × 106 cells in 1 ml RPMI-1640 medium with added fetal bovine serum (FBS) and 10 % DMSO using MR.FROSTY (Thermo Scientific) following the manufacturer’s instructions. The container was placed at −80 °C for 24–72 h prior to storage of cryovials in liquid nitrogen for a median of 13 months prior to analysis. The mouse mastocytoma cell line P815 (ATCC) is validated for assessing lymphocyte cytotoxicity, and the addition of CD3/OKT3 antibodies to the assay enables testing of CD3-redirected cytotoxic potency [23]. MDS-L (gift from Professor Tohyama, Kobe, Japan) is a confirmed, blastic MDS cell line derived from MDS92 [24] with common MDS genetic aberrations (including −7 and +8) [25].
Flow cytometry
These studies were conducted in a laboratory that operates under exploratory research principles. Fluorescent reagents are listed in Supplementary Table 1. The bone marrow screening assays were performed on bone marrow samples thawed and rested overnight in RPMI-1640 medium with 10 % FBS from a single lot previously qualified for low background in flow cytometry assays [13, 26]. Cells were thawed, stained, washed and analyzed within 20 h.
Peripheral blood and bone marrow samples were thawed, permeabilized, stained and fixed directly for the investigation of granule content, co-stimulatory ligands and receptors as well as surface markers of activation.
Combined cytotoxicity/degranulation assay was performed in accordance with previously published protocols [23]. Cryopreserved peripheral blood mononuclear cells, to serve as effectors, were thawed and rested overnight in RPMI-1640 medium with 10 % FBS. The following day, autologous bone marrow samples were thawed and lymphocytes eliminated from the bone marrow sample using MACS columns and beads (anti-CD3 and anti-CD56, both Miltenyi Biotech), following manufacturer’s instructions. Cells were counted using counting chambers, and a total of 3 × 106 cells were placed in 200 µl RPMI1640-medium with 10 % FBS in BD-Falcon 96-well U-bottom plates at an effector/target ratio of 2:1. Anti-CD3 was only added to the P815 well.
For degranulation assay, effector and target cells with added BD Golgi plug (Brefeldin A) were co-cultured for 4 h, subsequently washed, stained with extracellular antibodies and live/dead marker, washed, fixed (BD Fix/perm solution), stained with intracellular antibodies (in BD Perm/Wash solution), washed (BD Perm/Wash) and analyzed on flow cytometer. Effectors alone and effectors co-cultured with P815 without anti-CD3 were used as internal controls. The ability of lymphocytes to form stable conjugates with target cells is important for efficient cytotoxicity, and this can be measured objectively using flow cytometry [27].
For cytotoxicity assay, target cells were stained with reagents for cell tracing and detection of cells dead prior to assay (TFL4 and NFL1, respectively, both from OncoImmunin) according to manufacturer’s instructions. Effector and target cells were co-cultured for 1 h in media added non-fluorescent PanToxiLux substrate (PTL, OncoImmunin). PTL is rendered fluorescent in the presence of granzyme B and caspase 3 and can thus be used to detect cells that have received a ‘lethal hit.’ Background was reduced by using NFL1 to gate out cells dead by the start of the assay. Also, to correct for spontaneous apoptosis, PTL+ target cells in co-culture were corrected for the level of PTL+ target cells alone (percentage with effector–percentage without effector). For the MDS-L cell line, anti-CD34 was used to give a good separation of target and effector cells. For the bone marrow assays, anti-CD33, anti-CD34 and anti-CD45 were used in addition to NFL1, TFL4 and PTL. CD45+ and CD33+ cells were gated out to reduce contamination and false-positive results in the CD34+ subset, as CD33+ cells in both bone marrow and peripheral blood samples showed high autofluorescence in the PTL channel (data not shown). Peripheral blood cells did not contaminate the target cell gates (TFL4-gate for P815 and BMMC assays or CD34-gate for the MDS-L assay, data not shown). For two high-risk patients, bone marrow CD34− cells frozen down at the day of sampling were used as effector cells, because of lacking matched peripheral blood samples. Gating strategies are presented in Fig. 1.
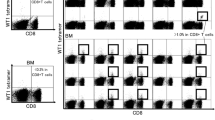
Gating strategy. a For the bone marrow CX3CR1 screening assay, BMMCs were gated on the lymphocyte subset based on scatter properties, subsequently dead cells were excluded, CD3+ cells selected and CD8+, and CD8− cells were gated based on expression of CD8. Finally, expression of CX3CR1 was assessed for the CD3+, CD3+CD8+ and CD3+CD8− subsets. b In the cytotoxicity assay, assessment of peripheral blood lymphocyte effectors was performed by primarily gating on lymphocytes based on scatter properties (right-arrow), and then, unwanted events were excluded using a dump channel designed to remove CD19+ (B cells), CD14+ (monocytes) and dead cells. Subsequently, T cells and NK cells were gated based on expression of CD3 and CD56, (T cells CD3+ and NK cells CD3−CD56+). CD3+ cells were subdivided into CD57+ and CD57− cells. Degranulation (CD107a) and production of TNF and IFNγ were then assessed from PBLs cultured alone or in the presence of target cells (inside black frame). For the T cells, graphs from samples cultured alone (above dotted line) and in the presence of P815 target cells with anti-CD3 (below dotted line) are shown. Analysis of granule content (granzymes and perforin), co-stimulatory receptors and markers of activation were investigated for the same subsets, but only for unstimulated PBLs. Isotype controls were only used for granzyme A, granzyme B and perforin. Investigation of doublet formation between target cells and lymphocytes was performed by first gating on target cells based on scatter properties (down-arrow), then doublets were selected (high FSC-A/FSC-H), and conjugates were defined based on the expression of lymphocyte markers (CD3+, CD4+, CD8+ and CD3+CD57+). c In the cytotoxicity assay, P815 target cell cytotoxicity was assessed by first gating on the target cells based on scatter properties; subsequently, the target cells were selected based on the target cell tracer dye TFL4 (in the APC channel) at the same time gating away cells dead prior to co-culture (NFL1 detected in the Pacific Orange channel), before target cell cytotoxicity was assessed by PanToxiLux fluorescence (converted by granzyme B/caspase-3 present in the target cell from a non-fluorescent substrate into a fluorescent product detectable in the FITC channel). Target cell cytotoxicity; d MDS-L target cells were initially gated out based on light scatter properties; subsequently, CD34 was used to identify the cells and at the same time gating away cells dead prior to initiation of the co-culture (NFL1). CD34 was chosen to identify the target cells as staining by the target cell tracer TFL4 was poor (data not shown). Finally, the MDS-L-directed cytotoxicity was investigated using PanToxiLux, as described for (c). e BMMC CD34+ target cells were gated out as the other target cells based on light scatter properties, TFL4/NFL1, and subsequently cells positive for CD45 and CD33 were gated away (selection of double-negative cells), penultimately CD34+ cells were selected and finally cytotoxicity assessed by PanToxiLux fluorescence as previously described
Data acquisition
Flow cytometry was performed on BD cytometers, Canto II (bone marrow screening) and Fortessa (granule content/co-stimulatory receptors/degranulation/cytotoxicity) both with FACS Diva software (BD biosciences, Franklin Lakes, NJ). Instrument setup was performed with CST beads. For the combined functional assay, voltages were tweaked to optimize the signal-to-noise ratio and reduce spillover between channels (PE/PE-ECD and BrV605/BrV655/BrV705/BrV785 in particular). Compensation was calculated by the FacsDiva software based on single-stained samples and beads. Raw data can be provided per request.
Data analysis
Flow cytometry data were analyzed using FlowJo v7.6.5 (Treestar Inc). Statistical analysis was performed using GraphPad Prism v5.02 for Windows (GraphPad Software). Nonparametric Mann–Whitney U test (M–W) was used comparing two categories: Kruskal–Wallis test (K–W) with Dunn’s posttest was used when comparing three categories and Wilcoxon signed-ranks test for paired samples. Correlation was assessed with Spearman’s correlation. p values below 0.05 were considered significant.
Results
Increased frequencies of CX3CR1+ T cells in the bone marrow of high-risk MDS patients compared to healthy donors
Our previous study revealed increased levels of CX3CR1+ T cells in the peripheral blood of MDS patients compared to healthy controls. Therefore, we hypothesized that cytotoxic CX3CR1+ lymphocytes may respond to and attempt to eradicate MDS clonal cells in MDS bone marrow. To evaluate the frequency of CX3CR1-expressing T cell subsets in MDS bone marrow, cryopreserved bone marrow aspirates from thirteen low-risk and nine high-risk MDS patients and five healthy controls were thawed, rested overnight and investigated for T cell expression of CX3CR1 by flow cytometry (Fig. 2a). Increased percentages of CX3CR1+ lymphocytes were observed in CD3, CD4+ and CD8+ lymphocyte subsets in bone marrow from high-risk MDS patients compared to healthy controls (Kruskal–Wallis with Dunn’s posttest, N = 25, p < 0.01, p < 0.05 and p < 0.05, respectively).
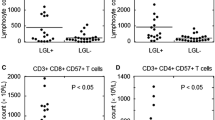
Frequencies of CX3CR1+ T cells in MDS bone marrow, and correlation of CX3CR1 and CD57 on T cells from MDS patients and healthy controls. a Levels of CX3CR1+ T cell subsets in cryopreserved bone marrow samples. Controls, low-risk and high-risk MDS patients represented as white, gray and black circles, respectively. Bars represent median values. Comparisons based on Kruskal–Wallis with Dunn’s posttest. b Spearman correlation (r = 0.9561, p < 0.0001, top left panel) and co-expression (top right panel, bar at median and whisker at interquartile range) of CX3CR1 and CD57 expression on CD3+ T cells peripheral blood from MDS patients and healthy controls. c Degranulation (as shown by CD107a expression) and cytokine production (TNF and IFN-γ) were assessed for CD3+CD57− and CD3+CD57+ subsets when co-cultured with P815 cells with added anti-CD3. Bars represent median and interquartile range (N = 28, Wilcoxon signed ranks) (*p < 0.05, **p < 0.01, ***p < 0.001)
Co-expression of CX3CR1 and CD57 defines T cells with strong cytotoxic potential
Co-culture of P815 cells and PBMCs was performed in order to investigate the functional properties of CX3CR1+ T cells. However, pilot assays revealed markedly diminished CX3CR1 staining intensity for T cells in co-culture with the P815 target cell line with anti-CD3 (data not shown), making CX3CR1 an unsuited subset marker in this functional setting. CX3CR1 and CD57 have both been used to define cytotoxic T cell subsets, and CD57 has previously been used successfully in similar functional assays. To determine whether CD57 could replace CX3CR1 for definition of this specific T cell subset, we examined expression of both surface markers and found that the expression was very closely correlated on CD3+ T cells from both healthy controls and MDS patients (Spearman, N = 28, r = 0.9561 (0.9041–0.9802), p < 0.0001). The same was confirmed by co-expression analysis; CD3+CD57+ and CD3+CX3CR1+ cells showed median positivity for CX3CR1 or CD57 of 85 % or 76 %, respectively, with scatter plots showing a predominant double-positive population (Fig. 2b). Thus, CX3CR1 and CD57 are co-expressed, and CD57 was therefore used to identify this subset in further functional assays. As previously described, the CD3+CD57+ cells demonstrated significantly higher degranulation and production of pro-inflammatory cytokines when co-cultured with P815 cells with anti-CD3 compared to their CD57− counterparts, showing that this subset is of particular interest when performing functional assays (Fig. 2c, Wilcoxon signed ranks, N = 28, CD107a, TNF and IFN-γ, all p < 0.0001). Of note, age was not correlated with peripheral blood levels of CD3+CD57+ or CD3+CX3CR1+ cells in our sample cohort, neither when all samples were investigated together (Spearman, N = 28, r = 0.1380 (−0.2665–0.5012), p = 0.4923 and r = 0.1778 (−0.2282–0.5311), p = 0.3750, respectively), nor when MDS patients and controls were investigated separately (data not shown).
CTL from MDS patients displays reduced cytotoxicity and forms fewer conjugates with target cells
To evaluate the in vitro cytotoxic potential of the CTL, we performed an assay for detection of overt target cell cytotoxicity as measured by the indirect detection of granzyme B and activated caspase-3 inside target cells. Samples from eleven low-risk, eight high-risk and eight healthy controls were used, and the results are presented in Fig. 3a. In this short-term co-culture, we found that CTLs from MDS patients were less potent in delivering ‘lethal hits’ directed at P815 cells with anti-CD3 compared to healthy controls (M–W, N = 27, p = 0.0062) and this was sustained for high-risk but not low-risk MDS patients when compared to healthy controls (K–W with Dunn’s test, N = 27, p < 0.05). Based on light scatter properties (high SSC-H/SSC-A), approximately 1 % of target P815 cells were found as doublets. The percentages of doublets formed between target P815 cells and CD3+, CD4+ and CD8+, but not CD3+CD57+ cell subsets, in the assay were significantly lower in MDS patients, compared to healthy controls (Fig. 3b, M–W, N = 27, p = 0.003, p = 0.002, p = 0.018 and p = 0.102). This was also true for CD3+, CD4+ and CD8+ for high-risk patients and CD4+ for low-risk patients when investigated separately (K–W, N = 27, p < 0.01, p < 0.01, p < 0.05 and p < 0.05, respectively).
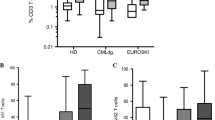
Analysis of TCR-induced redirected cytotoxicity and CD3+CD57+ T cell characteristics. Peripheral blood lymphocytes and P815 target cells with added anti-CD3 were co-cultured for 4 h, and target cell cytotoxicity was measured indirectly by flow cytometry using the PanToxiLux assay (OncoImmunin) for the assessment of intracellular granzyme B and caspase 3 activation in target cells. a Scatter plot showing P815 cytotoxicity following co-culture with PBMCs from healthy controls, low- and high-risk MDS patients (white, gray and black circles, respectively) (Kruskal–Wallis with Dunn’s posttest, N = 27, p < 0.05). b Scatter plot showing doublets of P815 cells and CD3+ cells as a percentage of all P815 cells following co-culture with PBMCs from healthy controls, low- and high-risk MDS patients (white, gray and black circles, respectively). (Kruskal–Wallis with Dunn’s post test. N = 27 *p < 0.05, **p < 0.01). c Effector cell degranulation and cytokine production by CD3+CD57+ T cells were measured in parallel co-cultures. Unstimulated CD3+CD57+ T cells were investigated for cytotoxic granule content (granzyme A, granzyme B and perforin), co-stimulatory receptors (DNAM-1, NKG2D and 2B4), co-inhibitory receptors (PD-1 and TIM3), adhesion molecules (CD11a, CD18 and ICAM) and extracellular markers of activation (HLA-DR, CD38, and CD45RA). Bars represent median and interquartile range (K–W with Dunn’s posttest, N = 24, *p < 0.05)
In order to further investigate potential causes of the observed reduced cytotoxic potency of CTL from high-risk MDS patients, we examined individual factors involved in the granzyme B cytotoxic pathway and other markers of CTL activation and function. The assay was performed in parallel with the in vitro cytotoxicity assay. All investigated PBMC samples showed cytokine production and degranulation of T cells when co-cultured with P815 cells added anti-CD3. Degranulation (CD107a) and TNF and IFN-γ production by CD3+CD57+ CTLs were similar when comparing patients and controls (Fig. 3b).
Untreated PBMCs from patients and controls were investigated for granule content, expression of co-stimulatory/inhibitory receptors, adhesion molecules and surface markers of activation in separate assays (Fig. 3b). No statistically significant differences were observed when comparing levels of granzyme A, granzyme B and perforin in CD3+CD57+ CTL from low- and high-risk MDS patients and healthy controls. Of the co-stimulatory receptors, the level of DNAM-1+ CTLs differed significantly between the groups and was lower in high-risk MDS patients compared to healthy controls (K–W with Dunn’s posttest, N = 24, p < 0.05). Levels of NKG2D+ and 2B4+ cells were not significantly different in these groups. Expression of the co-inhibitory receptor PD-1 was found to be increased on T cells from MDS patients compared to healthy controls for CD3+, CD4+ and CD8+ lymphocyte subsets, but not significantly for CD3+CD57+cells (M–W, N = 20, p = 0.04, p = 0.04, p = 0.03 and p = 0.30) (Fig. 3c). Expression of TIM3 and the adhesion molecules CD11a, CD18 and ICAM were all similar in healthy controls and MDS patients. With regard to markers of activation levels of HLA-DR+, CD38+, CD69+ and CD45RA+ T cells varied between the subsets, but the differences were not statistically significant (K–W with Dunn’s posttest, N = 24, p = 0.05, p = 0.08, p = 0.05 and p = 0.78, respectively). Thus, CD57+ T cells from MDS patients differed from healthy controls by lower expression of the co-stimulatory receptor DNAM-1 in high-risk patients.
P815-directed cytotoxicity varied greatly in these MDS patients (range 3.1–39.5 %). Nine patients had a P815-directed cytotoxicity below the lower 2.5 % confidence interval (11.9 %), based on healthy controls. These nine patients are referred to as the ‘low cytotoxicity’ group and consisted of one RARS, three RCMD, two RAEB-1 and three RAEB-2 patients. When comparing MDS patients in the ‘low cytotoxicity’ group with the remaining MDS patients (the ‘normal cytotoxicity’ group), we observed significantly reduced cytotoxicity (M–W, N = 21, p < 0.001), fewer conjugates between target P815-cells and CD3+CD57+cells (M–W, N = 21, p = 0.015), and reduced expression of CD11a, CD18 and ICAM on CD57+ T cells (M–W, N = 20, p = 0.018, p = 0.010 and p = 0.010, respectively).
NK cells degranulate and produce cytokines without mediating target cell cytotoxicity when co-cultured with the MDS-L cell line
We wanted to investigate whether the CTL was able to elicit an allogeneic immune response in 4-h co-cultures with the human MDS-L cell line. We found that co-culture induced degranulation and cytokine production by NK cells, but not T cells (data not shown). Target MDS-L and NK cell conjugation were significantly lower than T lymphocyte and P815 cell conjugation (M–W, N = 28, p < 0.0001). MDS-L-directed cytotoxicity was generally low (median 0.8 %, range 0–5 %), and there was no difference between controls and MDS patients (data not shown).
Unstimulated NK cells had significantly reduced levels of preformed perforin, granzyme A and surface expression of activating receptor DNAM-1 in high-risk patients compared to controls (K–W with Dunn’s posttest, N = 27, p < 0.001, p < 0.01 and N = 24, p < 0.01, respectively). Levels of 2B4 were reduced in both low- and high-risk MDS patients compared to healthy controls (both p < 0.05).
Cytotoxic T cells but not NK cells from MDS patients respond to autologous bone marrow cells
For six low-risk and four high-risk MDS patients matched samples of patient PBMCs and lymphocyte-depleted bone marrow cells were used to assess whether, and to what extent, cytotoxic lymphocytes recognize autologous bone marrow cells as targets (Fig. 4a). Our results revealed responses dominated by CD57+ T cells, with increased degranulation and production of TNF and IFN-γ compared to the CD57− T cell counterparts and NK cells (K–W with Dunn’s posttest, N = 10, all p < 0.01 and all p < 0.001, respectively). CD57− T cells also displayed increased cytokine production compared to NK cells (TNF p < 0.01 and IFN p < 0.05), but no statistical difference in degranulation was observed between these two subsets. Further, no significant differences in degranulation and cytokine production were observed when comparing low- and high-risk patients (data not shown).
Reactivity of PBLs toward autologous lymphocyte-depleted bone marrow. a Comparison of degranulation and cytokine production of CD57− and CD57+ T cells as well as NK cells following co-culture of PBMCs with autologous lymphocyte-depleted BMMCs. b Degranulation and production of TNF and IFNγ by CD57+ T cells from low-risk and high-risk MDS patients (gray and black bars, respectively). Bars represent median and interquartile range. c CD34+ BMMC-directed cytotoxicity was measured in co-culture of PBMCs and autologous lymphocyte-depleted BMMCs using the PanToxiLux assay (intracellular granzyme B and caspase 3 activation). No significant difference was observed when comparing PanToxiLux levels in CD34+ cells from low- and high-risk MDS patients (N = 11, M–W) (*p < 0.05, **p < 0.01, ***p < 0.001)
CD34+ BMMC-directed cytotoxicity was examined in parallel with the evaluation of degranulation and cytokine production, and granzyme B activity was observed in 0–13.9 % of CD34+CD33− cells (Fig. 4b). There was no significant difference in target cell cytotoxicity between low- and high-risk patients. The results taken together imply a T cell response dominated by CD57+ cells and a negligible response by NK cells. Despite significant levels of degranulation, the autologous CD34+ BMMC-directed cytotoxicity was generally low (Fig. 4c).
CTLs collected prior to treatment seem to display altered auto-reactivity when co-cultured with autologous bone marrow following demethylating therapy
Azacytidine therapy is routinely used in high-risk MDS patients. Increasing evidence points toward immunomodulating properties as a mechanism of action of this hypomethylating agent [28–31]. In the Nordic countries, azacytidine is administered on five consecutive days with a standard daily dose of 100 mg/m2 surface body area. The main effects of azacytidine occur after weeks and months, and multiple cycles of treatment are given if treatment is tolerated. Five of the patients included in the study were treated with azacytidine (in line with standard practice), and for these patients, bone marrow samples were collected prior to, as well as 3–6 months after initiation of treatment.
Bone marrow samples from these three time points were investigated for CD57 expression on T lymphocytes. Further, PBMCs collected prior to treatment were co-cultured with bone marrow samples from the same time points. Degranulation, cytokine production and CD34+ BMMC target cell cytotoxicity were assessed. Levels of CD3+CD57+cells (and CD3+CX3CR1+ cells, data not shown) in bone marrow samples were relatively stable following treatment with azacytidine (Fig. 5a). Degranulation of CD3+CD57+ T cells co-cultured with autologous lymphocyte-depleted bone marrow samples increased from baseline after initiation of azacytidine for three patients (square, up- and down-facing triangles). The two remaining patients (circle and diamond) showed high baseline degranulation, but falling levels after initiation of therapy (Fig. 5b). Production of TNF and IFN-γ followed the level of degranulation closely (data not shown). Levels of CD3+CD57+PD1+ cells increased from baseline for three patients (square, circle and diamond, the latter temporarily), while one showed a decrease from baseline (down-facing triangle). There was insufficient sample material to assess PD-1 expression for the last patient (Figure 5c). The two patients with high baseline degranulation showed 6–11 % CD34+ cytotoxicity, while negligible cytotoxicity (<1 %) was observed in two patients with low baseline degranulation (Fig. 5d). CD34+ BMMC cytotoxicity could not be assessed for the fifth patient. Expression of the co-stimulatory receptors DNAM-1, NKG2D and 2B4 on bone marrow-derived lymphocytes did not change when followed over time, and CD34+ BMMCs were negative for the corresponding ligands (CD112, MIC A/B and CD48, respectively) in the serial samples (data not shown).
Levels of bone marrow CD57+ T cells, CD57+ T cell degranulation and CD34+ BMMC-directed cytotoxicity following initiation of hypomethylating therapy. Assay of serial bone marrow samples taken prior to and following initiation of azacytidine therapy (samples 2 and 3 taken approximately 3–6 months after initiation of treatment). a Frequency of CD57+ T cells in bone marrow samples from the three time points. b Degranulation of CD57+ peripheral blood T cells collected prior to treatment with azacytidine when co-cultured with autologous T and NK cell-depleted BMMC samples from the given time points. c Expression of PD-1 on CD57+ T cells from bone marrow samples collected prior to and following treatment with azacytidine. d CD34+ BMMC-directed cytotoxicity using the PanToxiLux assay (granzyme B and caspase-3 activation) for co-cultures PBMCs collected prior to azacytidine treatment with autologous lymphocyte-depleted BMMCs from the given time points. Symbols used (diamond, square, circle, up- and down-facing triangles) correspond to individual patients in figures a–c
Thus, frequencies of cytotoxic CD57+ and CX3CR1+ T cells in BM appear stable when patients are treated with azacytidine. Peripheral blood CTL respond when co-cultured with lymphocyte-depleted bone marrow and degranulation of these CTL might increase following treatment with this drug.
Discussion
Aberrances in T cell immunology have been described in MDS, particularly with respect to T-helper and Treg subsets. We here describe distribution and function of CTL in peripheral blood and bone marrow of low-risk and high-risk MDS patients. Expansions of cytotoxic effector CD57+ T cells have previously been described in the bone marrow of MDS patients [11]. Corroborating these findings and our previous observations of increased levels of CX3CR1+ T cells in peripheral blood from high-risk MDS patients [13], we also find elevated levels of CX3CR1+ T cells in bone marrow of these patients. Remarkably, CTL derived from high-risk MDS patients exerted reduced cytotoxicity toward the P815 target cell line. On closer examination of CTL, no clear cause for the low cytotoxic potency could be identified by our battery of assays when MDS patients were investigated as one group, or by dividing the patients in two groups based on IPSS-R. However, when we investigated the MDS patients with the lowest target cell cytotoxicity, we found that CTL from these patients had significantly lower levels of CD11a, CD18 and ICAM, and formed fewer conjugates with the target cells compared to CTL from MDS patients with ‘normal’ cytotoxicity. Taken together, this suggests that defective adhesion to target cells as a result of reduced expression of one or more adhesion molecule(s) causes reduced P815 redirected cytotoxicity, in line with previous observations [32, 33]. Defective orientation of the cytotoxic synapse toward target cells could also cause reduced cytotoxic potency [34, 35], but has not been investigated in our study.
There are conflicting results in the literature regarding CTL inhibition of MDS BMMC colony formation. Zheng et al. [36] reported that CD57+ T cells inhibited BMMC colony formation in vitro, while Meers et al. [12] found no correlation between CD3+CD57+ numbers and colonies formed. Our observation of reduced CTL cytotoxic potency in high-risk MDS could shed some light on this controversy; Zheng et al. only included patients from low and intermediate-1 IPSS, where CTL is more likely to exert normal cytotoxicity, while Meers et al. included patients with excess of blasts where reduced CTL cytotoxic capacity could have confounded the analysis.
Co-culture with autologous bone marrow showed T cell auto-reactivity and cytokine production. The allogeneic response by NK cells toward MDS-L might be a result of down-regulation of MHC-I molecules or increased expression of killer immunoglobulin receptor (KIR) ligands, or other factors not assessed, for this severely mutated cell line [25]. In line with previous studies, NK cells from high-risk patients were found to contain lower levels of granzymes and perforin, as well as reduced surface expression of DNAM-1 [37, 38]. Reduced DNAM-1 expression on NK cells parallels our findings of reduced DNAM-1 expression also on CD3+CD57+ cells. Thus, a common factor may regulate DNAM-1 expression on cytotoxic lymphocytes. This is of clinical interest as DNAM-1 has previously been shown to be the main contributor in NK cell-mediated blast-directed cytotoxicity in MDS [38]. Combined evidence suggests reduced cytotoxic efficacy in both T and NK cells from subgroups of MDS patients, and the effect is greatest in high-risk patients and/or with increasing blasts [37, 38].
An immunosuppressive environment is a plausible cause for the reduced cytotoxic capacity of lymphocytes from high-risk MDS patients, but the exact mechanism remains unclear. Tregs [39, 40], MDSCs [26, 41] and leukemic blasts [42, 43] have all been suggested as important contributors. Regardless of the cause of the reduced cytotoxic potential of lymphocytes in high-risk MDS, there could be a therapeutic benefit in increasing it. Importantly, azacytidine, a constituent of the standard treatment for high-risk MDS patients, exhibits immunomodulating properties. Azacytidine has been found to both induce expression of tumor-associated antigens [19] and reduce Treg numbers and suppressive function [30]. However, azacytidine has also been found to increase the expression of immune checkpoint molecules PD-1 and PD-L1 [44, 45]. In the present cohort, MDS patients had higher levels of PD-1+ cells in the CD3+, CD4+ and CD8+ subsets, particularly for the high-risk patients. Our follow-up samples of azacytidine-treated patients did not show a uniform alteration in PD-1, although three of four patients had an increase from low levels (of these, one temporarily) and one decreased from a high baseline. Thus, azacytidine and PD-1-inhibition might be an interesting combination in future MDS clinical trials, and evaluation of baseline PD-1 expression could then be of particular interest, as suggested by Orskov et al. [45].
To conclude, our study indicates that frequencies of cytotoxic CX3CR1+ and CD57+ T cells are increased in MDS bone marrow, but these cells have reduced cytotoxic potential in high-risk MDS; reduced adhesion to target cells seems to be the main functional defect causing reduced cytotoxicity in our model.
Abbreviations
- AML:
-
Acute myeloid leukemia
- BMMC:
-
Bone marrow mononuclear cell
- CTL:
-
Cytotoxic T lymphocyte
- IPSS-R:
-
International prognostic scoring system revised
- K-W:
-
Kruskal–Wallis test
- M-W:
-
Mann–Whitney U test
- MDS:
-
Myelodysplastic syndromes
- PTL:
-
PanToxiLux substrate
- RAEB:
-
Refractory anemia with excess of blasts
- RARS:
-
Refractory anemia with ring sideroblasts
- RCMD:
-
Refractory anemia with multilineage dysplasia
- Treg:
-
T regulatory cell
References
Corey SJ, Minden MD, Barber DL, Kantarjian H, Wang JC, Schimmer AD (2007) Myelodysplastic syndromes: the complexity of stem-cell diseases. Nat Rev Cancer 7(2):118–129. doi:10.1038/nrc2047
Nimer SD (2008) Myelodysplastic syndromes. Blood 111(10):4841–4851. doi:10.1182/blood-2007-08-078139
Elghetany MT (1998) Surface marker abnormalities in myelodysplastic syndromes. Haematologica 83(12):1104–1115
Aggarwal S, van de Loosdrecht AA, Alhan C, Ossenkoppele GJ, Westers TM, Bontkes HJ (2011) Role of immune responses in the pathogenesis of low-risk MDS and high-risk MDS: implications for immunotherapy. Br J Haematol 153(5):568–581. doi:10.1111/j.1365-2141.2011.08683.x
Serio B, Risitano A, Giudice V, Montuori N, Selleri C (2014) Immunological derangement in hypocellular myelodysplastic syndromes. Transl Med UniSa 8:31–42
Kordasti SY, Afzali B, Lim Z, Ingram W, Hayden J, Barber L, Matthews K, Chelliah R, Guinn B, Lombardi G, Farzaneh F, Mufti GJ (2009) IL-17-producing CD4(+) T cells, pro-inflammatory cytokines and apoptosis are increased in low risk myelodysplastic syndrome. Br J Haematol 145(1):64–72. doi:10.1111/j.1365-2141.2009.07593.x
Kotsianidis I, Bouchliou I, Nakou E, Spanoudakis E, Margaritis D, Christophoridou AV, Anastasiades A, Tsigalou C, Bourikas G, Karadimitris A, Tsatalas C (2009) Kinetics, function and bone marrow trafficking of CD4+CD25+FOXP3+ regulatory T cells in myelodysplastic syndromes (MDS). Leukemia 23(3):510–518. doi:10.1038/leu.2008.333
Chen X, Eksioglu EA, Zhou J, Zhang L, Djeu J, Fortenbery N, Epling-Burnette P, Van Bijnen S, Dolstra H, Cannon J, Youn JI, Donatelli SS, Qin D, De Witte T, Tao J, Wang H, Cheng P, Gabrilovich DI, List A, Wei S (2013) Induction of myelodysplasia by myeloid-derived suppressor cells. J Clin Invest 123(11):4595–4611. doi:10.1172/JCI67580
Kordasti SY, Ingram W, Hayden J, Darling D, Barber L, Afzali B, Lombardi G, Wlodarski MW, Maciejewski JP, Farzaneh F, Mufti GJ (2007) CD4+CD25high Foxp3+ regulatory T cells in myelodysplastic syndrome (MDS). Blood 110(3):847–850. doi:10.1182/blood-2007-01-067546
Epperson DE, Nakamura R, Saunthararajah Y, Melenhorst J, Barrett AJ (2001) Oligoclonal T cell expansion in myelodysplastic syndrome: evidence for an autoimmune process. Leuk Res 25(12):1075–1083
Epling-Burnette PK, Painter JS, Rollison DE, Ku E, Vendron D, Widen R, Boulware D, Zou JX, Bai F, List AF (2007) Prevalence and clinical association of clonal T-cell expansions in Myelodysplastic Syndrome. Leukemia 21(4):659–667. doi:10.1038/sj.leu.2404590
Meers S, Vandenberghe P, Boogaerts M, Verhoef G, Delforge M (2008) The clinical significance of activated lymphocytes in patients with myelodysplastic syndromes: a single centre study of 131 patients. Leuk Res 32(7):1026–1035. doi:10.1016/j.leukres.2007.10.004
Sand KE, Rye KP, Mannsaker B, Bruserud O, Kittang AO (2013) Expression patterns of chemokine receptors on circulating T cells from myelodysplastic syndrome patients. Oncoimmunology 2(2):e23138. doi:10.4161/onci.23138
Nishimura M, Umehara H, Nakayama T, Yoneda O, Hieshima K, Kakizaki M, Dohmae N, Yoshie O, Imai T (2002) Dual functions of fractalkine/CX3C ligand 1 in trafficking of perforin +/granzyme B + cytotoxic effector lymphocytes that are defined by CX3CR1 expression. J Immunol 168(12):6173–6180
D’Haese JG, Demir IE, Friess H, Ceyhan GO (2010) Fractalkine/CX3CR1: why a single chemokine-receptor duo bears a major and unique therapeutic potential. Expert Opin Ther Targets 14(2):207–219. doi:10.1517/14728220903540265
Olsson B, Ridell B, Carlsson L, Jacobsson S, Wadenvik H (2008) Recruitment of T cells into bone marrow of ITP patients possibly due to elevated expression of VLA-4 and CX3CR1. Blood 112(4):1078–1084. doi:10.1182/blood-2008-02-139402
Ren J, Hou XY, Ma SH, Zhang FK, Zhen JH, Sun L, Sun YX, Hao YL, Cheng YF, Hou M, Xu CG, Zhang MH, Peng J (2014) Elevated expression of CX3C chemokine receptor 1 mediates recruitment of T cells into bone marrow of patients with acquired aplastic anaemia. J Intern Med 276(5):512–524. doi:10.1111/joim.12218
Kook H, Zeng W, Guibin C, Kirby M, Young NS, Maciejewski JP (2001) Increased cytotoxic T cells with effector phenotype in aplastic anemia and myelodysplasia. Exp Hematol 29(11):1270–1277
Goodyear O, Agathanggelou A, Novitzky-Basso I, Siddique S, McSkeane T, Ryan G, Vyas P, Cavenagh J, Stankovic T, Moss P, Craddock C (2010) Induction of a CD8+ T-cell response to the MAGE cancer testis antigen by combined treatment with azacitidine and sodium valproate in patients with acute myeloid leukemia and myelodysplasia. Blood 116(11):1908–1918. doi:10.1182/blood-2009-11-249474
Leclercq S, Gueugnon F, Boutin B, Guillot F, Blanquart C, Rogel A, Padieu M, Pouliquen D, Fonteneau JF, Gregoire M (2011) A 5-aza-2′-deoxycytidine/valproate combination induces cytotoxic T-cell response against mesothelioma. Eur Respir J 38(5):1105–1116. doi:10.1183/09031936.00081310
Chou J, Voong LN, Mortales CL, Towlerton AM, Pollack SM, Chen X, Yee C, Robbins PF, Warren EH (2012) Epigenetic modulation to enable antigen-specific T-cell therapy of colorectal cancer. J Immunother 35(2):131–141. doi:10.1097/CJI.0b013e31824300c7
Britten CM, Janetzki S, Butterfield LH, Ferrari G, Gouttefangeas C, Huber C, Kalos M, Levitsky HI, Maecker HT, Melief CJ, O’Donnell-Tormey J, Odunsi K, Old LJ, Ottenhoff TH, Ottensmeier C, Pawelec G, Roederer M, Roep BO, Romero P, van der Burg SH, Walter S, Hoos A, Davis MM (2012) T cell assays and MIATA: the essential minimum for maximum impact. Immunity 37(1):1–2. doi:10.1016/j.immuni.2012.07.010
Chiang SC, Theorell J, Entesarian M, Meeths M, Mastafa M, Al-Herz W, Frisk P, Gilmour KC, Ifversen M, Langenskiold C, Machaczka M, Naqvi A, Payne J, Perez-Martinez A, Sabel M, Unal E, Unal S, Winiarski J, Nordenskjold M, Ljunggren HG, Henter JI, Bryceson YT (2013) Comparison of primary human cytotoxic T-cell and natural killer cell responses reveal similar molecular requirements for lytic granule exocytosis but differences in cytokine production. Blood 121(8):1345–1356. doi:10.1182/blood-2012-07-442558
Tohyama K, Tsutani H, Ueda T, Nakamura T, Yoshida Y (1994) Establishment and characterization of a novel myeloid cell line from the bone marrow of a patient with the myelodysplastic syndrome. Br J Haematol 87(2):235–242
Matsuoka A, Tochigi A, Kishimoto M, Nakahara T, Kondo T, Tsujioka T, Tasaka T, Tohyama Y, Tohyama K (2010) Lenalidomide induces cell death in an MDS-derived cell line with deletion of chromosome 5q by inhibition of cytokinesis. Leukemia 24(4):748–755. doi:10.1038/leu.2009.296
Kittang AO, Kordasti S, Sand KE, Costantini B, Kramer AM, Perezabellan P, Seidl T, Rye KP, Hagen KM, Kulasekararaj A, Bruserud O, Mufti GJ (2016) Expansion of myeloid derived suppressor cells correlates with number of T regulatory cells and disease progression in myelodysplastic syndrome. Oncoimmunology 5(2):e1062208. doi:10.1080/2162402X.2015.1062208
Shaw S, Luce GE, Quinones R, Gress RE, Springer TA, Sanders ME (1986) Two antigen-independent adhesion pathways used by human cytotoxic T-cell clones. Nature 323(6085):262–264. doi:10.1038/323262a0
Gao XN, Lin J, Wang LL, Yu L (2009) Demethylating treatment suppresses natural killer cell cytolytic activity. Mol Immunol 46(10):2064–2070. doi:10.1016/j.molimm.2009.02.033
Bontkes HJ, Ruben JM, Alhan C, Westers TM, Ossenkoppele GJ, van de Loosdrecht AA (2012) Azacitidine differentially affects CD4(pos) T-cell polarization in vitro and in vivo in high risk myelodysplastic syndromes. Leuk Res. doi:10.1016/j.leukres.2012.03.026
Costantini B, Kordasti SY, Kulasekararaj AG, Jiang J, Seidl T, Abellan PP, Mohamedali A, Thomas NS, Farzaneh F, Mufti GJ (2013) The effects of 5-azacytidine on the function and number of regulatory T cells and T-effectors in myelodysplastic syndrome. Haematologica 98(8):1196–1205. doi:10.3324/haematol.2012.074823
Wang LX, Mei ZY, Zhou JH, Yao YS, Li YH, Xu YH, Li JX, Gao XN, Zhou MH, Jiang MM, Gao L, Ding Y, Lu XC, Shi JL, Luo XF, Wang J, Wang LL, Qu C, Bai XF, Yu L (2013) Low dose decitabine treatment induces CD80 expression in cancer cells and stimulates tumor specific cytotoxic T lymphocyte responses. PLoS ONE 8(5):e62924. doi:10.1371/journal.pone.0062924
Koneru M, Monu N, Schaer D, Barletta J, Frey AB (2006) Defective adhesion in tumor infiltrating CD8 + T cells. J Immunol 176(10):6103–6111
Zhao C, Morgan M, Haeryfar SM, Blay J, Hoskin DW (2003) Exposure to paclitaxel or vinblastine down-regulates CD11a and CD54 expression by P815 mastocytoma cells and renders the tumor cells resistant to killing by nonspecific cytotoxic T lymphocytes induced with anti-CD3 antibody. Cancer Immunol Immunother 52(3):185–193. doi:10.1007/s00262-002-0357-4
Radoja S, Saio M, Schaer D, Koneru M, Vukmanovic S, Frey AB (2001) CD8(+) tumor-infiltrating T cells are deficient in perforin-mediated cytolytic activity due to defective microtubule-organizing center mobilization and lytic granule exocytosis. J Immunol 167(9):5042–5051
Ou-Yang CW, Zhu M, Fuller DM, Sullivan SA, Chuck MI, Ogden S, Li QJ, Zhang W (2012) Role of LAT in the granule-mediated cytotoxicity of CD8 T cells. Mol Cell Biol 32(14):2674–2684. doi:10.1128/MCB.00356-12
Zheng Y, Watanabe N, Nagamura-Inoue T, Igura K, Nagayama H, Tojo A, Tanosaki R, Takaue Y, Okamoto S, Takahashi TA (2003) Ex vivo manipulation of umbilical cord blood-derived hematopoietic stem/progenitor cells with recombinant human stem cell factor can up-regulate levels of homing-essential molecules to increase their transmigratory potential. Exp Hematol 31(12):1237–1246
Epling-Burnette PK, Bai F, Painter JS, Rollison DE, Salih HR, Krusch M, Zou J, Ku E, Zhong B, Boulware D, Moscinski L, Wei S, Djeu JY, List AF (2007) Reduced natural killer (NK) function associated with high-risk myelodysplastic syndrome (MDS) and reduced expression of activating NK receptors. Blood 109(11):4816–4824. doi:10.1182/blood-2006-07-035519
Carlsten M, Baumann BC, Simonsson M, Jadersten M, Forsblom AM, Hammarstedt C, Bryceson YT, Ljunggren HG, Hellstrom-Lindberg E, Malmberg KJ (2010) Reduced DNAM-1 expression on bone marrow NK cells associated with impaired killing of CD34+ blasts in myelodysplastic syndrome. Leukemia 24(9):1607–1616. doi:10.1038/leu.2010.149
Fu J, Xu D, Liu Z, Shi M, Zhao P, Fu B, Zhang Z, Yang H, Zhang H, Zhou C, Yao J, Jin L, Wang H, Yang Y, Fu YX, Wang FS (2007) Increased regulatory T cells correlate with CD8 T-cell impairment and poor survival in hepatocellular carcinoma patients. Gastroenterology 132(7):2328–2339. doi:10.1053/j.gastro.2007.03.102
Geffner L, Basile JI, Yokobori N, Sabio YGC, Musella R, Castagnino J, Sasiain MC, de la Barrera S (2014) CD4(+) CD25(high) forkhead box protein 3(+) regulatory T lymphocytes suppress interferon-gamma and CD107 expression in CD4(+) and CD8(+) T cells from tuberculous pleural effusions. Clin Exp Immunol 175(2):235–245. doi:10.1111/cei.12227
Spallanzani RG, Dalotto-Moreno T, Raffo Iraolagoitia XL, Ziblat A, Domaica CI, Avila DE, Rossi LE, Fuertes MB, Battistone MA, Rabinovich GA, Salatino M, Zwirner NW (2013) Expansion of CD11b(+)Ly6G (+)Ly6C (int) cells driven by medroxyprogesterone acetate in mice bearing breast tumors restrains NK cell effector functions. Cancer Immunol Immunother 62(12):1781–1795. doi:10.1007/s00262-013-1483-x
Wu RC, Hwu P, Radvanyi LG (2012) New insights on the role of CD8(+)CD57(+) T-cells in cancer. Oncoimmunology 1(6):954–956. doi:10.4161/onci.20307
Wu RC, Liu S, Chacon JA, Wu S, Li Y, Sukhumalchandra P, Murray JL, Molldrem JJ, Hwu P, Pircher H, Lizee G, Radvanyi LG (2012) Detection and characterization of a novel subset of CD8(+)CD57(+) T cells in metastatic melanoma with an incompletely differentiated phenotype. Clin Cancer Res 18(9):2465–2477. doi:10.1158/1078-0432.CCR-11-2034
Yang H, Bueso-Ramos C, DiNardo C, Estecio MR, Davanlou M, Geng QR, Fang Z, Nguyen M, Pierce S, Wei Y, Parmar S, Cortes J, Kantarjian H, Garcia-Manero G (2014) Expression of PD-L1, PD-L2, PD-1 and CTLA4 in myelodysplastic syndromes is enhanced by treatment with hypomethylating agents. Leukemia 28(6):1280–1288. doi:10.1038/leu.2013.355
Orskov AD, Treppendahl MB, Skovbo A, Holm MS, Friis LS, Hokland M, Gronbaek K (2015) Hypomethylation and up-regulation of PD-1 in T cells by azacytidine in MDS/AML patients: a rationale for combined targeting of PD-1 and DNA methylation. Oncotarget 6(11):9612–9626
Acknowledgments
We thank Professor Einar Kristoffersen, Department of Clinical Science, University of Bergen for scientific advice, and Kristin Paulsen and Marie Hagen for technical assistance in the laboratory. We also thank Professor Kaoru Tohyama, Kobe University Graduate School of Medicine, Kobe, Japan, for generously supplying the MDS-L cell line. The flow cytometry for the cytotoxicity assay was performed at the Molecular Imaging Center, Dept. of Biomedicine, University of Bergen. This work was supported by the Western Norway Regional Health Authority (Helse Vest), University of Bergen and the Norwegian Cancer Society (Kreftforeningen).
Author contributions
K Sand performed research, analyzed and interpreted data and drafted the manuscript; J. Theorell performed research, interpreted data and drafted the manuscript; Ø. Bruserud designed research, interpreted data and drafted the manuscript; Y. Bryceson designed research, interpreted data and drafted the manuscript; and A. Olsnes Kittang designed research, provided patient material and clinical data, interpreted data, drafted the manuscript and was responsible for preparing the final version of the manuscript.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
No potential conflicts of interest to disclose.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Sand, K., Theorell, J., Bruserud, Ø. et al. Reduced potency of cytotoxic T lymphocytes from patients with high-risk myelodysplastic syndromes. Cancer Immunol Immunother 65, 1135–1147 (2016). https://doi.org/10.1007/s00262-016-1865-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00262-016-1865-y