Abstract
Malignant melanoma is highly aggressive cancer with poor prognosis and few therapeutic options. Interferon alpha (IFN-α) has been tested as adjuvant immunotherapy in high-risk melanoma patients in a number of studies, but its beneficial role is controversial. Although IFN-α treatment can prolong relapse-free survival, the effect on overall survival is not significant. However, a small subset of patients benefits from the treatment, signifying the need for biomarkers able to identify a responding subgroup. Here we evaluated whether serum osteopontin (OPN) could function as a biomarker identifying patients with poor prognosis that might benefit from IFN-α. The choice of osteopontin was based on the knowledge about the dual role of this protein in cancer and immune response, an apparent association between OPN and IFN signaling and a prognostic value of OPN in multiple other tumor types. Serum samples from 275 high-risk melanoma patients enrolled in the Nordic Adjuvant IFN Melanoma trial were analyzed for circulating OPN concentrations and OPN promoter polymorphisms in position −443. The potential relation between serum OPN levels, the genotypes and survival in non-treated patients and patients receiving adjuvant IFN-α was investigated. Although slightly better survival was observed in the treated patients that had high levels of OPN, the difference was not statistically significant. In conclusion, serum OPN (its level or the genotype) cannot distinguish melanoma patients with poor prognosis, or patients that might benefit from adjuvant treatment with IFN-α.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Malignant melanoma is among the most aggressive human cancers with increasing incidence worldwide [1]. The prognosis for melanoma patients with stage II or stage III disease (by American Joint Committee on Cancer (AJCC) staging) is adverse with reported 5-year survival of 24–67 % [2]. To improve prognosis of such high-risk patients, efficient adjuvant therapies are needed. Interferon alpha (IFN-α) as adjuvant therapy has been tested in a number of clinical studies during the last 15 years [3]. However, the real clinical benefit of this therapy is a subject of debate. Although adjuvant IFN-α can prolong relapse-free survival (RFS), the effect on overall survival (OS) is not significant, even though a small subset of patients seems to benefit [4–6]. Given that IFN-α treatment is associated with substantial dose-dependent toxicity, use in a non-selected patient group is questionable, and selection of “right” patients that might benefit is essential. Therefore, the future of adjuvant interferon therapy depends on identification of factors that could select the group of patients likely to respond.
IFN-α is a cytokine acting by promoting Th1 response and activating anti-melanoma cytotoxic T lymphocytes, thereby boosting the patients immune system against the cancer [7]. Another important modulator of the immune system, stimulating Th1 response by enhancing IFN-α production, is osteopontin (OPN) [8, 9]. OPN is a member of small integrin-binding ligand N-linked glycoprotein (SIBLING) family. The protein is expressed by a variety of normal cells, such as macrophages, endothelial cells, lymphocytes and tumor cells from different cancer types. OPN is involved in various physiological processes, such as cell adhesion, wound healing, angiogenesis, immune response, and also plays a role in multiple pathologies, including autoimmune, inflammatory, muscular, bone diseases as well as cancer [10, 11]. It has been reported that in chronic hepatitis C, where IFN-α therapy is also applied, serum OPN levels and gene polymorphisms function as predictors for response to IFN-α [12]. OPN is also known as a factor associated with cancer progression and metastasis [13], and it has been evaluated as a clinical marker for patient survival in various cancer forms, also melanoma [14–16]. Meta-analysis of the published data on OPN as a cancer marker has revealed an association between poor prognosis and elevated levels of OPN (both in tumors and in serum/plasma) in all cancers combined and in some individual cancers [17, 18]. Interestingly, OPN was identified by bioinformatics analysis as a top candidate gene being at the interface between the immune system and cancer [19]. Due to apparent association between OPN and IFN signaling, and the dual role of the protein in cancer and immunity, we hypothesized that osteopontin could be a candidate biomarker in high-risk melanoma patients receiving IFN-α immunotherapy.
In the present study, the potential relation between serum OPN levels, the gene polymorphisms and survival was investigated in malignant melanoma patients from the Nordic Adjuvant IFN Melanoma trial. The aim was to evaluate whether serum OPN could function as a biomarker providing prognostic information in non-treated patients or patients treated with IFN-α.
Materials and methods
Melanoma patient cohort, treatment regimes and serum samples
Serum samples were collected from 275 melanoma patients with high-risk resected cutaneous melanoma of stage II or stage III that were enrolled into the Nordic Adjuvant IFN Melanoma trial (ClinicalTrials.gov number NCT01259934) undertaken between 1996 and 2004 at 35 centers in the Nordic countries (855 patients in total were enrolled). All criteria for patient inclusion into the trial and patient characteristics have been specified previously [4]. The trial was approved by the appropriate local research ethics committees and undertaken according to good clinical practice and the principles of the Declaration of Helsinki. All patients provided written or oral informed consent according to the requirements in each participating country.
Patients were randomly assigned to one of three study groups: group A, observation only; group B, IFN-α 10 million unit subcutaneously 5 days per week for 1 month (induction) followed by the same dose 3 days per week for 12 months (1-year maintenance); group C, induction as in group B, but followed by maintenance for 24 months (2-year maintenance). The type of interferon alpha-2b used was Intron A (Merck, Whitehouse Station, NJ, USA). Patients were followed up every 3 months for 2 years, then every 6 months for 3 years and once per year for additional 5 years. After the first relapse, the treatment was discontinued and patients were followed up with respect to survival, but no further relapses were recorded. The primary endpoint was overall survival, and the secondary endpoint was relapse-free survival defined as the first verified relapse at any site [4].
For serum isolation, blood samples were taken before the start of the treatment, left at room temperature for clotting for 30 min and centrifuged at 2000g for 15 min. The serum was collected and stored at −80 °C before analysis.
Measurement of serum osteopontin concentration
The OPN levels in stored serum samples were measured with the enzyme-linked immunosorbent assay (ELISA) kit Quantikine Human Osteopontin Immunoassay DOST00 (R&D Systems Inc., Minneapolis, Minnesota, USA), according to the manufacturer’s manual. In brief, a serum sample from each patient was diluted 1:10 with Diluent RD5-24 and incubated in an OPN antibody-coated microtiter plate for 2 h at room temperature followed by washing. Subsequently, 200 µl OPN conjugate (polyclonal antibody against OPN conjugated to horseradish peroxidase) was added to each well and incubated for 2 h at room temperature. Following wash, 200 µl substrate solution (hydrogen peroxide and chromogen) was added to each well and incubated for 30 min at room temperature. The samples were measured on a plate reader Victor 1420 Multilabel Counter (Wallac/PerkinElmer Life Sciences, Turku, Finland) at 450 nm with wavelength correction at 570 nm. Standard curve and sample values were calculated by use of the Wallac MultiCalc program.
Analysis of polymorphisms in the osteopontin promoter
For analysis of the −443 OPN promoter polymorphism rs17730582, 5 μl of serum was denaturated at 95 °C for 5 min and amplified by adding 40 μl of PCR master mix, consisting of 1 × PCR buffer IV (ABgene/Thermo Scientific, Massachusetts, USA), 3 mM MgCl2, 100 µM dNTP mix (VWR, Pennsylvania, USA), 300 nM reverse primer 5′-CTA TTG TTC AAG CCT GCA A-3′ and 200 nM forward primer FAM-5′-CGC CCG CCG CGC CCC GCG CCC GTC CCG CCG CCC CCG CCC GAG CTT GAG TAG TAA AGG ACA-3′ (both from Integrated DNA Technologies, Iowa, USA), 0.005U Pfu and 0.08U Taq DNA polymerases (both in house production). Amplification was performed in a DNA Engine Tetrad 2 Thermal Cycler (Bio-Rad, California, USA) with a 5 min 95 °C hot start, 35 cycles of—denaturation at 95 °C, annealing at 62 °C and extension at 72 °C, each 30 s—followed by a 10 min final extension at 72 °C.
Analyses of the rs117730582 polymorphism were done by cycling temperature capillary electrophoresis using a modified MegaBACE 1000 (Amersham Biosciences Uppsala, Sweden) capillary sequencing instrument. Samples were subjected to electrophoresis at a mean separating temperature of 52.5 °C, with amplitudes of 3 °C and cycled 20 times. An internal standard specific for the rs117730582 polymorphism was used to verify the different allelic variants as described previously [20].
Statistical analyses
Survival was estimated according to the Kaplan–Meier method, and survival curves were compared using the log-rank test. Means were compared using independent samples t test or one-way ANOVA as appropriate. Data analysis was performed using SPSS version 21.0 (SPSS Inc., Chicago, USA), and p values <0.05 were considered statistically significant.
Results
Patent characteristics
Clinicopathological parameters of the patient cohort used in this study are summarized in Table 1. The cohort consisted of 275 patients (100 females and 175 males) with high-risk resected cutaneous melanoma of AJCC stage II (tumor thickness ≥ 4 mm without lymph node metastases) or stage III (with regional lymph node metastases). In this cohort, 50 % of the patients represented group A (observation only), 25 % were from the treatment group B (interferon alpha-2b with 1-year maintenance), and 25 % were from the treatment group C (interferon alpha-2b with 2-year maintenance). Thirty blood donors (17 females and 13 males) constituted the healthy control group.
Evaluation of serum OPN concentration and its association with outcome
To determine concentration of circulating OPN, serum samples from the melanoma patients and the healthy volunteers were investigated by ELISA. It can be noted that our preliminary study, where OPN concentration was measured in the frozen samples immediately, after a round of thawing–freezing, or after incubation at room temperature for several hours, showed no significant differences in the OPN level (data not shown). This indicates that OPN is a relatively stable protein, which has been reported also by others [15, 21]. In the patients, mean and median values of serum OPN concentration were 27.5 and 25.5 ng/ml, respectively. In the healthy volunteers, the serum OPN concentration was lower, 22.98 ng/ml (mean) and 20.3 ng/ml (median) (Fig. 1). The difference in serum OPN levels between the patients and the healthy individuals was statistically significant (p = 0.027). It can be noted that in the patients, there was no statistically significant association between the OPN level and patient characteristics such as age, sex, ulceration, AJCC stage or treatment/no treatment with IFN-α (data not shown).
Osteopontin concentration in serum from the melanoma patients (n = 275) and the healthy volunteers (n = 30). Data show median values (horizontal lines within the boxes), the edges of the boxes represent the 25th and 75th percentile, and the whiskers represent the highest and lowest values that are not outliers or extreme values. Outliers (values that are between 1.5 and three times the interquartile range) and extreme values (values that are more than three times the interquartile range) are represented by dots beyond the whiskers
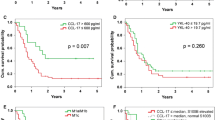
To investigate whether serum OPN level could be of prognostic significance, we divided the non-treated patients (group A) into high OPN and low OPN and compared relapse-free survival (RFS) and overall survival (OS) between these two subgroups. Patients were considered to be high OPN, when their serum OPN concentration exceeded 25 ng/ml i.e. the median value observed in the patients. There was found no statistically significant association between the OPN level and RFS (p = 0.75) or OS (p = 0.87) (Fig. 2). Likewise, there was no significant association between OPN level and survival, when the group A patients were divided into stage II or stage III and analyzed separately (data not shown).
Previously, it was reported that patients receiving interferon alpha (from the groups B and C combined, or the group B only) showed improved RFS, but not OS compared with the observation group A [4]. To evaluate whether serum OPN level could identify a subgroup that benefits from adjuvant IFN-α, we analyzed RFS and OS in the treated patients stratified into high-OPN and low-OPN subgroups. While we saw that high-OPN patients that received IFN-a showed a better clinical outcome, the association between the OPN levels and RFS or OS was not statistically significant (Fig. 3, p = 0.16 and p = 0.35, respectively). Likewise, we found no statistically significant association when we analyzed the patients from the treatment group B only (data not shown).
It should be noted that no significant difference in survival between low-OPN and high-OPN patients was found when OPN level of 20.3 ng/ml (median in healthy individuals), 22.98 ng/ml (mean in healthy individuals) or 27.5 ng/ml (mean in patients) was used as a cutoff to divide the patients (data not shown).
Taken together, our data indicate that serum OPN level, which was higher in melanoma patients than healthy individuals, was not significantly associated with survival in the non-treated patients, or the patients treated with adjuvant IFN-α.
Analysis of OPN promoter polymorphisms and association with survival
Previously, it has been reported that single-nucleotide polymorphisms (SNPs) in OPN promoter can affect OPN expression levels, and that SNP in position −433 has a prognostic value in human hepatocellular carcinoma and lung cancer [22, 23] and a predictive value in IFN-treated hepatitis C patients [12]. To evaluate OPN promoter polymorphisms at this position in our patient cohort, SNP analysis was performed on the serum samples. Overall, from 239 analyzed samples, 49 % were heterozygous (−443C/T), 25.9 % were homozygous for CC, and 25.1 % were homozygous for TT (Table 2). There was no association between the genotype and patient characteristics, such as age, sex, ulceration and treatment/no treatment with IFN-α (data not shown). However, a statistically significant (p = 0.016) association between the genotype and AJCC stage was observed, where 29.9 % of patients with CT genotype had stage II disease, compared with 11 % for CC and 20 % for TT, respectively (Table 2). There was no association between the genotype and the serum OPN levels that were measured to be 27.6, 28 and 27.3 ng/ml, respectively (p = 0.9, one-way ANOVA) (Table 2). Further, we analyzed survival of patients from non-treated (group A) and IFN-α-treated groups (B + C combined) based on their genotypes. Although we saw a better survival for −433C/T genotype, the association was not statistically significant, either in non-treated or in IFN-α-treated patients (Fig. 4).
Discussion
Good prognostic/predictive biomarkers, that could be detected in patient blood and supplement complicated imaging modalities used to monitor high-risk patients, are of great clinical interest. In the present study, we investigated circulating OPN levels and gene polymorphisms as candidate biomarkers by analyzing serum samples from a cohort of melanoma patients from the Nordic IFN trial [4]. We found elevated levels of serum OPN in the melanoma patients compared with the healthy individuals. This is in line with what has been reported by others, revealing a positive association between circulating OPN levels and cancer/metastasis. Several studies reported higher concentrations of plasma OPN in metastatic melanoma patients, particularly of stage IV, compared with non-metastatic patients or healthy individuals [14, 15, 24]. It can be noted that the observed OPN levels in serums were approximately half the levels measured by others in plasma, and a similar difference has been reported previously [21]. Even though there is some contention whether serum or plasma is the best, OPN stability in both types of specimens was shown to be similar [21], and the ability of OPN to discriminate cancer patients from healthy individuals using serum or plasma was also comparable [25]. In addition, an association between OPN level and clinical outcomes was reported using either specimen type [26, 27], indicating that diagnostic, prognostic or predictive value of OPN can be disclosed by analyzing serum.
The observation that OPN is associated with advanced cancer/metastasis boosted interest in this protein, both intratumoral and in circulation, as a potential prognostic biomarker. In our study, we did not find a statistically significant link between serum OPN concentration and patient survival. Likewise, Filia et al. [15] did not observe a significant association between high plasma OPN levels and poor prognosis in the Leeds Melanoma Cohort. Several reports proposed that circulating OPN level might be a better indicator of prognosis in melanoma if employed in a panel with other plasma markers [14, 24]. However, a comprehensive overview of the literature conclude that results are conflicting, and marker panels including OPN for clinical use have still not been found [28].
While prognostic significance of circulating OPN in melanoma is uncertain, tumor-associated OPN (detected by immuohistochemistry in primary cutaneous melanoma) was found to be an independent prognostic marker [16]. However, that study did not evaluate the impact of circulating OPN in the same patient cohort. Interestingly, Rud et al. [29] compared prognostic significance of OPN levels in primary tumors versus serum in patients with non small cell lung cancer, and found that tumor OPN expression, but not serum OPN, was significantly associated with poor survival. This proposes that the role and the prognostic value of intratumoral OPN and circulating OPN (which derives from both tumor and stromal cells) might be different.
Despite many studies investigating OPN as a candidate prognostic factor, its usefulness for predicting therapy response, particularly immunotherapy, has been little explored in cancer. Knowing that OPN regulates IFN-α production and Th1 response, we hypothesized that OPN level could be of importance for the effect of IFN-α treatment. Although we saw that IFN-α treated patients with high levels of serum OPN showed slightly better survival than patients with low-OPN levels, the difference did not reach statistical significance. Thus, our data do not validate serum OPN level as a factor identifying patients that might benefit from adjuvant IFN-a treatment. Interestingly, using a subcohort from the same Nordic IFN trial, another study evaluated matrix metalloproteinase-8 (MMP-8) as a candidate serum marker for response to IFN-a. Likewise in our study, the MMP-8 study revealed that high levels of MMP-8 indicated some survival benefit from adjuvant IFN-a, but the differences were not statistical significant either [30].
There might be several reasons why serum OPN level failed as a prognostic indicator. OPN is a multifunctional protein produced by various cells, both tumor and stroma, and plays different biological roles intracellularly and as a secreted factor [11]. It might be that OPN positive effects on response to IFN-α are counteracted by other influences. For example, Sangaletti et al. [31] have recently shown that host-derived OPN, particularly from myeloid cells, can render immunosuppression in vivo. In addition, the level of circulating OPN might be influenced by other systemic sources than the tumor itself, e.g., inflammation that often accompanies cancer development and is known to involve osteopontin [32]. Finally, OPN is subjected to abundant modifications on genetic and epigenetic levels, and some of these modifications might be of significance with respect to prognostic/predictive impact. One such genetic alteration, hypothesized as a risk factor for disease outcome, is OPN gene polymorphisms. Particularly, SNP in position −443 has been linked to tumor aggressiveness, poor prognosis and modulated response to chemotherapy [22, 23, 33]. We identified −443C/T as the most common genotype in our patient cohort, but we did not find any significant association with serum OPN level or patient survival, either in the non-treated or in the IFN-α treated patients. In contrast, in hepatitis C patients, the −443T/T genotype was found to be linked to better response to IFN-α treatment [12]. Generally, data on the SNP in position −443 and its significance for the gene expression or as a prognostic indicator are conflicting. Possibly, SNP plays a complex role, different in different diseases or cancer types, which needs further exploration.
In conclusion, we evaluated serum OPN, its level and polymorphisms as biomarkers in high-risk melanoma patients receiving adjuvant IFN-α. No significant association with relapse-free or overall survival was found, indicating that circulating osteopontin cannot identify melanoma patients that might benefit from adjuvant interferon alpha.
Abbreviations
- AJCC:
-
American Joint Committee on Cancer
- ELISA:
-
Enzyme-linked immunosorbent assay
- IFN-α:
-
Interferon alpha
- MMP:
-
Matrix metalloproteinase
- OPN:
-
Osteopontin
- OS:
-
Overall survival
- RFS:
-
Relapse-free survival
- SIBLING:
-
Small integrin-binding ligand N-linked glycoprotein
- SNP:
-
Single-nucleotide polymorphism
References
Forsea AM, del Marmol V, de Vries E, Bailey EE, Geller AC (2012) Melanoma incidence and mortality in Europe: new estimates, persistent disparities. Br J Dermatol 167:1124–1130
Balch CM, Gershenwald JE, Soong SJ et al (2009) Final version of 2009 AJCC melanoma staging and classification. J Clin Oncol 27:6199–6206
Ascierto PA, Chiarion-Sileni V, Muggiano A, Mandala M, Pimpinelli N, Del Vecchio M, Rinaldi G, Simeone E, Queirolo P (2014) Interferon alpha for the adjuvant treatment of melanoma: review of international literature and practical recommendations from an expert panel on the use of interferon. J Chemother 26:193–201
Hansson J, Aamdal S, Bastholt L, Brandberg Y, Hernberg M, Nilsson B, Stierner U, von der Maase H (2011) Two different durations of adjuvant therapy with intermediate-dose interferon alpha-2b in patients with high-risk melanoma (Nordic IFN trial): a randomised phase 3 trial. Lancet Oncol 12:144–152
Eggermont AM, Suciu S, Santinami M et al (2008) Adjuvant therapy with pegylated interferon alpha-2b versus observation alone in resected stage III melanoma: final results of EORTC 18991, a randomised phase III trial. Lancet 372:117–126
Mocellin S, Pasquali S, Rossi CR, Nitti D (2010) Interferon alpha adjuvant therapy in patients with high-risk melanoma: a systematic review and meta-analysis. J Natl Cancer Inst 102:493–501
Asmana Ningrum R (2014) Human interferon alpha-2b: a therapeutic protein for cancer treatment. Scientifica (Cairo) 2014:1–8
Ashkar S, Weber GF, Panoutsakopoulou V et al (2000) Eta-1 (osteopontin): an early component of type-1 (cell-mediated) immunity. Science 287:860–864
Shinohara ML, Lu L, Bu J, Werneck MB, Kobayashi KS, Glimcher LH, Cantor H (2006) Osteopontin expression is essential for interferon-alpha production by plasmacytoid dendritic cells. Nat Immunol 7:498–506
Bandopadhyay M, Bulbule A, Butti R et al (2014) Osteopontin as a therapeutic target for cancer. Expert Opin Ther Targets 18:883–895
Shevde LA, Samant RS (2014) Role of osteopontin in the pathophysiology of cancer. Matrix Biol 37:131–141
Naito M, Matsui A, Inao M et al (2005) SNPs in the promoter region of the osteopontin gene as a marker predicting the efficacy of interferon-based therapies in patients with chronic hepatitis C. J Gastroenterol 40:381–388
Rittling SR, Chambers AF (2004) Role of osteopontin in tumour progression. Br J Cancer 90:1877–1881
Maier T, Laubender RP, Sturm RA, Klingenstein A, Korting HC, Ruzicka T, Berking C (2012) Osteopontin expression in plasma of melanoma patients and in melanocytic tumours. J Eur Acad Dermatol Venereol 26:1084–1091
Filia A, Elliott F, Wind T et al (2013) Plasma osteopontin concentrations in patients with cutaneous melanoma. Oncol Rep 30:1575–1580
Rangel J, Nosrati M, Torabian S, Shaikh L, Leong SP, Haqq C, Miller JR 3rd, Sagebiel RW, Kashani-Sabet M (2008) Osteopontin as a molecular prognostic marker for melanoma. Cancer 112:144–150
Weber GF, Lett GS, Haubein NC (2010) Osteopontin is a marker for cancer aggressiveness and patient survival. Br J Cancer 103:861–869
Weber GF, Lett GS, Haubein NC (2011) Categorical meta-analysis of Osteopontin as a clinical cancer marker. Oncol Rep 25:433–441
Clancy T, Pedicini M, Castiglione F, Santoni D, Nygaard V, Lavelle TJ, Benson M, Hovig E (2011) Immunological network signatures of cancer progression and survival. BMC Med Genomics 4:28
Bjorheim J, Gaudernack G, Giercksky KE, Ekstrom PO (2003) Direct identification of all oncogenic mutants in KRAS exon 1 by cycling temperature capillary electrophoresis. Electrophoresis 24:63–69
Lanteri P, Lombardi G, Colombini A, Grasso D, Banfi G (2012) Stability of osteopontin in plasma and serum. Clin Chem Lab Med 50:1979–1984
Dong QZ, Zhang XF, Zhao Y et al (2013) Osteopontin promoter polymorphisms at locus −443 significantly affect the metastasis and prognosis of human hepatocellular carcinoma. Hepatology 57:1024–1034
Chen Y, Liu H, Wu W, Li Y, Li J (2013) Osteopontin genetic variants are associated with overall survival in advanced non-small-cell lung cancer patients and bone metastasis. J Exp Clin Cancer Res 32:45
Kluger HM, Hoyt K, Bacchiocchi A et al (2011) Plasma markers for identifying patients with metastatic melanoma. Clin Cancer Res 17:2417–2425
Grigoriu BD, Scherpereel A, Devos P et al (2007) Utility of osteopontin and serum mesothelin in malignant pleural mesothelioma diagnosis and prognosis assessment. Clin Cancer Res 13:2928–2935
Isa S, Kawaguchi T, Teramukai S et al (2009) Serum osteopontin levels are highly prognostic for survival in advanced non-small cell lung cancer: results from JMTO LC 0004. J Thorac Oncol 4:1104–1110
Mack PC, Redman MW, Chansky K et al (2008) Lower osteopontin plasma levels are associated with superior outcomes in advanced non-small-cell lung cancer patients receiving platinum-based chemotherapy: SWOG Study S0003. J Clin Oncol 26:4771–4776
Weber GF (2011) The cancer biomarker osteopontin: combination with other markers. Cancer Genomics Proteomics 8:263–288
Rud AK, Boye K, Oijordsbakken M et al (2013) Osteopontin is a prognostic biomarker in non-small cell lung cancer. BMC Cancer 13:540
Vihinen P, Tervahartiala T, Sorsa T et al (2014) Benefit of adjuvant interferon alpha-2b (IFN-alpha) therapy in melanoma patients with high serum MMP-8 levels. Cancer Immunol Immunother 64:173–180
Sangaletti S, Tripodo C, Sandri S et al (2014) Osteopontin shapes immunosuppression in the metastatic niche. Cancer Res 74:4706–4719
Lund SA, Giachelli CM, Scatena M (2009) The role of osteopontin in inflammatory processes. J Cell Commun Signal 3:311–322
Hao Y, Liu J, Wang P, Wang F, Yu Z, Li M, Chen S, Ning F (2014) OPN polymorphism is related to the chemotherapy response and prognosis in advanced NSCLC. Int J Genomics 2014:846142
Acknowledgments
The project was supported by the Norwegian Cancer Society, the Research Council of Norway and the South East Regional Health Authority. We thank Karen-Marie Heintz and Geir Frode Øy from the genotyping core facility for performing SNP analysis.
Conflict of interest
Johan Hansson and Lars Bastholt have been involved in advisory board activities for Merck MSD. Lars Bastholt has received congress-related travel support from Merck MSD. The other authors have no conflicts of interest to report.
Author information
Authors and Affiliations
Corresponding author
Additional information
Lina Prasmickaite and Gisle Berge have contributed equally.
Rights and permissions
About this article
Cite this article
Prasmickaite, L., Berge, G., Bettum, I.J. et al. Evaluation of serum osteopontin level and gene polymorphism as biomarkers: analyses from the Nordic Adjuvant Interferon alpha Melanoma trial. Cancer Immunol Immunother 64, 769–776 (2015). https://doi.org/10.1007/s00262-015-1686-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00262-015-1686-4