Abstract
The disialoganglioside GD2 is a well-established target antigen for passive immunotherapy in neuroblastoma (NB). Despite the recent success of passive immunotherapy with the anti-GD2 antibody ch14.18 and cytokines, treatment of high-risk NB remains challenging. We expanded the approach of GD2-specific, antibody-based immunotherapy to an application of a GD2-specific natural killer (NK) cell line, NK-92-scFv(ch14.18)-zeta. NK-92-scFv(ch14.18)-zeta is genetically engineered to express a GD2-specific chimeric antigen receptor generated from ch14.18. Here, we show that chimeric receptor expression enables NK-92-scFv(ch14.18)-zeta to effectively lyse GD2+ NB cells also including partially or multidrug-resistant lines. Our data suggest that recognition of GD2 by the chimeric receptor is the primary mechanism involved in NK-92-scFv(ch14.18)-zeta-mediated lysis and is independent of activating NK cell receptor/ligand interactions. Furthermore, we demonstrate that NK-92-scFv(ch14.18)-zeta is able to mediate a significant anti-tumor response in vivo in a drug-resistant GD2+ NB xenograft mouse model. NK-92-scFv(ch14.18)-zeta is an NB-specific NK cell line that has potential for future clinical development due to its high stability and activity toward GD2+ NB cell lines.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Neuroblastoma (NB) is an aggressive childhood malignancy accounting for 8–10 % of all childhood cancers [1] and approximately 15 % of all pediatric oncology deaths [2]. Although multimodal therapy has improved outcome [3], treatment of high-risk NB patients remains challenging. Unfortunately, relapsed tumors are often refractory toward salvage chemotherapy due to multidrug resistance [4]. These problems in high-risk NB treatment reveal the need for novel and effective therapeutic strategies.
Immunological approaches are increasingly attractive in this respect. Recently, the combination of the disialoganglioside (GD2)-specific chimeric antibody ch14.18, interleukin-2 (IL-2), granulocyte–macrophage colony-stimulating factor (GM-CSF) and 13-cis-retinoic acid resulted in a significant increase in the 2-year event-free survival rate for high-risk NB patients from 46 to 66 % [5]. These results show the potential of immunotherapy but also highlight the need for refinement of therapy regimens, as more than 30 % of high-risk NB patients still do not survive the disease.
GD2 is a ganglioside highly expressed on NB. Physiologically, expression of GD2 is restricted to the neurons of the brain, protected by the blood–brain barrier, and peripheral nerve fibers [6], which results in manageable side effects following GD2-specific immunotherapeutic treatment [7]. Taken together, GD2 is a suitable target antigen for immunotherapy.
Natural killer (NK) cells are innate immune cells known to lyse virus-infected or malignant cells and produce immunoregulatory cytokines [interferon-γ (IFN-γ), tumor necrosis factor-α (TNF-α) and GM-CSF)] [8, 9]. Regulation of their cytotoxic activity is based on the interaction of activating and inhibitory NK cell receptors with respective ligands on target cells. NK cells mediate apoptosis of target cells following both the granzyme B-induced intrinsic pathway [10] and the extrinsic pathway induced by interaction of death receptors [Fas, TNF-related apoptosis-inducing ligand (TRAIL)-receptor (TRAIL-R)] on tumor cells with their respective ligands [Fas-ligand (FasL) and TRAIL] on NK cells [11]. In contrast to T cells, NK cells are able to immediately execute their cytotoxic activity without prior priming or sensitization [12]. These functional characteristics underline the potential of NK cells as effector cells in an immunotherapeutic approach.
NK-92 is a continuously growing, IL-2-dependent human NK cell line, which exhibits both the phenotypic and functional characteristics of activated NK cells [13] and presents a high cytotoxic activity toward a broad spectrum of tumor cell lines and primary tumor cells [14–16]. Irradiated NK-92 cells have been demonstrated to be safe and tolerable in a clinical phase I trial [17, 18].
However, shedding of activating ligands [19] and production of inhibitory cytokines by tumor cells can result in the down-modulation of activating NK cell receptors and ligands and result in resistance to NK cell-mediated lysis [20]. Chimeric antigen receptor (CAR) technology can be employed to overcome NK cell resistance and to specifically target a certain tumor entity. CARs consist of a single-chain Fv (scFv) fragment containing the heavy and light chain of a target antigen-specific antibody, connected by a flexible linker with a signaling component such as the cluster of differentiation (CD) 3-zeta chain [21]. Although current CAR-based immunotherapeutic approaches generally focus on T cells as effector cells, the use of NK cells is increasingly attractive. To circumvent obstacles associated with autologous or allogeneic primary NK cells, NK-92 cells were transduced to express a GD2-specific CAR [NK-92-scFv(ch14.18)-zeta]. This generation of GD2-specific NK-92-scFv(ch14.18)-zeta was based on transduction with the retroviral vector pL-scFv(ch14.18)HL-ζ-SN, encoding for a ch14.18-derived single-chain fragment variable, a Myc-tag, the hinge region of CD8α and the CD3ζ-chain [22].
In this study, we demonstrate that GD2-specific NK-92 effectively lyse GD2+ human NB cells. Importantly, this effect is mediated by antigen (GD2)-specific activation of engineered NK-92 cells through CAR signaling. This enables GD2-specific, CAR-expressing NK-92 to kill NB cells that are resistant to drug treatment and otherwise less responsive to NK cell lysis. Further, these engineered NK cells mediate a significant anti-tumor response in vivo in a GD2+ NB xenograft model.
Materials and methods
Cell lines
The GD2+ NB lines LA–N-1, LA–N-5, SK-N-BE(2), the GD2− NB line SK-N-SH as well as the erythroleukemia line K562 were cultured in Roswell Park Memorial Institute (RPMI) 1640 medium (Hyclone Laboratories, Thermo Scientific, Logan, Utah, USA) with 10 % fetal bovine serum (FBS) (GIBCO, Invitrogen, USA). The GD2-positive NB lines CHLA-79, CHLA-20 and CHLA-136 were cultured in Iscove’s modified Dulbecco’s medium (IMDM) (Hyclone Laboratories, Thermo Scientific) with 20 % FBS, 4 mM l-glutamine (Cellgro, Mediatech Inc., Manassas, VA, USA) and 1 × ITS (1000×: 100 mg insulin, 100 mg transferrin, 100 µg selenous acid, BD Biosciences, Bedford, MA, USA). All NB lines were obtained from the Children’s Oncology Group (COG) cell line repository (www.COGcell.org). Cell line identities were confirmed by short tandem repeat (STR) profiling and verified against the COG cell line STR database and routinely tested negative for mycoplasma. Previous studies analyzed the drug-resistant patterns of the above-mentioned NB cell lines and reported drug resistance of CHLA-20 and multidrug resistance of SK-N-BE(2), CHLA-79 and CHLA-136 [4, 23, 24].
NK-92 cells were cultured in X-VIVO-10 (Lonza, Walkersville, MD, USA) with 5 % human AB serum (PAA, Dartmouth, MA, USA) and 100 international units (IU)/ml recombinant human IL-2 (Novartis, East Hanover, NJ, USA). The genetically engineered NK cell line NK-92-scFv(ch14.18)-zeta [22] as well as empty vector control NK-92-pLXSN and ErbB2-specific NK-92-scFv(FRP5)-zeta [25] (kindly provided by Dr. Tina Müller, Georg-Speyer-Haus, Frankfurt, Germany) were cultured in X-VIVO-10 media with 5 % human AB serum, 100 IU/ml recombinant human IL-2 and 0.6 mg/ml G418 (Sigma-Aldrich, St. Louis, MO, USA). All cell lines were cultured at 37 °C in a humidified incubator (20 % O2 and 5 % CO2).
Flow cytometric analysis
For analysis of CAR surface expression, 1 × 106 NK-92-scFv(ch14.18)-zeta, NK-92 (parental cell line), NK-92-pLXSN (empty vector transduced NK-92) and NK-92-scFv(FRP5)-zeta (ErbB2-specific CAR) were incubated with either 1 µg of anti-idiotypic antibody (anti-IdAb) ganglidiomab [26] or 1 µg of anti-Myc-tag antibody (clone 9E10, Sigma-Aldrich, Steinheim, Germany) followed by incubation with phycoerythrin (PE)-labeled rat anti-mouse immunoglobulin G (IgG1) antibody (clone A85-1, BD Pharmingen, San Diego, CA, USA). Mouse IgG1 (clone 11711, R&D Systems, Minneapolis, MN, USA) was utilized as isotype control.
For analysis of GD2 surface expression, cells were stained with ch14.18/Chinese hamster ovary (CHO) (1 µg/1 × 106 cells) followed by incubation with PE-labeled mouse antihuman IgG antibody (clone G18-145, BD Pharmingen). The anti-CD20 chimeric antibody rituximab (1 µg/1 × 106 cells, MabThera, Roche, Mannheim, Germany) was used as isotype control for ch14.18/CHO. GD2 synthesis was inhibited using the selective glucosylceramide synthase (GCS) inhibitor PPPP (1-phenyl-2-hexadecanoylamino-3-pyrrolidino-1-propanol (kindly provided by Dr. Barry J. Maurer, Texas Tech University Health Sciences Center Cancer Center, Lubbock, TX, USA). For this purpose, NB cells [CHLA-20 or SK-N-BE(2)] were seeded into a 6-well plate in a concentration of 1 × 106 cells in 3 ml per well and allowed to attach over night. PPPP was added with a final concentration of 1 µM. The control cells were treated with the same volume of 100 % ethanol (vehicle control). Cells were incubated with PPPP or vehicle for 3 days and analyzed for GD2 expression as described above. Comparison of GD2 expression was based on the mean fluorescence intensity ratio (MFI sample/isotype).
Discrimination of dead cells was based on propidium iodide (PI) or 4′,6-diamino-2-phenylindole (DAPI). For each sample, 20,000 live cells were analyzed using a BD fluorescence-activated cell sorting (FACS) CANTO II and FACS Diva software (BD Biosciences, Heidelberg, Germany). Data were analyzed with FlowJo 7.6.1 software (Treestar, Ashland, OR, USA).
Calcein release cytotoxicity assay
Calcein labeling of target cells was performed as previously described [26]. Target cells and effector cells were resuspended in X-VIVO-10/5 % human serum and co-cultured at an effector cell-to-target cell (E/T) ratio of 6.3:1. Maximum calcein release was induced by disruption of target cells with ultrasound for 30 s. After incubation for 5 h at 37 °C without CO2, calcein fluorescence in the supernatant was analyzed at an excitation wavelength of 495 nm and emission wavelength of 515 nm using a microplate reader (Synergy HT multi-mode microplate reader, BioTek, Bad Friedrichshall, Germany). Specific cytotoxicity was calculated according to the formula: \( \left[ {\left( {{\text{experimental lysis}} - {\text{spontaneous lysis}}} \right)/\left( {{\text{maximal lysis}} - {\text{spontaneous lysis}}} \right)} \right] \times 100 \). CAR was blocked by addition of 10 µg/ml anti-IdAb or mouse IgG1 as isotype control during co-incubation. Blocking of GD2 was done by addition of 10 µg/ml ch14.18/CHO during co-incubation. Rituximab was used as isotype control for ch14.18/CHO.
51Cr release cytotoxicity assay
Target cells were loaded with 51Cr (0.125 mCi/5 × 105 cells in 500 µl, PerkinElmer, Billerica, MA, USA) and incubated for 2 h at 37 °C. Cells were washed twice with X-VIVO-10 + 5 % human AB serum, and 5 × 103 target cells were co-cultured with NK-92-scFv(ch14.18)-zeta at an E/T ratio of 6.3:1 for 6 h. Radioactivity was measured in supernatants using gamma counter Wizard 2 (PerkinElmer, Billerica, MA, USA). Maximum 51Cr release was induced by addition of 5 % sodium dodecyl sulfate (SDS) solution (Promega, Madison, Wisconsin, USA) to target cell suspension. Specific cytotoxicity was calculated according to the formula:
Granzyme B and perforin ELISA
Gangliosides (100 ng GD2 or monosialoganglioside GM2) (Sigma-Aldrich, St. Louis, MO, USA) were coated in a 24-well plate (100 µl, 1 µg/ml, 100 % methanol, 1 h, 56 °C). NK-92-scFv(ch14.18)-zeta or NK-92-pLXSN (5 × 105) were added (500 µl X-VIVO-10, 5 % human AB serum, 6 h, 37 °C). As positive control, NK cells were activated with 10 ng/ml phorbol-12-myristate-13-acetate (PMA) (Calbiochem, EMD Biosciences Inc., La Jolla, CA, USA) and 1 µg/ml ionomycin (Sigma-Aldrich, St. Louis, MO, USA). CAR was blocked with 10 µg/ml anti-IdAb ganglidiomab compared to mouse IgG1 isotype control. Supernatants collected after 6 h were analyzed using granzyme B and perforin enzyme-linked immunosorbent assay (ELISA) kits (MabTech, Cincinnati, OH, USA).
Efficacy of NK-92-scFv(ch14.18)-zeta in a xenograft mouse model
Female NSG (NOD.Cg-Prkdc scid Il2rg tm1WjI/SzJ) mice were obtained at 6–8 weeks of age from Charles River Laboratories (Sulzfeld, Germany) and maintained under pathogen-free conditions. Experiments were performed in compliance with the German Law for Welfare of Laboratory Animals.
For induction of a primary subcutaneous tumor, 1 × 106 CHLA-20 cells diluted at a ratio of 1:2 in Matrigel (BD Biosciences, Bedford, MA, USA) were subcutaneously injected into the left flank of 28 mice (day 0). Treatment consisted of peritumoral injections of 2 × 107 NK-92-scFv(ch14.18)-zeta and 200 IU recombinant human IL-2 compared to NK-92-scFv(FRP5)-zeta and IL-2, IL-2 only and PBS (days 3, 7, 11, 15, 19, 26, 33, 40). IL-2 treatment was continued by intraperitoneal injection of 1,000 IU (days 4, 8, 12, 16, 20, 27, 34, 41). Tumor growth was monitored and calculated according to the formula: length x (width)2 × 0.5 until 800 mm3 or necrosis.
Statistical analysis
Data were analyzed using GraphPad Prism software (GraphPad Software, San Diego, CA, USA). Statistical analysis for comparison of two groups was performed using unpaired t test. For comparison of more than two groups, one-way analysis of variance (ANOVA) test was used. p values <0.05 were considered significant (*p < 0.05; **p < 0.01; ***p < 0.001). Survival curves were analyzed with a log-rank (Mantel–Cox) test. Due to multiple comparisons of survival curves, p values less than the Bonferroni-corrected significance level of 0.008 were considered significant (*p < 0.008; **p < 0.002; ***p < 0.0002).
Results
Chimeric antigen receptor expression on NK-92-scFv(ch14.18)-zeta
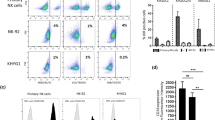
CAR expression on NK-92-scFv(ch14.18)-zeta was determined with anti-IdAb ganglidiomab, specific for the antigen-binding regions of the GD2-specific CAR and with anti-Myc-tag antibody. Both staining strategies revealed homogenous signals with NK-92-scFv(ch14.18)-zeta (Fig. 1). In contrast, NK-92-scFv(FRP5)-zeta stained only with an anti-Myc-tag antibody, demonstrating expression of a CAR with a different specificity. No CAR expression was detected on parental NK-92 and NK-92-pLXSN.
Chimeric antigen receptor expression on NK-92-scFv(ch14.18)-zeta and control cell lines. Flow cytometric analysis of CAR expression was performed by staining with 1 µg/1 × 106 cells of either anti-IdAb ganglidiomab (filled black curve, top row) or anti-Myc-tag antibody (filled black curve, bottom row), followed by staining with PE-labeled secondary antibody. Controls were stained with mouse IgG1 (1 µg/1 × 106) that was used as isotype control (dashed gray curve). Results are shown as representative histograms chosen from several individual experiments
Cytotoxicity of NK-92-scFv(ch14.18)-zeta toward GD2-expressing NB cell lines
We analyzed cytotoxic activity of GD2-specific NK-92-scFv(ch14.18)-zeta toward GD2+ CHLA-20 and GD2− SK-N-SH NB cell lines (Fig. 2a). NK-92-scFv(ch14.18)-zeta (E/T ratio 6.3:1) effectively lysed GD2+ CHLA-20 (specific cytotoxicity 55 %) in contrast to NK control cell lines NK-92, NK-92-pLXSN and NK-92-scFv(FRP5)-zeta (with specific cytotoxicities ranging from 22 to 25 %). Lysis of GD2− SK-N-SH was comparable for all NK cell lines with specific cytotoxicities ranging from 19 % [NK-92-pLXSN) to 22 % (NK-92)] (Fig. 2b).
Specific cytotoxicity of NK-92-scFv(ch14.18)-zeta toward GD2+ neuroblastoma cell lines. a, c GD2 expression of target NB cell lines was confirmed using flow cytometry. Cells were stained with either ch14.18 (1 µg/1 × 106 cells, filled black curve) or anti-CD20 chimeric antibody rituximab (1 µg/1 × 106 cells, dashed black curve) as isotype control, followed by staining with PE-labeled secondary antibody. b Specific cytotoxicities of NK-92-scFv(ch14.18)-zeta and control NK cell lines toward GD2+ CHLA-20 (black bars) and GD2− SK-N-SH (gray bars) were determined in calcein release cytotoxicity assays (E/T ratio 6.3:1). Differences in specific cytotoxicity of NK-92-scFv(ch14.18)-zeta toward CHLA-20 compared to control cell lines were statistically significant (***p < 0.001). NK-92-scFv(ch14.18)-zeta-mediated lysis of CHLA-20 was significantly higher compared to lysis of GD2− SK-N-SH (***p < 0.001). Results are presented as mean specific cytotoxicity in % ± SD of three independent experiments. d Cytotoxic activity of NK-92-scFv(ch14.18)-zeta toward six human GD2+ NB cell lines was analyzed in 51Cr release assays (E/T ratio 6.3:1). NK-92-scFv(ch14.18)-zeta-mediated lysis was normalized against control NK cells. Results are shown as mean GD2-specific cytotoxicity ± SD of at least three independent experiments
These results demonstrate GD2-specific cytotoxicity of NK-92-scFv(ch14.18)-zeta. This experiment showed that cytotoxic activity of the different control NK cell lines was comparable, and therefore, only one control NK cell line was used in the following cytotoxicity assays.
We further confirmed the GD2-specific cytotoxic activity of NK-92-scFv(ch14.18)-zeta in 51Cr release cytotoxicity assays with six GD2-expressing NB cell lines (Fig. 2c). To show GD2-specific lysis mediated by NK-92-scFv(ch14.18)-zeta, specific cytotoxicity of control NK cell lines was set to zero and NK-92-scFv(ch14.18)-zeta-mediated lysis was normalized to control NK cells. Therefore, specific cytotoxic activities represent the difference in lysis between control NK cell lines and GD2-specific NK-92-scFv(ch14.18)-zeta (Fig. 2d).
51Cr release assays revealed that at an E/T ratio of 6.3:1 NK-92-scFv(ch14.18)-zeta expressing a GD2-specific CAR effectively lysed all six GD2+ NB cell lines with specific cytotoxicities ranging from 34 % (CHLA-79) to 66 % (CHLA-136) over control NK cell lines. These data show that NK-92-scFv(ch14.18)-zeta are able to effectively lyse GD2+ target cells that otherwise exhibit only a low sensitivity toward NK cell lysis in general, suggesting that in our setting CAR expression enables NK effector cells to overcome potential target cell escape mechanisms.
Role of GD2 antigen recognition by chimeric receptor on NK-92-scFv(ch14.18)-zeta
We analyzed whether NK-92-scFv(ch14.18)-zeta-mediated cytotoxicity depends on binding to the target antigen GD2 expressed on CHLA-20 in contrast to K562 controls (Fig. 3a). The addition of anti-IdAb (10 µg/ml) almost completely abrogated GD2-specific cytotoxicity of NK-92-scFv(ch14.18)-zeta toward CHLA-20 (11 % specific cytotoxicity) compared to isotype control (38 % specific cytotoxicity), indicating that binding of the target antigen GD2 is the main primary mechanism of NK-92-scFv(ch14.18)-zeta cell activation (Fig. 3b). In contrast, GD2-independent cytotoxicity against NK cell-sensitive K562 (Fig. 3c) was not affected by anti-IdAb application (37 % specific cytotoxicity in controls compared to 36 % with anti-IdAb), indicating in this case that GD2-independent cell killing mechanisms are involved.
Effect of competitive CAR and GD2 antigen blocking on specific cytotoxicity of NK-92-scFv(ch14.18)-zeta. a GD2 expression of CHLA-20 (left histogram) and K562 (right histogram) was confirmed using flow cytometry. Cells were stained with either ch14.18 (1 µg/1 × 106 cells, filled black curve) or rituximab (1 µg/1 × 106 cells, dashed black curve) as isotype control, followed by staining with PE-labeled secondary antibody. b, c CAR on NK-92-scFv(ch14.18)-zeta was blocked by addition of 10 µg/ml anti-IdAb during co-incubation with GD2+ target cell lines CHLA-20 (b) and GD2− target cells K562 (c). Differences in specific cytotoxicity induced by CAR-blocking with anti-IdAb were statistically significant (***p < 0.001). Results are presented as mean specific cytotoxicity in % ± SD of at least three independent experiments. Data were normalized against specific cytotoxicity of controls without addition of anti-IdAb. Target antigen GD2 was blocked by addition of 10 µg/ml ch14.18/CHO during co-incubation of the GD2+ target cell line CHLA-20 (d) and GD2− K562 (e) target cells with NK-92-scFv(ch14.18)-zeta. Addition of ch14.18/CHO resulted in significantly abrogated cytotoxicity of NK-92-scFv(ch14.18)-zeta (***p < 0.001). Results are presented as mean specific cytotoxicity in % ± SD of three independent experiments. Data were normalized against specific cytotoxicity of controls without addition of antibody
Blocking GD2 was our second concurrent method to confirm the role of antigen recognition. Hence, we performed competitive calcein release cytotoxicity assays with the GD2+ NB cell line CHLA-20 (Fig. 3d) and GD2− K562 (Fig. 3e), in which GD2-specific antibody ch14.18/CHO was added during co-incubation with NK-92-scFv(ch14.18)-zeta to prevent the binding of the CAR to GD2. Since NK-92 lack expression of Fc-gamma receptors (FcγRs) [13], binding of ch14.18/CHO to GD2 will not induce antibody-dependent cellular cytotoxicity (ADCC) of tumor cells. Blocking of GD2 by addition of 10 µg/ml ch14.18/CHO resulted in almost complete abrogation of NK-92-scFv(ch14.18)-zeta-mediated lysis of the GD2-positive cell line CHLA-20 (14 % specific cytotoxicity with the addition of ch14.18 compared to 54 % specific cytotoxicity in controls). Addition of the same amount of rituximab isotype control did not have any effect (57 % specific lysis). NK-92-scFv(ch14.18)-zeta-mediated lysis of K562 was unaffected by addition of ch14.18/CHO (51 % specific cytotoxicity in controls compared to 52 % with ch14.18/CHO), confirming that GD2-independent mechanisms are involved.
This finding was further supported by assessing GD2-dependent release of effector molecules (granzyme B and perforin) with ELISA. For this purpose, NK-92-scFv(ch14.18)-zeta cells were incubated with immobilized GD2 and GM2. Only GD2 induced a significant increase in granzyme B and perforin release in NK-92-scFv(ch14.18)-zeta compared to controls (Fig. 4a, b). Blocking of the CAR with an anti-IdAb significantly inhibited GD2-induced effects, relative to isotype control (Fig. 4c, e). Importantly, this was only detectable for the CAR-expressing cell line NK-92-scFv(ch14.18)-zeta, in contrast to empty vector control cell line NK-92-pLXSN, while both cell lines reacted to non-specific stimulation with PMA/ionomycin (Fig. 4d, f). Together, these findings demonstrate antigen-specific release of effector molecules and exclude contributions by other activating stimuli, such as activating NK cell ligands.
Granzyme B and perforin production of NK-92-scFv(ch14.18)-zeta and pLXSN in response to activation. Granzyme B (a) and perforin (b) production of NK-92-scFv(ch14.18)-zeta in response to incubation with 100 ng of plate-bound gangliosides GM2 and GD2 for 5 h, was quantified in supernatants by ELISA. Results are presented as mean effector molecule concentration in ng/ml ± SD from three independent experiments. NK-92-scFv(ch14.18)-zeta incubated without plate-bound gangliosides (control) were used to normalize data from independent experiments. GD2-induced increase in effector molecule production was statistically significant (***p < 0.001). c, e NK-92-scFv(ch14.18)-zeta was incubated with immobilized GD2, and CAR was blocked by addition of 10 µg/ml anti-IdAb. Same amounts of mouse IgG1 were used as isotype control. Production of effector molecules granzyme B (c) and perforin (e) was analyzed with ELISA. Results are presented as mean effector molecule concentration in ng/ml ± SD from three independent experiments. Data of independent experiments were normalized against basal effector molecule release in the absence of GD2. Production of effector molecules was significantly higher upon GD2 stimulation and was abrogated when CAR was blocked (***p < 0.001). Fold increase in production of effector molecules granzyme B (d) and perforin (f) of NK-92-scFv(ch14.18)-zeta and NK-92-pLXSN compared to respective controls incubated in the absence of GD2. Results are presented as mean fold increase ± SD of three independent experiments
We also determined the role of GD2 antigen recognition in NK-92-scFv(ch14.18)-zeta-mediated lysis by blocking ganglioside synthesis. For this purpose, two GD2+ cell lines [SK-N-BE(2), CHLA-20] were treated with the selective glucosylceramide synthase (GCS) inhibitor PPPP (1-phenyl-2-hexadecanoylamino-3-pyrrolidino-1-propanol), and down-regulation of GD2 surface expression was determined by flow cytometry (Fig. 5a, b). PPPP- and vehicle-treated control cells were used as target cells in a cytotoxicity assay with NK-92-scFv(ch14.18)-zeta as effector cells (E/T ratio 6.3:1). Reduced GD2 expression resulted in significantly decreased sensitivity of SK-N-BE(2) and CHLA-20 toward NK-92-scFv(ch14.18)-zeta-mediated lysis (14 and 39 % specific cytotoxicity, respectively) compared to vehicle-treated target cells (37 and 63 % specific lysis, respectively) (Fig. 5c), again emphasizing the role of GD2 recognition by the CAR in cell killing of GD2+ target cells.
Effect of GCS inhibition on GD2 expression on target cells and NK-92-scFv(ch14.18)-zeta-mediated lysis. Target cells SK-N-BE(2) and CHLA-20 were treated with 1 µM PPPP or the same volume of 100 % ethanol as vehicle control for 3 days. For analysis of GD2 expression, cells were stained with 1 µg ch14.18/CHO or rituximab as isotype control. Cytotoxic activity of NK-92-scFv(ch14.18)-zeta toward PPPP-treated and vehicle-treated target cells was determined in 51Cr release assays (E/T ratio 6.3:1). a Representative histograms for each cell line (black curve: isotype control; black dotted curve: PPPP-treated cells; black filled curve: vehicle-treated cells). b Mean fluorescence intensity (MFI) ratio of GD2 expression was calculated according to the formula: MFI of GD2-stained sample/MFI of isotype control. PPPP-induced decrease in GD2 expression was statistically significant [SK-N-BE(2) **p = 0.0037, CHLA-20 *p = 0.01]. Results are presented as mean MFI ratio ± SD of five independent experiments. c Cytotoxic activity of NK-92-scFv(ch14.18)-zeta toward PPPP- and vehicle-treated target cells. Reduction in sensitivity of target cells to NK-92-scFv(ch14.18)-mediated lysis in response to PPPP treatment was statistically significant (***p < 0.001). Cytotoxic activity of NK-92-scFv(ch14.18)-zeta toward vehicle-treated cells was used to normalize data of independent experiments. Results are shown as mean specific cytotoxicity in % ± SD of three independent experiments
Anti-tumor efficacy of NK-92-scFv(ch14.18)-zeta
The therapeutic efficacy of NK-92-scFv(ch14.18)-zeta on primary tumor growth was determined in a xenograft mouse model with the drug-resistant NB cell line CHLA-20. Subcutaneous tumors were induced by injection of 1 × 106 CHLA-20 into the left flank. Mice were treated with NK-92-scFv(ch14.18)-zeta and IL-2. Control groups received either PBS only (untreated), IL-2 only or a combination of the ErbB2-specific NK control cell line NK-92-scFv(FRP5)-zeta and IL-2 (Fig. 6c, lower panel).
Anti-tumor efficacy of NK-92scFv(ch14.18)-zeta in a GD2+ NB xenograft. Female NSG mice (group size n = 7) received subcutaneous injection of 1 × 106 GD2+ CHLA-20 on day 0. Mice were subjected to eight peritumoral subcutaneous injections of NK-92-scFv(ch14.18)-zeta in combination with 200 IU of human recombinant IL-2 on days 3, 7, 11, 15, 19, 26, 33, 40. Additionally, 1,000 IU of recombinant human IL-2 were intraperitoneally injected 24 h after each NK cell injection. Control mice received either PBS only (untreated control) or IL-2 only or ErbB2-specific NK-92-scFv(FRP5)-zeta in combination with IL-2 (treatment schedule is shown in 6C, lower panel). a Survival curves of NK-92-scFv(ch14.18)-zeta-treated mice (black solid curve), NK-92-scFv(FRP5)-zeta-treated mice (black dashed curve), IL-2 control mice (gray solid curve) and untreated mice (gray dashed curve). Difference in survival of NK-92-scFv(ch14.18)-zeta-treated mice compared to untreated mice (**p = 0.0009), IL-2-treated mice (**p = 0.0008) and NK-92-scFv(FRP5)-zeta-treated mice (*p = 0.002) was statistically significant. b Comparison of tumor growth, analyzed as time from tumor cell inoculation until development of an established tumor with a volume of ≥150 mm3. Tumor growth in NK-92-scFv(ch14.18)-zeta-treated mice was significantly delayed compared to control groups (***p < 0.001). Results are presented as mean time until tumor volume of 150 mm3 was reached [d] ± SD (n = 7), c Tumor growth of individual mice of the NK-92-scFv(ch14.18)-zeta-treated group (black solid lines) and NK-92-scFv(FRP5)-zeta control group (black dashed lines). The treatment schedule indicates days of peritumoral application of NK cells and IL-2 (black arrow) as well as intraperitoneal injections of IL-2 (gray arrows)
IL-2 was included in our experimental setting to ensure survival and stable CAR expression of the IL-2-dependent NK cell lines NK-92-scFv(ch14.18)-zeta and NK-92-scFv(FRP5)-zeta in vivo.
Median survival time of control mice was 30 days (untreated, IL-2 control) and 34 days (NK-92-scFv(FRP5)-zeta and IL-2). In contrast, treatment with GD2-specific CAR-expressing NK-92-scFv(ch14.18)-zeta resulted in significantly increased survival with a median survival time of 52 days (Fig. 6a).
Tumor growth of individual mice of the NK-92-scFv(FRP5)-zeta-treated group and mice receiving NK-92-scFv(ch14.18)-zeta is shown in Fig. 6c. Our data demonstrate that CHLA-20 tumor growth was quite aggressive, but could be effectively delayed by application of GD2-specific NK-92-scFv(ch14.18)-zeta. The average time to the appearance of the first palpable tumors after CHLA-20 injection was longer in NK-92-scFv(ch14.18)-zeta-treated mice (30 ± 10 days) compared to control groups (untreated group 13 ± 2 days, IL-2 group 15 ± 3 days, NK-92-scFv(FRP5)-zeta group 21 ± 4 days). To further demonstrate the effect of the NK-92-scFv(ch14.18)-zeta treatment, we compared all experimental groups regarding the time until tumors reached a volume ≥150 mm3 (Fig. 6b). This analysis showed that tumor growth was significantly delayed in NK-92-scFv(ch14.18)-zeta-treated mice (49 ± 11 days) compared to control groups (untreated group 29 ± 5 days, IL-2 group 27 ± 4 days, NK-92-scFv(FRP5)-zeta group 29 ± 4 days).
Taken together, our results demonstrate the efficacy of chimeric receptor expressing NK-92-scFv(ch14.18)-zeta to slow down tumor growth and thereby prolong survival in a GD2+ NB xenograft model.
Discussion
The treatment of high-risk NB patients remains challenging, despite recent progress in developing and refining conventional multimodal therapy protocols. In particular, a prospective randomized study conducted by the Children’s Oncology Group demonstrated that a combination of 13-cis-retinoic acid and immunotherapy with the GD2-specific chimeric antibody ch14.18 and adjuvant cytokines, significantly increased the 2-year event-free survival rate to 66 % compared to 46 % with 13-cis-retinoic acid alone [5]. This provided definitive clinical evidence supporting immunological targeting of GD2 for therapy of high-risk NB. However, further improvement is needed and may be achieved by novel cellular GD2-targeting approaches.
The use of NK cells in NB is appealing because of the missing self hypothesis, since major histocompatibility complex (MHC) class I expression is rather low [27]. Immunological approaches using NK cells involve the adoptive transfer of ex vivo expanded autologous, IL-2 stimulated NK cells (LAK) or the application of allogeneic NK cells in a haploidentical setting. Autologous attempts showed only modest success, probably due to the inhibition of NK cell activity in the tumor microenvironment [28, 29]. It could be shown that NK cells from patients with malignancies exhibit only an insufficient cytotoxicity [30, 31]. The use of allogeneic haploidentical NK cells is more promising than human leukocyte antigen (HLA)-identical NK cells, due to the absence of inhibitory signaling [32–34]. Nevertheless, there are still obstacles to overcome, because tumors can evade cytotoxicity mediated by NK cells through escape mechanisms, such as shedding of activating ligands, e.g., the MHC class I-related protein A (MICA) [35].
This problem can be addressed by the application of genetically modified NK cells that express a chimeric antigen receptor (CAR) and thereby specifically target and lyse tumor cells independently from inhibitory and/or activating ligands. Currently, CAR-based approaches mainly utilize T cells as effector cell populations. Nevertheless, this approach has been expanded to NK cells [36, 37]. Protocols for isolation and expansion of NK cells have been improved to compensate for initially lower NK cell numbers compared to T cells [38, 39]. NK cells might be particularly advantageous in an allogeneic setting, since they exhibit reactivity in the graft versus tumor direction but simultaneously prevent graft versus host disease [28, 40, 41]. The shorter life span of NK cells, compared to T cells, might also be beneficial with regard to long-term side effects potentially caused by memory-like CAR-expressing T cells [42, 43].
Several attempts have been made to genetically modify primary NK cells of healthy donors to express CARs. However, there are limitations regarding extensive variations in transfection efficiency and CAR functionality depending on the donor [36, 37]. Furthermore, ex vivo expansion and activation of autologous or allogeneic NK cells requires time for each patient and consumes substantial resources.
Therefore, the use of genetically modified NK-92 is a promising alternative to bypass these difficulties. NK-92 is well tolerated in patients [17] and can be easily expanded under good manufacturing practices (GMP) [15]. The application of NK-92 in a recent phase I trial demonstrated persistence of NK-92 for up to 48 h after infusion and revealed that the tendency of NK-92 to induce a humoral immune response is only low [18]. Importantly, CAR-expressing NK-92 could be ready on demand in a standardized quality. Furthermore, NK-92 lacks inhibitory killer cell immunoglobulin-like receptors (KIR) [44] and therefore has an uninhibited alloreactivity toward tumor antigen-expressing target cells. Several attempts have already shown the in vitro and in vivo efficacy of genetically modified NK-92 with different antigen specificities. To target malignant cells of hematopoietic origin, genetically modified NK-92 expressing a CD20-specific CAR were derived and demonstrated effective lysis of NK-resistant target antigen-expressing cell lines and primary cells in vitro and significantly delayed tumor growth in vivo [45]. NK-92 cells expressing an ErbB2-specific CAR effectively lysed ErbB2-expressing malignant cells of epithelial origin in vitro and mediated a specific anti-tumor effect in vivo [25].
Here, we expand the approach to NB using GD2, which is a suitable and promising antigen for immunotherapy of high-risk NB. With regard to the potential tumorigenic risk of NK-92 cells, previous studies revealed that irradiation successfully prevented proliferation without compromising cytotoxicity [22, 44]. This represents an important prerequisite for safe application in patients and tolerability to repeated GD2-specific NK-92 applications. Importantly, limited persistence of irradiated non-proliferating GD2-specific NK-92 appears to be advantageous with respect to long-term side effects associated with persisting memory-like CAR-T cells, such as CD19-directed CAR-T cell caused B cell aplasia [43].
We investigated the mechanism and the cytotoxic activity of NK-92-scFv(ch14.18)-zeta toward NB in vitro and in vivo. Our results demonstrated that NK-92-scFv(ch14.18)-zeta specifically target GD2. Further, we showed that immobilized GD2 is sufficient to activate NK-92-scFv(ch14.18)-zeta in the absence of activating NK receptor/ligand interactions. Experimental disruption of the CAR/GD2 interaction resulted in almost complete abrogation of NK-92-scFv(ch14.18)-zeta-mediated lysis of GD2+ NB cells. These findings suggest that in our experimental setting activation of NK-92-scFv(ch14.18)-zeta is independent of activating NK cell receptor/ligand interactions and cytotoxic activity of NK-92-scFv(ch14.18)-zeta is mainly mediated by recognition of GD2 through the CAR.
GD2-CAR-expressing NK-92 cells were able to effectively lyse human NB cells that are partially or multidrug resistant. This indicates that CAR-expressing NK-92-scFv(ch14.18)-zeta might be able to overcome some of the escape mechanisms that NB cells can acquire during intensive therapy. Most importantly, we showed in a GD2+ xenograft mouse model that NK-92-scFv(ch14.18)-zeta mediate a significant anti-tumor effect toward an aggressively growing, drug-resistant NB cell line, resulting in a significantly delayed tumor growth and a prolonged survival of treated mice. In summary, our data demonstrate that NK-92-scFv(ch14.18)-zeta effectively kill GD2+ NB cell lines in vitro and are able to significantly slow down primary tumor growth in vivo revealing the potential for and providing an important baseline for a clinical application of NK-92-scFv(ch14.18)-zeta.
Abbreviations
- ADCC:
-
Antibody-dependent cellular cytotoxicity
- ANOVA:
-
Analysis of variance
- anti-IdAb:
-
Anti-idiotypic antibody
- BSA:
-
Bovine serum albumin
- CAR:
-
Chimeric antigen receptor
- CD:
-
Cluster of differentiation
- CHO:
-
Chinese hamster ovary
- COG:
-
Children’s Oncology Group
- DAPI:
-
4′,6-Diamino-2-phenylindole
- EDTA:
-
Ethylenediaminetetraacetic acid
- ELISA:
-
Enzyme-linked immunosorbent assay
- E/T ratio:
-
Effector cell-to-target cell ratio
- FACS:
-
Fluorescence-activated cell sorting
- FasL:
-
Fas ligand
- FBS:
-
Fetal bovine serum
- FcγR:
-
Fc-gamma receptor
- GCS:
-
Glucosylceramide synthase
- GD2:
-
Disialoganglioside
- GM2:
-
Monosialoganglioside
- GM-CSF:
-
Granulocyte–macrophage colony-stimulating factor
- GMP:
-
Good manufacturing practices
- HLA:
-
Human leukocyte antigen
- IFN-γ:
-
Interferon-γ
- IgG:
-
Immunoglobulin G
- IL-2:
-
Interleukin-2
- IMDM:
-
Iscove’s modified Dulbecco’s medium
- IU:
-
International unit
- KIR:
-
Killer cell immunoglobulin-like receptor
- LAK:
-
Lymphokine-activated killer cells
- MFI:
-
Mean fluorescence intensity
- MHC:
-
Major histocompatibility complex
- MICA:
-
MHC class I-related protein A
- NB:
-
Neuroblastoma
- NK:
-
Natural killer
- NSG:
-
NOD.Cg-Prkdc scid Il2rg tm1WjI/SzJ
- PBS:
-
Phosphate-buffered saline
- PE:
-
Phycoerythrin
- PI:
-
Propidium iodide
- PMA:
-
Phorbol-12-myristate-13-acetate
- PPPP:
-
1-Phenyl-2-hexadecanoylamino-3-pyrrolidino-1-propanol
- RPMI:
-
Roswell Park Memorial Institute
- scFv:
-
Single-chain fragment variable
- SDS:
-
Sodium dodecyl sulfate
- STR:
-
Short tandem repeat
- TNF:
-
Tumor necrosis factor
- TRAIL:
-
TNF-related apoptosis-inducing ligand
- TRAIL-R:
-
TNF-related apoptosis-inducing ligand receptor
References
Westermann F, Schwab M (2002) Genetic parameters of neuroblastomas. Cancer Lett 184:127–147
Maris JM, Hogarty MD, Bagatell R, Cohn SL (2007) Neuroblastoma. Lancet 369:2106–2120
van Noesel MM, Versteeg R (2004) Pediatric neuroblastomas: genetic and epigenetic ‘danse macabre’. Gene 325:1–15
Keshelava N, Seeger RC, Groshen S, Reynolds CP (1998) Drug resistance patterns of human neuroblastoma cell lines derived from patients at different phases of therapy. Cancer Res 58:5396–5405
Yu AL, Gilman AL, Ozkaynak MF, London WB, Kreissman SG, Chen HX, Smith M, Anderson B, Villablanca JG, Matthay KK, Shimada H, Grupp SA, Seeger R, Reynolds CP, Buxton A, Reisfeld RA, Gillies SD, Cohn SL, Maris JM, Sondel PM (2010) Anti-GD2 antibody with GM-CSF, interleukin-2, and isotretinoin for neuroblastoma. N Engl J Med 363:1324–1334
Mujoo K, Cheresh DA, Yang HM, Reisfeld RA (1987) Disialoganglioside GD2 on human neuroblastoma cells: target antigen for monoclonal antibody-mediated cytolysis and suppression of tumor growth. Cancer Res 47:1098–1104
Yang RK, Sondel PM (2010) Anti-GD2 strategy in the treatment of neuroblastoma. Drugs Future 35:665
Biron CA, Nguyen KB, Pien GC, Cousens LP, Salazar-Mather TP (1999) Natural killer cells in antiviral defense: function and regulation by innate cytokines. Annu Rev Immunol 17:189–220
Trinchieri G (1989) Biology of natural killer cells. Adv Immunol 47:187–376
Cullen SP, Martin SJ (2008) Mechanisms of granule-dependent killing. Cell Death Differ 15:251–262
Schmitz I, Kirchhoff S, Krammer PH (2000) Regulation of death receptor-mediated apoptosis pathways. Int J Biochem Cell Biol 32:1123–1136
Kim S, Iizuka K, Aguila HL, Weissman IL, Yokoyama WM (2000) In vivo natural killer cell activities revealed by natural killer cell-deficient mice. Proc Natl Acad Sci USA 97:2731–2736
Gong JH, Maki G, Klingemann HG (1994) Characterization of a human cell line (NK-92) with phenotypical and functional characteristics of activated natural killer cells. Leukemia 8:652–658
Klingemann HG, Wong E, Maki G (1996) A cytotoxic NK-cell line (NK-92) for ex vivo purging of leukemia from blood. Biol Blood Marrow Transplant 2:68–75
Tam YK, Martinson JA, Doligosa K, Klingemann HG (2003) Ex vivo expansion of the highly cytotoxic human natural killer-92 cell-line under current good manufacturing practice conditions for clinical adoptive cellular immunotherapy. Cytotherapy 5:259–272
Yan Y, Steinherz P, Klingemann HG, Dennig D, Childs BH, McGuirk J, O’Reilly RJ (1998) Antileukemia activity of a natural killer cell line against human leukemias. Clin Cancer Res 4(11):2859–2868
Arai S, Meagher R, Swearingen M, Myint H, Rich E, Martinson J, Klingemann H (2008) Infusion of the allogeneic cell line NK-92 in patients with advanced renal cell cancer or melanoma: a phase I trial. Cytotherapy 10(6):625–632
Tonn T, Schwabe D, Klingemann HG, Becker S, Esser R, Koehl U, Suttorp M, Seifried E, Ottmann OG, Bug G (2013) Treatment of patients with advanced cancer with the natural killer cell line NK-92. Cytotherapy 15(12):1563–1570
Groh V, Wu J, Yee C, Spies T (2002) Tumour-derived soluble MIC ligands impair expression of NKG2D and T-cell activation. Nature 419:734–738
Lee JC, Lee KM, Kim DW, Heo DS (2004) Elevated TGF-beta1 secretion and down-modulation of NKG2D underlies impaired NK cytotoxicity in cancer patients. J Immunol 172:7335–7340
Uherek C, Groner B, Wels W (2001) Chimeric antigen receptors for the retargeting of cytotoxic effector cells. J Hematother Stem Cell Res 10:523–534
Esser R, Muller T, Stefes D, Kloess S, Seidel D, Gillies SD, Aperlo-Iffland C, Huston JS, Uherek C, Schonfeld K, Tonn T, Huebener N, Lode HN, Koehl U, Wels WS (2012) NK cells engineered to express a GD2 -specific antigen receptor display built-in ADCC-like activity against tumour cells of neuroectodermal origin. J Cell Mol Med 16(3):569–581
Keshelava N, Zuo JJ, Chen P, Waidyaratne SN, Luna MC, Gomer CJ, Triche TJ, Reynolds CP (2001) Loss of p53 function confers high-level multidrug resistance in neuroblastoma cell lines. Cancer Res 61(16):6185–6193
Keshelava N, Davicioni E, Wan Z, Ji L, Sposto R, Triche TJ, Reynolds CP (2007) Histone deacetylase 1 gene expression and sensitization of multidrug-resistant neuroblastoma cell lines to cytotoxic agents by depsipeptide. J Natl Cancer Inst 99(14):1107–1119
Uherek C, Tonn T, Uherek B, Becker S, Schnierle B, Klingemann HG, Wels W (2002) Retargeting of natural killer-cell cytolytic activity to ErbB2-expressing cancer cells results in efficient and selective tumor cell destruction. Blood 100(4):1265–1273
Lode HN, Schmidt M, Seidel D, Huebener N, Brackrock D, Bleeke M, Reker D, Brandt S, Mueller HP, Helm C, Siebert N (2013) Vaccination with anti-idiotype antibody ganglidiomab mediates a GD(2)-specific anti-neuroblastoma immune response. Cancer Immunol Immunother 62(6):999–1010
Raffaghello L, Prigione I, Airoldi I, Camoriano M, Morandi F, Bocca P, Gambini C, Ferrone S, Pistoia V (2005) Mechanisms of immune evasion of human neuroblastoma. Cancer Lett 228(1–2):155–161
Farag SS, Fehniger TA, Ruggeri L, Velardi A, Caligiuri MA (2002) Natural killer cell receptors: new biology and insights into the graft-versus-leukemia effect. Blood 100:1935–1947
Papamichail M, Perez SA, Gritzapis AD, Baxevanis CN (2004) Natural killer lymphocytes: biology, development, and function. Cancer Immunol Immunother 53:176–186
Suck G (2006) Novel approaches using natural killer cells in cancer therapy. Semin Cancer Biol 16:412–418
Sutlu T, Alici E (2009) Natural killer cell-based immunotherapy in cancer: current insights and future prospects. J Intern Med 266:154–181
Ruggeri L, Mancusi A, Capanni M, Martelli MF, Velardi A (2005) Exploitation of alloreactive NK cells in adoptive immunotherapy of cancer. Curr Opin Immunol 17:211–217
Velardi A, Ruggeri L, Mancusi A, Aversa F, Christiansen FT (2009) Natural killer cell allorecognition of missing self in allogeneic hematopoietic transplantation: a tool for immunotherapy of leukemia. Curr Opin Immunol 21:525–530
Voskens CJ, Watanabe R, Rollins S, Campana D, Hasumi K, Mann DL (2010) Ex-vivo expanded human NK cells express activating receptors that mediate cytotoxicity of allogeneic and autologous cancer cell lines by direct recognition and antibody directed cellular cytotoxicity. J Exp Clin Cancer Res 29:134
Kloess S, Huenecke S, Piechulek D, Esser R, Koch J, Brehm C, Soerensen J, Gardlowski T, Brinkmann A, Bader P, Passweg J, Klingebiel T, Schwabe D, Koehl U (2010) IL-2-activated haploidentical NK cells restore NKG2D-mediated NK-cell cytotoxicity in neuroblastoma patients by scavenging of plasma MICA. Eur J Immunol 40(11):3255–3267
Altvater B, Landmeier S, Pscherer S, Temme J, Schweer K, Kailayangiri S, Campana D, Juergens H, Pule M, Rossig C (2009) 2B4 (CD244) signaling by recombinant antigen-specific chimeric receptors costimulates natural killer cell activation to leukemia and neuroblastoma cells. Clin Cancer Res 15(15):4857–4866
Imai C, Iwamoto S, Campana D (2005) Genetic modification of primary natural killer cells overcomes inhibitory signals and induces specific killing of leukemic cells. Blood 106:376–383
Liu Y, Wu HW, Sheard MA, Sposto R, Somanchi SS, Cooper LJ, Lee DA, Seeger RC (2013) Growth and activation of natural killer cells ex vivo from children with neuroblastoma for adoptive cell therapy. Clin Cancer Res 19(8):2132–2143
Fujisaki H, Kakuda H, Shimasaki N, Imai C, Ma J, Lockey T, Eldridge P, Leung WH, Campana D (2009) Expansion of highly cytotoxic human natural killer cells for cancer cell therapy. Cancer Res 69(9):4010–4017
Benjamin JE, Gill S, Negrin RS (2010) Biology and clinical effects of natural killer cells in allogeneic transplantation. Curr Opin Oncol 22(2):130–137
Ruggeri L, Capanni M, Urbani E, Perruccio K, Shlomchik WD, Tosti A, Posati S, Rogaia Frassoni F, Aversa F, Martelli MF, Velardi A (2002) Effectiveness of donor natural killer cell alloreactivity in mismatched hematopoietic transplants. Science 295(5562):2097–2100
Campbell KS, Hasegawa J (2013) Natural killer cell biology: an update and future directions. J Allergy Clin Immunol 132(3):536–544
Porter DL, Levine BL, Kalos M, Bagg A, June CH (2011) Chimeric antigen receptor-modified T cells in chronic lymphoid leukemia. N Engl J Med 365(8):725–733
Tonn T, Becker S, Esser R, Schwabe D, Seifried E (2001) Cellular immunotherapy of malignancies using the clonal natural killer cell line NK-92. J Hematother Stem Cell Res 10:535–544
Muller T, Uherek C, Maki G, Chow KU, Schimpf A, Klingemann HG, Tonn T, Wels WS (2008) Expression of a CD20-specific chimeric antigen receptor enhances cytotoxic activity of NK cells and overcomes NK-resistance of lymphoma and leukemia cells. Cancer Immunol Immunother 57(3):411–423
Acknowledgments
We thank Dr. Barry J. Maurer for providing the PPPP; Tito Woodburn, Heather Kimmons, Heather Davidson, Malkanthi Mudannayake and Christin Eger for excellent technical assistance; Merck Serono and Merck KGaA for providing scFv(ch14.18) constructs; and the SIOPEN group for providing ch14.18/CHO. This work was financially supported by the German Cancer Foundation (Deutsche Krebshilfe, Holger N. Lode) and the South Plains Foundation (Nicole Huebener). Further support was provided by the University Medicine of Greifswald (Holger N. Lode), the Hector Stiftung (Nicole Huebener, Nikolai Siebert, Holger N. Lode), the Kind-Philipp-Stiftung für Leukämieforschung (Diana Seidel), Apeiron Biologics (Holger N. Lode) and National Cancer Institute grant CA82830 (C. Patrick Reynolds).
Conflict of interest
The authors have no conflict of interest to declare.
Ethical standard
All applicable international, national, and/or institutional guidelines for the care and use of animals were followed. This article does not contain any studies with human participants performed by any of the authors.
Author information
Authors and Affiliations
Corresponding author
Additional information
Nicole Huebener and Holger N. Lode have share senior authorship.
Rights and permissions
About this article
Cite this article
Seidel, D., Shibina, A., Siebert, N. et al. Disialoganglioside-specific human natural killer cells are effective against drug-resistant neuroblastoma. Cancer Immunol Immunother 64, 621–634 (2015). https://doi.org/10.1007/s00262-015-1669-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00262-015-1669-5