Abstract
Background
We previously reported overexpression of heat-shock protein (HSP) 70 in hepatitis C virus (HCV)-related hepatocellular carcinoma (HCC) using proteomic profiling and immunohistochemical staining (IHS). This suggested that HSP70 could be a molecular target for treatment of HCC.
Methods
Twelve patients with HCV-related HCC were enrolled in a phase 1 clinical trial. Dendritic cells (DCs) transfected with HSP70 mRNA (HSP70-DCs) induced by electroporation were injected intradermally. Patients were treated three times every 3 weeks. The number of HSP70-DCs injected was 1 × 107 as the lowest dose, then 2 × 107 as the medium dose, and then 3 × 107 as the highest dose. Immunological analyses were performed.
Findings
No adverse effects of grade III/IV, except one grade III liver abscess at the 3 × 107 dose, were observed. Thus, we added three more patients to confirm whether 3 × 107 is an appropriate dose. Eventually, we chose 3 × 107 as the recommended dose of DCs. Complete response (CR) without any recurrence occurred in two patients, stable disease in five, and progression of disease in five. The two patients with CR have had no recurrence for 44 and 33 months, respectively. IHS in one patient who underwent partial hepatectomy showed infiltration of CD8+ T cells and granzyme B in tumors, indicating that the dominant immune effector cells were cytotoxic T lymphocytes with tumor-killing activity.
Interpretation
This study demonstrated that HSP70-DCs therapy is both safe and feasible in patients with HCV-related HCC. Further clinical trials should be considered.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Hepatocellular carcinoma (HCC) is one of the most common fatal malignancies worldwide with an annual mortality of approximately 600,000 [1]. Chronic infection with hepatitis B virus (HBV) or hepatitis C virus (HCV) is the most clearly established risk factor for HCC [2]. In Japan, approximately 80 % of HCC patients are attributable to chronic HCV infection [3, 4]. In spite of recent advancements in patient management, the 5-year survival rate of HCC patients is low (26–50 %) after curative surgery [5]. Despite many studies, HCC prognosis has seen little improvement because HCC tends to metastasize frequently in the liver as well as distant organs, resulting in death [6–10]. Furthermore, patients with HCV-related HCC usually have liver dysfunction, which restricts chemotherapy because of potential liver damage. Novel therapies with fewer adverse effects should therefore be considered.
Since HCC has been shown to be immunogenic, T cell-based immunotherapy is considered promising [11, 12]. Activation of an HCC-specific response can be accomplished by strategies targeting tumor-associated antigens (TAA). It is critical to find molecular targets or TAAs for the successful development of immunotherapies. We previously found that the heat-shock protein (HSP) 70 family is overexpressed in HCC tissues associated with HCV infection compared with normal liver tissue samples using proteomic analysis [13] and that 92 % of HCCs express HSP70 according to immunohistochemical staining [14]. These findings suggest that HSP70 may be a target molecule for HCV-related HCC.
DCs loaded with tumor-specific or tumor-associated antigens have been shown to induce immune responses. DCs pulsed with DNA or RNA of a certain antigen could lead to prolonged presentation of the antigen and generate a high-affinity tumor-reactive CTL response [15]. It has been shown that DCs transfected with RNA encoding a specific TAA can induce potent antigen- and tumor-specific T cell responses directed against multiple epitopes [16]. Electroporation is the most efficient technique for transfecting human DCs [17]. These reports suggest that the introduction of HSP mRNA to DCs may be useful for treatment of HCC.
We therefore conducted a phase 1 immunotherapy study using HSP70 mRNA-transfected DCs (HSP70-DCs) via electroporation to treat patients with HCV-related unresectable or recurrent HCC.
Patients and methods
Study design
This was a single-center, phase 1 immunotherapy study using HSP70-DCs in patients with HCV-related unresectable or recurrent HCC. Patients were treated at the Department of Digestive Surgery and Surgical Oncology (Department of Surgery II) of Yamaguchi University Graduate School of Medicine between 2007 and 2011. Primary objectives were to evaluate safety, feasibility, and toxicity. Secondary objectives were to examine immune function such as cytotoxic T cell (CTL) induction and changes in lymphocyte subsets in PBMC. Additionally, clinical response was evaluated according to the Response Evaluation Criteria in Solid Tumors guideline version 1.1 (RECIST).
Patients with stable disease (SD) were eligible. HSP70-DCs were intradermally administered three times at intervals of 3 weeks. Subject to HSP70-DCs therapy yields, a 3-tiered dose escalation strategy using three cohorts of patients was planned with low, medium, and high doses of 1 × 107, 2 × 107, and 3 × 107 DCs, respectively. Adverse effects were evaluated according to the Common Terminology Criteria for Adverse Events version 3.0 (CTCAE). Patients were observed and vital signs monitored before, during, and for 30 min after each injection. If grade 3 or 4 adverse effects appeared in one of three patients, another three patients were added. If grade 3 or 4 toxicities were observed in two of three patients or in four of six patients, the dose escalation was discontinued.
Clinical and computed tomography (CT) evaluations were performed at baseline, before and after the first three DC administrations, and 1 month after the final injection according to RECIST [18].
Pretreatment evaluation and follow-up
Pretreatment evaluation included a complete medical history, physical examination, imaging of measurable tumors using CT and magnetic resonance imaging (MRI), complete blood cell count, and a biochemical screening profile. During treatment, patient monitoring included assessment of clinical toxicities, complete blood cell count, serum chemistry, and physical examination. Tumor markers such as α-fetoprotein (AFP) and protein induced by vitamin K absence or antagonist II (PIVKA-II) were measured. Adverse effects were evaluated according to CTCAE.
Patient eligibility
During 2007–2011, 12 patients with unresectable or recurrent HCV-related HCC were enrolled in this trial. The main eligibility criteria included the following: measurable lesions according to the RECIST criteria, life expectancy of more than 3 months, age between 20 and 85, Eastern Cooperative Oncology Group Performance Status (ECOG PS) of 0–1, no previous therapy such as resection, radiofrequency ablation (RFA), transcatheter arterial chemoembolization (TACE), or sorafenib within 4 weeks prior to the study, written informed consent to participate in the study, no major complications or active concurrent malignancies, and no history of drug allergy.
The study protocol was approved by the Institutional Review Board for Human Use of Yamaguchi University School of Medicine.
Generation of HSP70-mRNA
RNAs of HSP70 were transcribed in vitro. A full-HSP70 cDNA was cloned into the pcDNA3.1 plasmid. Clones containing the HSP70 cDNA were generated using a Quantum Prep™ Plasmid Midiprep Kit (Bio Rad, Hercules, CA). In vitro transcription was then carried out using an mMessage mMachine® T7 Ultra Kit (Ambion, Austin, TX) to cap RNAs with 3′-O-Me-m7G(5′) pppG and to add a poly(A) tail according to the manufacturer’s protocol.
Preparation of HSP70-DCs
Peripheral blood mononuclear cells (PBMCs) were harvested with the COBE Spectra Apheresis System (COBE BCT, Inc., Lakewood, CO) every 3 weeks. The PBMCs from 3000 ml of blood were enriched by density gradient centrifugation with Ficoll-Paque (Amersham Pharmacia Biotech, Uppsala, Sweden). The PBMCs were incubated for 45 min in a 5 % CO2 atmosphere at 37 °C in serum-free AIM-V medium (Gibco, Paisley, Scotland). Plastic-adherent cells were cultured in AIM-V medium containing 800 units/mL granulocyte–macrophage colony-stimulating factor (GM-CFS) (Osteogenetics GmbH, Wurzburg, Germany) and 500 units/mL interleukin-4 (IL-4) (Osteogenetics GmbH). On day 6, immature DCs were cultured in AIM-V medium containing 300 units/mL tumor necrosis factor-α (TNF-α; R&D Systems, Minneapolis, MN). Cultures were checked for endotoxins, mycoplasma, and bacterial contamination prior to administration. On day 10, floating and loosely adherent cells were collected as mature DCs. Maturated DCs (2 × 106 cells/400 µL) and 10 µg of HSP70 mRNA were mixed and electroporated for 500 µs with 400 V (Harvard Apparatus, Holliston, MA). DCs were washed three times with saline, suspended in 2 mL saline, and injected intradermally in the inguinal region.
Analysis of DC and lymphocyte subsets using flow cytometry
Subsets of immature and HSP70-DCs were analyzed with monoclonal antibodies against surface antigens using a flow cytometer (MACSQuant Analyzer, Miltenyi Biotec, Bergisch Gladbach, Germany). All monoclonal antibodies were purchased from Coulter Immunology (Hialeah, FL). Fluorescein isothiocyanate (FITC)-conjugated anti-CD80 (B7-1), anti-CD83 (HB-15), anti-CD14 (B1), anti-HLA-ABC, and anti-HLADR (I2) were used. PE-conjugated anti-CD86 (B7-2) and anti-CD40 were also used according to the manufacturer’s instructions.
Isolation of PBMCs
Blood from each patient was drawn at four time points before starting the treatment and after the first, second, and third treatments. The drawn blood was processed, and PBMCs were isolated by a Pancoll® gradient (PAN Biotech, Aidenbach, Germany). Right after the isolation, PBMCs were frozen with CellBanker (Nippon Zenyaku Kogyo, Fukushima, Japan). These samples were used for enzyme-linked immunospot (ELISPOT) assays and flow cytometric analysis.
Enzyme-linked immunospot (ELISPOT)
PBMCs as effector cells (2 × 105 cells) responding to PBMCs as stimulator cells (2 × 105 cells) electroporated with HSP70 mRNA or PBMCs as a negative control of stimulator cells electroporated without mRNA were measured for interferon gamma (IFN-γ) using an ELISPOT assay according to the manufacturer’s instructions (Mabtech, Cincinnati, OH). PBMCs were plated and incubated for 18–20 h in an incubator with stimulator cells. Assays were performed in duplicate. The number of spots on a plate was counted by an Eliphoto Scan (Minerva Tech, Tokyo, Japan). HSP70-specific spots of IFN-γ were calculated as described below.
A: PBMCs co-cultured with PBMCs electroporated with HSP70 mRNA and B: PBMCs co-cultured with PBMCs electroporated without mRNA.
Population kinetics of immune effector cells in patients
Surface markers on PBMCs were also measured before treatment and after the first treatment, second treatment, and 1 month after the third treatment using a flow cytometer. The following staining monoclonal antibodies (mAb) were used: anti-CD3, anti-CD56, anti-CD57, and anti-NKG2D (Coulter Immunology), and the cells were counted on a flow cytometer. Matched isotype control antibodies were used.
CD107a staining and intracellular cell staining for IFN-γ
The PBMCs were processed according to the manufacturer’s instructions (BD Biosciences). These cells were stained with CD3, CD8, CD56, and CD107a. The cells were then stained with IFN-γ-PE (BD Biosciences). Cells were assayed on a flow cytometer.
Immunohistochemical staining of HSP70, HLA class I, CD8, CD56, and granzyme B
Patient 12 underwent partial hepatectomy after this immunotherapy. Immunohistochemical staining (IHS) was performed using an EnVision+ kit according to the manual provided by Dako (Carpinteria, CA). Anti-Hsp70 mAb, anti-HLA class 1 ABC mAb, anti-CD8 mAb, anti-granzyme B mAb, and anti-CD56 mAb (Abcam, Cambridge, UK) were used for the staining.
Statistical analysis
A p value of <0.05 was considered significant. Values are presented as mean ± standard error (SE). Kaplan–Meier analysis was used to estimate cumulative survival. Statistical analysis was performed with SPSS version 20.
Results
Patient characteristics
The clinicopathological features of the patients are described in Tables 2 and 3. Briefly, eligible patients were aged 56–82 years (male 10 and female 2); all patients were Child–Pugh score A or B and never received sorafenib.
Immune profiles of induced dendritic cells
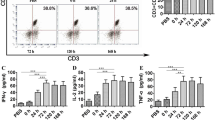
Table 1 shows a comparison of surface marker expression between immature DCs (IM-DCs) and mature DCs (M-DCs). Data show the percentage of each surface marker on day 6, showing IM-DCs, and on day 9, showing M-DCs. CD80, CD83, and CD86 were vastly increased in M-DCs compared with IM-DCs (p < 0.001). HLA class II expression was slightly higher in M-DCs than in IM-DCs (p < 0.05), but HLA class I expression was identical between the two (Table 2).
Toxicity
Twelve patients received HSP70-DCs. Five of the patients had adverse effects (Table 3). At the low dose (1 × 107 DC cells) and medium dose (2 × 107), no grade 3 or 4 toxicities were observed. At the high dose (3 × 107), one out of three patients had grade 3 toxicity with liver abscess, while another three patients treated with the high dose had no severe toxicities. The patient who received the grade 3 toxicity with liver abscess was successfully treated with antibiotics. The administered DCs of the patient with the liver abscess were double-checked and confirmed in vitro that there was no contamination. In addition, the location of the abscess was different than the site of the treated tumor, indicating that the abscess was not associated with tumor lysis with this treatment. There was no local toxicity, which is notable because other vaccines have been associated with some local toxicity.
Tumor responses
Tumor response was evaluated by tumor markers and CT or MRI in all patients (Table 3). Two patients achieved a complete response (CR), five had SD, and five had progressive disease (PD). Recurrence or multi-centric carcinogenesis in the two CR patients has not been observed for more than 3 years. Details of these CR patients are as follows.
Patient 1: A 69-year-old female, who had received a partial hepatectomy for HCC 8 months prior, had recurrent HCC with a tumor size of 20 mm in segment (S) 8. AFP and PIVKA-II levels were 9.2 ng/mL (normal: <20) and 143 mAU/mL (normal: <40), respectively (Supplementary Fig. 1). These levels became within the normal range 1 month after treatment. MRI showed CR 1 month after treatment (Fig. 1a). The patient has had no liver tumors as of 51 months after treatment.
a Chronological MRI of patient 1. Early phase of dynamic-enhanced EOB-MRI shows patient 1 with CR 1 month after treatment. Upper left image shows the pretreatment state with enhanced lesion. Upper right image shows the state of 1 month after treatment with disappearance of the former lesion. Lower left shows the state 1 year after treatment with the same state as before. The patient has had no liver tumor after 44 months of follow-up. b Chronological CT of patient 4. Early phase of dynamic CT shows patient 4 with CR 3 months after treatment. Upper left CT shows the pretreatment state with enhanced lesion. Upper right CT shows the state 1 month after treatment with a 37.5 % reduction in the former lesion. Lower left CT shows the state 3 months after treatment with complete disappearance of the lesion. Lower right shows the state 2 years after treatment with the same state as before. The patient has had no liver tumors as of a 38-month follow-up
Patient 4: A 59-year-old male underwent partial hepatectomy for tumors located in S5 and S8, and ablation for HCC in S8. Since follow-up CT had revealed unsuccessful ablation for HCC in S8, the patient received HSP70 mRNA-DC therapy 1 month after the surgery. Figure 1b shows CT at 1 month prior to treatment and after 1 month, 3 months, and 2 years of follow-up. Dynamic CT showed a typical HCC image such as enhancement at the early phase and washout at the delayed phase. The tumor size was decreased after 1 month and disappeared 3 months later. AFP levels decreased from 199.1 to 99.1 ng/mL; however, this level is above the normal range and only returned to normal 10 months after therapy, maintaining this level for 33 months at present (Supplementary Fig. 2). The patient has had no liver tumors as of a 37-month follow-up by dynamic CT.
Enzyme-linked immunospot analysis
IFN-γ ELISPOT assays were performed to evaluate the activity of immune effector cells. The number of spots was 0.3 ± 0.3, 10.3 ± 5.1, 9.3 ± 4.3, and 9.0 ± 6.3 prior to treatment, after first treatment, after second treatment, and at 1 month after the third treatment, respectively (Fig. 2a). The number of spots in ELISPOT assays at the two times showed that CR had 39 spots, SD had 8.4 (± 7.4, SE), and PD had 7.2 (± 3.9, SE) (Fig. 2b). These were not statistically significant. Although IFN-γ activity was increased according to this assay, it was still unclear which cells had high activity, such as CTL or NK cells.
a Enzyme-linked immunospot analysis. The number of spots is shown. Time points tested were prior to treatment, after the first treatment, after the second treatment, and 1 month after the third treatment. Data are mean ± standard error. b Number of spots in ELISPOT assays at two time points. CR had 39 spots, SD had 8.4 (±7.4, SE), and PD had 7.2(±3.9, SE). c Population kinetics of immune effector cells in patients. Patients with CR and SD tended to have a higher percentage of CD4+/FoxP3+ T cells. In patients with CR and SD, the percentage of CD3−/CD56+ cells, which are supposed to be NK cells, were higher than in PD. For NKG2D-expressing NK cells, the percentages in the patients of CR and SD increased, but not in PD. CD57+ NK cells from the group of CR and SD had the same trend, indicating that activated NK cells were induced by treatment. However, these trends were not statistically significant
Population kinetics of immune effector cells in patients
To specify the immune effector cells, we performed flow cytometric analysis using PBMCs from patients. The percentages of each immune effector cell are shown in Fig. 2c. In particular, CD3-/CD56+ cells, which are supposed to be NK cells in patients with CR and SD, were significantly higher than those in the patients with PD at the second administration (p < 0.046).
The percentages of patients with CR and SD tended to have higher percentages of CD4+/FoxP3+ T cells. For NKG2D-expressing NK cells, the percentages in patients with CR and SD increased, but not in PD. CD57-positive NK cells from CR and SD had the same trend, indicating that activated NK cells were induced by treatment. However, these trends were not statistically significant.
CD107a staining and intracellular cell staining for IFN-γ
We next sought to discover which effector cells were activated by this treatment. We performed staining of IFN-γ and CD107a for PBMCs from patient 12. This patient showed a significant number of IFN-γ spots from the ELISPOT assay (Fig. 2a). The percentages of IFN-γ+/CD3+/CD8+ and IFN-γ+/CD3−/CD56+ cells were 66.8 and 61.6 %, respectively (control of each: 41.6, 43.1 %). The percentage of CD107a+/CD3+/CD8+ was 12.9 % (control: 5.5 %) (Fig. 3).
Enhanced IFN-γ production and cytotoxic T lymphocyte activity in patient 12. PBMCs from patient 12 were analyzed by flow cytometry. a and d show the percentage of IFN-γ+/CD3+/CD8+ cells. b and e show the percentage of IFN-γ+/CD3−/CD56+ cells. c and f show the percentage of CD107a+/CD3+/CD8+ cells. a–c show data from responder PBMCs co-cultured with stimulator PBMCs without HSP70-mRNA (mRNA(−)). d–f show data of responder PBMCs co-cultured with stimulator PBMCs encoding HSP70-mRNA (mRNA(+)). The production of IFN-γ and the expression of CD107a were observed in both CTL and NK cells. In CTL, these were relatively highly produced and expressed
Immunohistochemical expression of HSP70, HLA class 1, CD8, granzyme B, and CD56 in patient 12
Patient 12 underwent partial hepatectomy after this immunotherapy. The tumor site of the resected specimen expressed HSP70 and HLA class 1 by IHC. At the site of these expressions, dense CD8+ T cells were infiltrated intratumorally and widely spread. These dense infiltrated cells expressed granzyme B as well. A few CD56+ cells were detected intratumorally (Fig. 4). Based on data of the ELISPOT assay, flow cytometry, and IHC in patient 12, we determined that CTLs are the dominant immune effector cells, rather than NK cells at the tumor site.
Immunohistochemical expression of HSP70, HLA class 1, CD8, granzyme B, and CD56 in patient 12. The tumor site of the resected specimen expresses HSP70 (a) and HLA class 1 (b) by IHC. At the site of these expressions, dense CD8+ T cells had infiltrated intratumorally and were widely spread (c). These dense infiltrated cells expressed granzyme B as well (d). A few CD56+ cells were detected intratumorally (e)
Discussion
Although surgical resection and ablation are the primary treatments for HCC, recurrence is still common and is the main cause of patient death. Effective adjuvant chemotherapy for HCC is needed to improve survival. However, clear benefits of adjuvant or chemopreventive therapy have not been definitively demonstrated [19].
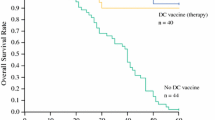
We performed a phase 1 study using HSP70-DCs for 12 patients with unresectable or recurrent HCV-related HCC. All patients completed the regimen, and no severe adverse effects except for a grade 3 liver abscess were observed. Seven out of 12 patients had CR or SD, suggesting that this therapy is potentially powerful and effective. Surprisingly, two CR patients have been followed up without a new lesion in the liver and without another distant metastasis in over 3 years after treatment.
The recommended dose of DC therapy is generally 107 cells as according to previous reports [20, 21]. The maximum dose for which we can show clear patient benefit is 3 × 107 cells. We did not observe a maximum tolerated dose until 3 × 107 cells and therefore decided that this dose is the recommended maximum based on safety. Efficacy was not dependent on the dosage in this study; as such, is why we decided that the recommended dose should not be based on efficacy.
The immune system has great potential for the specific destruction of tumors with no toxicity to normal tissue and for a long-term memory that can prevent cancer recurrence. Xie et al. [22] evaluated about six immunotherapies against HCC using meta-analysis. They concluded that adjuvant immunotherapy with cytokines that induces killer cells or lymphokine-activated killer cells may reduce recurrence in postoperative HCC patients, but may not improve survival. Tada et al. [23] reported a multiple TAAs-pulsed DC vaccine using AFP, glypican-3, and MAGE-1 recombinant fusion proteins for advanced HCC. All five patients showed T cell responses against TAAs. One clinical benefit case was observed as SD out of the five patients. Palmer et al. [24] reported adaptive immunotherapy using DCs pulsed with tumor cell line lysate (HepG2) in patients with HCC. They reported that one patient was PR, and six patients were SD out of 25 patients. Compared with other solid tumors, the success rate of immunotherapy against HCC is relatively low.
We found that HSP70 is overexpressed on HCC tissues associated with HCV infection compared with normal liver tissue samples using proteomic analysis [13]. Theoretically, HSP70 overexpression is induced by stress, and cancer cells exist in a stressful environment. In fact, elevated levels of HSP70 have been reported in human cancer cells [25], and it has been reported that early HCCs overexpress HSP70 in the liver [26]. We also reported that 92 % of HCV-related HCCs express HSP70 by immunohistochemical staining [14]. Recently, HSP70 has attracted attention from tumor immunology researchers because it has been revealed to function as an endogenous danger signal that can increase the immunogenicity of tumors and induce CTL responses. It has also been reported that HSP70 activates innate immunity in a mouse model [27, 28]. HSPs can bind and present TAA to professional antigen-presenting cells (APCs) through MHC class I and class II molecules. This mechanism leads to the activation of anti-tumor cytotoxic and helper T cells [29]. HSP70-peptide complexes coordinately activate innate immune responses and deliver antigens for representation by MHC class I and II molecules on the APC surface, leading to specific anti-tumor adaptive immunity [30]. We therefore decided to use HSP70 as a target to treat HCV-related HCC.
Zhang et al. [31] reported that DCs transfected with AFP mRNA induce an AFP-specific T cell response. Gilboa et al. [32] found that mRNA transfection is superior to other antigen-loading techniques in generating immunopotent DCs. These findings encouraged our study in terms of using mRNA-encoding DCs. In mouse model, it has been demonstrated that HSP70 can stimulate the immune system through CD40 effectively [33].
HSP70-DCs may be a powerful immune stimulator and useful for the treatment of HCC. In fact, we observed two CR patients without recurrence over 3 years. Although our treatment was effective, the precise mechanism remains unclear. Immunological analysis demonstrated that PBMCs isolated from patients who received HSP70-DCs therapy had an HSP70-specific reaction. ELISPOT assays showed that immune effector cells were activated by this immunotherapy. In the population kinetic study for immune effector cells, the number of Foxp3+/CD4+ cells was higher in patients with CR and SD. The reason may be that the population of Foxp3+/CD4+ cells not only consists of Treg but also includes activated CD4+ T cells. NK, NKG2D-positive NK, and activated NK cells had higher numbers in patients with CR and SD, suggesting that this treatment at least induces innate immunity.
We next evaluated the innate and adaptive immunities of patient 12, in which we obtained significant numbers of IFN-γ spots from ELISPOT assays. The percentages of IFN-γ+ on CD8+ T and NK cells were much higher compared with the control, which was from patient 12 PBMCs without electroporation. Although these data demonstrate an immune reaction in only one patient, they support the notion that IFN-γ expression in the ELISPOT assay was from CD8+ T cells and NK cells, showing a representative patient when taken together with IFN-γ and CD8+/NK population data. CD107a expression on CD8+ T cells was also high, suggesting that CD8+ T cells from patient 12 had high killing activity.
In addition, we confirmed the high expression of HLA class I and HSP70 in cancer lesion tissue from this patient by IHS. CD8+ T cells with granzyme B expression were detected broadly in the cancer lesion, indicating that this immunotherapy preferentially activated an adaptive immune response resulting in anti-tumor efficacy.
In conclusion, immunotherapy using HSP70-DCs for HCC associated with HCV is effective and safe based on this phase 1 clinical trial. Immunological studies suggested that this immunotherapy might preferentially induce adaptive immunity. Our next step will be phase 2 clinical trials in an adjuvant setting after resection of HCC.
Abbreviations
- AFP:
-
α-Fetoprotein
- CR:
-
Complete response
- CT:
-
Computed tomography
- CTCAE:
-
Common Terminology Criteria for Adverse Events version 3.0
- CTL:
-
Cytotoxic T cell
- DCs:
-
Dendritic cells
- ECOG PS:
-
Eastern Cooperative Oncology Group Performance Status
- ELISPOT:
-
Enzyme-linked immunospot
- FITC:
-
Fluorescein isothiocyanate
- GM-CFS:
-
Granulocyte–macrophage colony-stimulating factor
- HBV:
-
Hepatitis B virus
- HCC:
-
Hepatocellular carcinoma
- HCV:
-
Hepatitis C virus
- HSP:
-
Heat-shock protein
- HSP70-DCs:
-
HSP70 mRNA-transfected DCs
- IFN-γ:
-
Interferon gamma
- IHS:
-
Immunohistochemical staining
- IL-4:
-
Interleukin-4
- IM-DCs:
-
Immature DCs
- mAb:
-
Monoclonal antibodies
- M-DCs:
-
Mature DCs
- MRI:
-
Magnetic resonance imaging
- PBMCs:
-
Peripheral blood mononuclear cells
- PD:
-
Progression of disease
- PIVKA-II:
-
Vitamin K absence or antagonist II
- RECIST:
-
Response Evaluation Criteria in Solid Tumors guideline version 1.1
- RFA:
-
Radiofrequency ablation
- SD:
-
Stable disease
- SE:
-
Standard error
- TAA:
-
Tumor-associated antigens
- TACE:
-
Transcatheter arterial chemoembolization
- TNF-α:
-
Tumor necrosis factor-α
References
Parkin DM, Bray F, Ferlay J, Pisani P (2005) Global cancer statistics, 2002. CA Cancer J Clin 55(2):74–108
Thorgeirsson SS, Grisham JW (2002) Molecular pathogenesis of human hepatocellular carcinoma. Nat Genet 31(4):339–346. doi:10.1038/ng0802-339
Ohishi W, Kitamoto M, Aikata H, Kamada K, Kawakami Y, Ishihara H, Kamiyasu M, Nakanishi T, Tazuma S, Chayama K (2003) Impact of aging on the development of hepatocellular carcinoma in patients with hepatitis C virus infection in Japan. Scand J Gastroenterol 38(8):894–900
Ikai I, Arii S, Ichida T, Okita K, Omata M, Kojiro M, Takayasu K, Nakanuma Y, Makuuchi M, Matsuyama Y, Yamaoka Y (2005) Report of the 16th follow-up survey of primary liver cancer. Hepatol Res 32(3):163–172. doi:10.1016/j.hepres.2005.04.005
Thomas MB, Abbruzzese JL (2005) Opportunities for targeted therapies in hepatocellular carcinoma. J Clin Oncol 23(31):8093–8108. doi:10.1200/JCO.2004.00.1537
Llovet JM, Burroughs A, Bruix J (2003) Hepatocellular carcinoma. Lancet 362(9399):1907–1917. doi:10.1016/S0140-6736(03)14964-1
Iizuka N, Oka M, Yamada-Okabe H, Nishida M, Maeda Y, Mori N, Takao T, Tamesa T, Tangoku A, Tabuchi H, Hamada K, Nakayama H, Ishitsuka H, Miyamoto T, Hirabayashi A, Uchimura S, Hamamoto Y (2003) Oligonucleotide microarray for prediction of early intrahepatic recurrence of hepatocellular carcinoma after curative resection. Lancet 361(9361):923–929. doi:10.1016/S0140-6736(03)12775-4
Iizuka N, Hamamoto Y, Oka M (2004) Predicting individual outcomes in hepatocellular carcinoma. Lancet 364(9448):1837–1839. doi:10.1016/S0140-6736(04)17455-2
Itoh Y, Ohkubo K, Iuchi H, Michitaka K, Horiike N, Onji M (2002) Chronological changes of causes of death and distant metastasis in hepatocellular carcinoma. Oncol Rep 9(2):331–335
Yang Y, Nagano H, Ota H, Morimoto O, Nakamura M, Wada H, Noda T, Damdinsuren B, Marubashi S, Miyamoto A, Takeda Y, Dono K, Umeshita K, Nakamori S, Wakasa K, Sakon M, Monden M (2007) Patterns and clinicopathologic features of extrahepatic recurrence of hepatocellular carcinoma after curative resection. Surgery 141(2):196–202. doi:10.1016/j.surg.2006.06.033
Unitt E, Marshall A, Gelson W, Rushbrook SM, Davies S, Vowler SL, Morris LS, Coleman N, Alexander GJ (2006) Tumour lymphocytic infiltrate and recurrence of hepatocellular carcinoma following liver transplantation. J Hepatol 45(2):246–253. doi:10.1016/j.jhep.2005.12.027
Takayama T, Sekine T, Makuuchi M, Yamasaki S, Kosuge T, Yamamoto J, Shimada K, Sakamoto M, Hirohashi S, Ohashi Y, Kakizoe T (2000) Adoptive immunotherapy to lower postsurgical recurrence rates of hepatocellular carcinoma: a randomised trial. Lancet 356(9232):802–807. doi:10.1016/S0140-6736(00)02654-4
Takashima M, Kuramitsu Y, Yokoyama Y, Iizuka N, Toda T, Sakaida I, Okita K, Oka M, Nakamura K (2003) Proteomic profiling of heat shock protein 70 family members as biomarkers for hepatitis C virus-related hepatocellular carcinoma. Proteomics 3(12):2487–2493. doi:10.1002/pmic.200300621
Yoshida S, Hazama S, Tokuno K, Sakamoto K, Takashima M, Tamesa T, Torigoe T, Sato N, Oka M (2009) Concomitant overexpression of heat-shock protein 70 and HLA class-I in hepatitis C virus-related hepatocellular carcinoma. Anticancer Res 29(2):539–544
Liao X, Li Y, Bonini C, Nair S, Gilboa E, Greenberg PD, Yee C (2004) Transfection of RNA encoding tumor antigens following maturation of dendritic cells leads to prolonged presentation of antigen and the generation of high-affinity tumor-reactive cytotoxic T lymphocytes. Mol Ther 9(5):757–764. doi:10.1016/j.ymthe.2004.02.011
Boczkowski D, Nair SK, Snyder D, Gilboa E (1996) Dendritic cells pulsed with RNA are potent antigen-presenting cells in vitro and in vivo. J Exp Med 184(2):465–472
Van Tendeloo VF, Ponsaerts P, Lardon F, Nijs G, Lenjou M, Van Broeckhoven C, Van Bockstaele DR, Berneman ZN (2001) Highly efficient gene delivery by mRNA electroporation in human hematopoietic cells: superiority to lipofection and passive pulsing of mRNA and to electroporation of plasmid cDNA for tumor antigen loading of dendritic cells. Blood 98(1):49–56
Eisenhauer EA, Therasse P, Bogaerts J, Schwartz LH, Sargent D, Ford R, Dancey J, Arbuck S, Gwyther S, Mooney M, Rubinstein L, Shankar L, Dodd L, Kaplan R, Lacombe D, Verweij J (2009) New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1). Eur J Cancer 45(2):228–247. doi:10.1016/j.ejca.2008.10.026
Tan A, Aucejo F, Kim R (2010) Is there a role for adjuvant treatment after hepatic resection for hepatocellular carcinoma? Oncology 78(3–4):161–171. doi:10.1159/000315577
Litzow MR, Dietz AB, Bulur PA, Butler GW, Gastineau DA, Hoering A, Fink SR, Letendre L, Padley DJ, Paternoster SF, Tefferi A, Vuk-Pavlovic S (2006) Testing the safety of clinical-grade mature autologous myeloid DC in a phase I clinical immunotherapy trial of CML. Cytotherapy 8(3):290–298. doi:10.1080/14653240600735743
Olin MR, Low W, McKenna DH, Haines SJ, Dahlheimer T, Nascene D, Gustafson MP, Dietz AB, Clark HB, Chen W, Blazar B, Ohlfest JR, Moertel C (2014) Vaccination with dendritic cells loaded with allogeneic brain tumor cells for recurrent malignant brain tumors induces a CD4(+)IL17(+) response. J Immunother Cancer 2:4. doi:10.1186/2051-1426-2-4
Xie F, Zhang X, Li H, Zheng T, Xu F, Shen R, Yan L, Yang J, He J (2012) Adoptive immunotherapy in postoperative hepatocellular carcinoma: a systemic review. PLoS One 7(8):e42879. doi:10.1371/journal.pone.0042879
Tada F, Abe M, Hirooka M, Ikeda Y, Hiasa Y, Lee Y, Jung NC, Lee WB, Lee HS, Bae YS, Onji M (2012) Phase I/II study of immunotherapy using tumor antigen-pulsed dendritic cells in patients with hepatocellular carcinoma. Int J Oncol 41(5):1601–1609. doi:10.3892/ijo.2012.1626
Palmer DH, Midgley RS, Mirza N, Torr EE, Ahmed F, Steele JC, Steven NM, Kerr DJ, Young LS, Adams DH (2009) A phase II study of adoptive immunotherapy using dendritic cells pulsed with tumor lysate in patients with hepatocellular carcinoma. Hepatology 49(1):124–132. doi:10.1002/hep.22626
Ferrarini M, Heltai S, Zocchi MR, Rugarli C (1992) Unusual expression and localization of heat-shock proteins in human tumor cells. Int J Cancer 51(4):613–619
Chuma M, Sakamoto M, Yamazaki K, Ohta T, Ohki M, Asaka M, Hirohashi S (2003) Expression profiling in multistage hepatocarcinogenesis: identification of HSP70 as a molecular marker of early hepatocellular carcinoma. Hepatology 37(1):198–207. doi:10.1053/jhep.2003.50022
Elsner L, Muppala V, Gehrmann M, Lozano J, Malzahn D, Bickeboller H, Brunner E, Zientkowska M, Herrmann T, Walter L, Alves F, Multhoff G, Dressel R (2007) The heat shock protein HSP70 promotes mouse NK cell activity against tumors that express inducible NKG2D ligands. J Immunol 179(8):5523–5533
Takemoto S, Nishikawa M, Guan X, Ohno Y, Yata T, Takakura Y (2010) Enhanced generation of cytotoxic T lymphocytes by heat shock protein 70 fusion proteins harboring both CD8(+) T cell and CD4(+) T cell epitopes. Mol Pharm 7(5):1715–1723. doi:10.1021/mp1001069
Ciocca DR, Cayado-Gutierrez N, Maccioni M, Cuello-Carrion FD (2012) Heat shock proteins (HSPs) based anti-cancer vaccines. Curr Mol Med 12(9):1183–1197
Calderwood SK, Theriault JR, Gong J (2005) Message in a bottle: role of the 70-kDa heat shock protein family in anti-tumor immunity. Eur J Immunol 35(9):2518–2527. doi:10.1002/eji.200535002
Zhang HM, Zhang LW, Ren J, Fan L, Si XM, Liu WC (2006) Induction of alpha-fetoprotein-specific CD4- and CD8-mediated T-cell response using RNA-transfected dendritic cells. Cell Immunol 239(2):144–150. doi:10.1016/j.cellimm.2006.05.004
Gilboa E, Vieweg J (2004) Cancer immunotherapy with mRNA-transfected dendritic cells. Immunol Rev 199:251–263. doi:10.1111/j.0105-2896.2004.00139.x
Binder RJ (2009) CD40-independent engagement of mammalian hsp70 by antigen-presenting cells. J Immunol 182(11):6844–6850. doi:10.4049/jimmunol.0900026
Acknowledgments
This study was funded by KAKEN 20591612. We thank Ms. Akiko Sano, Ms. Kaori Kaneyasu, and Ms. Yuko Yanai for their excellent technical assistance.
Conflict of interest
The authors have no conflict of interest.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Maeda, Y., Yoshimura, K., Matsui, H. et al. Dendritic cells transfected with heat-shock protein 70 messenger RNA for patients with hepatitis C virus-related hepatocellular carcinoma: a phase 1 dose escalation clinical trial. Cancer Immunol Immunother 64, 1047–1056 (2015). https://doi.org/10.1007/s00262-015-1709-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00262-015-1709-1