Abstract
Clinical outcomes from cancer vaccine trials in patients with advanced melanoma have so far been disappointing. This appears at least partially due to a state of immunosuppression in these patients induced by an expansion of regulatory cell populations including regulatory T cells (Tregs). We have previously demonstrated potent immunogenicity of the NY-ESO-1/ISCOMATRIX™ vaccine in patients with resected melanoma (study LUD99-08); however, the same vaccine induced only a few vaccine antigen-specific immune responses in patients with advanced disease (study LUD2002-013). Pre-clinical models suggest that the alkylating agent cyclophosphamide can enhance immune responses by depleting Tregs. Therefore, we have enrolled a second cohort of patients with advanced melanoma in the clinical trial LUD2002-013 to investigate whether pre-treatment with cyclophosphamide could improve the immunogenicity of the NY-ESO-1/ISCOMATRIX™ vaccine. The combination treatment led to a significant increase in vaccine-induced NY-ESO-1-specific CD4+ T cell responses compared with the first trial cohort treated with vaccine alone. We could not detect a significant decline in regulatory T cells in peripheral blood of patients 14 days after cyclophosphamide administration, although a decline at an earlier time point cannot be excluded. Our observations support the inclusion of cyclophosphamide in combination trials with vaccines and other immune-modulatory agents.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Melanoma is recognized as a highly immunogenic cancer which responds to a variety of immunotherapies. These can achieve durable tumour responses in 10–30 % of patients with metastatic disease [1–4]. Cancer vaccine trials in malignant melanoma patients have so far failed to demonstrate meaningful clinical benefits, although a recent randomized clinical trial of a gp100 peptide vaccine in combination with IL-2 compared to high dose IL-2 alone led to a higher response rate and progression-free survival in patients in the combination arm, supporting the concept that cancer vaccines provide clinical benefit in this setting [5].
It is of paramount importance for successful therapeutic vaccination to identify melanoma-associated antigens that are good tumour rejection antigens. NY-ESO-1, a cancer–testis antigen, has many favourable characteristics as an immunological target. In addition to being highly immunogenic, it is expressed in approximately 30–50 % of metastatic melanomas and no normal tissues apart from testis [6, 7]. Adoptive T cell therapy trials using NY-ESO-1-specific CD4+ T cell clones or genetically modified CD8+ T cells have demonstrated major tumour regressions in patients with metastatic melanoma and have validated NY-ESO-1 as an excellent tumour rejection antigen [8, 9]. This is further supported by recent studies showing that pre-existing NY-ESO-1-specific immunity is associated with clinical response to the immune-stimulatory CTLA-4 blocking antibody ipilimumab [10] and that the presence of NY-ESO-specific T cells is an independent prognostic marker for overall survival in patients with metastatic melanoma [11]. Pre-existing NY-ESO-1-specific humoral and cellular immune responses are frequently detected in patients with tumours expressing NY-ESO-1, and various vaccination strategies targeting this antigen have demonstrated high immunogenicity in patients with low disease burden [12–14].
Anti-tumour immune responses in patients with advanced melanoma are compromised by several immune-regulatory mechanisms [15]. In particular, a subset of CD4+ T cells identified by the transcription factor FOXP3 and defined as natural regulatory T cells (nTregs) is thought to play an important role in suppressing anti-tumour responses in patients with advanced solid malignancies, including melanoma. Several studies have identified increased nTreg numbers in peripheral blood, lymph nodes and metastatic lesions of patients with advanced melanoma [16, 17]. Pre-clinical studies have demonstrated that depletion of nTregs in combination with vaccination can lead to major tumour regression in a mouse melanoma model [18]. Moreover, nTreg depletion in vitro leads to an expansion of NY-ESO-1-specific Th1 effector T cells in patients vaccinated with a NY-ESO-1 DNA vaccine [19]. Recently, supporting the importance of nTregs in controlling anti-tumour immune responses in patients with melanoma, the frequency of CD4+ CD25+ FoxP3+ was shown to be negatively correlated with clinical responses in patients enrolled in adoptive T cell therapy studies [20]. There is also some evidence that vaccination itself can lead to induction and expansion of Tregs [21–23]. Hence, the integration of an nTreg-depleting agent in vaccination protocols could improve the efficacy of current vaccine approaches.
We have previously performed clinical trials with a vaccine of full-length NY-ESO-1 protein formulated with ISCOMATRIX™ adjuvant (CSL Limited). The vaccine generated strong, broad cellular and humoral NY-ESO-1-specific immune responses in patients with fully resected melanoma [12] with long-lasting NY-ESO-1-specific immunity detectable several years after treatment [24]. However, the same vaccine evaluated in a phase II clinical trial, LUD2002-013, in patients with measurable Stage III or IV melanoma demonstrated poor cellular immunogenicity and clinical efficacy [16]. This was mainly attributed to systemic immunosuppression due to an expansion of CD4+ CD25+ FoxP3+ regulatory T cells in the peripheral blood of these patients compared to the patients with a low disease burden in the former trial.
Cyclophosphamide is an alkylating cytotoxic agent that has been shown to have immune-modulatory properties [25]. Depending on the dose, either immunosuppressive or immune-enhancing effects have been observed. Prior to the current definition and characterization of nTregs, low-dose cyclophosphamide in combination with an autologous whole tumour vaccine was associated with some clinical efficacy in patients with advanced melanoma [26]. Recent pre-clinical studies have confirmed its selective nTreg-depleting activity [27]. Further supporting the nTreg-depleting property of cyclophosphamide, a peptide vaccine trial in patients with renal carcinoma demonstrated a reduced frequency of nTregs in patients treated with the vaccine in combination with cyclophosphamide [28]. Similarly, the frequency of tumour antigen-specific CD8+ T cell responses to a whole tumour vaccine has been enhanced by inclusion of cyclophosphamide into the vaccination regimen in patients with advanced pancreatic carcinoma [29].
We have subsequently enrolled a second cohort of patients with metastatic melanoma in the LUD2002-013 trial, with the objective of investigating whether administration of low-dose cyclophosphamide leads to an improved efficacy of the NY-ESO-1/ISCOMATRIX™ vaccine in this patient population.
Materials and methods
Study design
The LUD2002-013 clinical trial was an open-label, 2-centre phase II study intended to evaluate the safety and immunogenicity of the NY-ESO-1/ISCOMATRIX™ vaccine in patients with advanced melanoma. The first cohort of patients (Cohort 1) received NY-ESO-1/ISCOMATRIX™ vaccine alone, and the results of this cohort were reported previously [16]. The second cohort (Cohort 2) was added after evaluation of responses in Cohort 1 and received NY-ESO-1/ISCOMATRIX™ vaccine in combination with low-dose cyclophosphamide. All patients received three injections of NY-ESO-1 ISCOMATRIX™ preceded, in Cohort 2, by cyclophosphamide at a dose of 300 mg/m2 every 4 weeks (Supplementary Figure 1).
Assessment of clinical and immunological responses was undertaken at week 11. If there was no disease progression that warranted other systemic treatments, patients were offered additional cycles of therapy. The response assessment was performed according to the response evaluation criteria in solid tumours (RECIST) criteria [30]. The LUD2002-013 study was approved by the Human Research Ethics Committees of Austin Health and the Peter MacCallum Cancer Centre, Melbourne, Australia. All patients gave written informed consent prior to inclusion in the study. For myeloid-derived suppressor cell (MDSC) experiments, peripheral blood mononuclear cells (PBMC) from buffy coats of healthy donors (Red Cross Blood Bank, Melbourne, Australia) were prepared by Ficoll-Paque density gradient centrifugation (GE Healthcare, Little Chalfont, United Kingdom). Use of these cells was granted ethical approval by the Human Research Ethics Committee of Austin Health.
Patients
Cohort 1 of the LUD2012-003 study included 27 patients and Cohort 2 nineteen patients. The main inclusion criteria were: Stage IV (metastatic) or unresectable Stage III melanoma with measurable disease, detection of NY-ESO-1 or LAGE-1 expression by immunohistochemistry (IHC) or reverse transcription-PCR (RT-PCR) in melanoma specimen, life expectancy of more than 4 months, adequate organ function and a Karnofsky performance status of more than 70 %. Patients with chemotherapy, radiotherapy or immunotherapy within 4 weeks before study week 1, other malignancy within 3 years before study entry, known immunodeficiency, other serious illnesses and pregnancy or concomitant treatment with immunosuppressive drugs were excluded.
Investigational agents
Recombinant NY-ESO-1 protein was produced in Escherichia coli, as previously described [31]. The vaccine, which was administered intramuscularly (i.m.), comprised 200 µg/mL NY-ESO-1 protein formulated with 240 µg/mL ISCOMATRIX™ adjuvant. A total volume of 0.5 mL vaccine was injected i.m. with each vaccination.
Cyclophosphamide (Baxter Healthcare Ltd.,) was administered intravenously (i.v.) 24 h prior to vaccination at a dose of 300 mg/m2.
Serology
Peripheral blood NY-ESO-1-specific antibodies were measured using a standardized ELISA method, as described [12], and the level of antibody was expressed as reciprocal titter. The lower limit of the assay detection was set at 2,000. Patients with pre-treatment titters >5,000 were deemed to have a pre-existing response, while patients were deemed to have had a positive humoral response to vaccination if they developed a titter >5,000 and had no pre-existing response [12].
Analysis of T cell responses
Blood was taken on day 0, 14, 42, 70 and 154 of study treatment, and PBMC were isolated by Ficoll-Paque density gradient centrifugation and cryopreserved until used. In vitro culture followed by intracellular cytokine staining (ICS) was used to assess T cell responses within patient PBMC samples and to identify the epitopes recognized, as previously described [32].
Briefly, two peptide libraries were synthesized, each of which covered the entire sequence of NY-ESO-1 with an overlap of either 12 amino acids (for the 18mer library) or 11 amino acids (for the 13mer library). Cryopreserved PBMC were thawed and pulsed with pools of 3–4 18mer peptides at 10 µM for 1 h at 37 °C and then cultured in the presence 25 U/mL IL-2. Cultures were first screened for responses on ~day 11 by re-stimulating with the individual peptides of the 18mer peptide pools used for culture, in the presence of 10 µg/mL Brefeldin A, followed by staining for CD4, CD8 and intracellular IFN-γ. Based on these responses, further examination was performed using 13mers within the relevant 18mer region.
CD4+ and CD8+ T lymphocytes have been delineated by gating in the first step on the lymphocyte population followed by gating on the CD4+ or CD8+ cell population.
Responses were defined as positive when a clear population of strongly positive IFN-γ-producing CD4+ or CD8+ lymphocytes could be discerned on the flow cytometry dot plot, and this population was at least 0.1 % of gated CD8+ or CD4+ events. In all responses that were defined as positive, the detected population was at least twice the background staining.
Flow cytometry
The following antibodies were obtained from BD Biosciences (Franklin Lakes, NJ): CD4 (clone RPA-T4), CD25 (clone 2A3) and CD127 (clone hIL7R-M21). The antibody to FOXP3 (clone PCH101) was purchased from e-bioscience (San Diego, CA, USA).
For lymphocyte subpopulation and MDSC staining, cryopreserved PBMC were thawed out and stained immediately at 4 °C. After 30-min incubation, samples were washed twice in PBS + 1 % FBS solution and analysed using a FACSCalibur instrument (BD).
MDSC have been defined by gating on the whole PBMC gate followed by gating on the CD14+ and HL-DR−/low population.
NTreg staining was performed as previously described [16]. The nTreg population has been defined by gating on the total lymphocyte population followed by gating on the CD4+/FoxP3+ or the CD4+/CD127dim population. Data were analysed using the FlowJo software package (TreeStar).
Suppression assay
Frozen PBMC samples were thawed and rested in culture for 48 h. Live cells were collected using Lymphoprep, stained and FACS sorted as follows: nTreg (CD4+CD25+CD127−) and non-Treg (CD4+CD25−CD127−). FACS sorted, buffy coat-derived responder (CD8+CD127+CD25−) CD8+ T cells were labelled with 5 μM CFSE, and 4,000 cells were cultured with 2,000 nTreg in 96-well plates pre-coated with 1 μg/mL anti-CD3 (OKT3) antibody. The percentage of divided CD8+ T cells (CFSE-low) was determined after 4 days’ culture and used to calculate percentage of suppression as follows: 1−(% divided test/ % divided max) × 100, where ‘test’ was the suppressor population being tested and ‘max’ was the non-Treg control.
Statistical analysis
Frequency of immune-regulatory cell populations and the suppressive capacity of nTregs at different time points were compared using the Wilcoxon signed-rank test and calculated using Prism software (GraphPad Software, La Jolla, CA, USA). The frequency of vaccine-induced immune responses between both study cohorts was compared using a paired t test. P values <0.05 were considered significant.
Results
Patients, toxicity and clinical outcome
Nineteen patients with progressive metastatic melanoma were enrolled as an additional cohort (Cohort 2) in the clinical trial LUD2002-013 and received the NY-ESO-1/ISCOMATRIX™ vaccine in combination with low-dose cyclophosphamide. Treatment with the same vaccine in the first cohort of patients in this study demonstrated poor immunogenicity and clinical efficacy [16]. The patient and disease characteristics were similar to those of patients enrolled in Cohort 1 [16] with the majority of patients having Stage IV melanoma (Table 1). Four patients came off the study due to disease progression prior to completing their first vaccination cycle. Fifteen patients received at least one vaccination cycle consisting of three i.m. NY-ESO-1/ISCOMATRIX™ injections. Six of these patients completed two cycles and one patient received a total of 13 cycles. Only patients who had completed at least one cycle were evaluated for NY-ESO-1-specific immune responses.
As in the previously treated cohort of patients [16], the treatment was well tolerated with only a few patients developing vaccine-related side effects such as myalgia and injection site pain. Inclusion of cyclophosphamide in the treatment regimen did not change the number or the grade of adverse events compared to Cohort 1 of this study.
No objective responses could be observed, although six patients had stable disease at the first assessment at week 11 and two of these patients demonstrated prolonged disease control in the following restaging (Table 2).
Effect of low-dose cyclophosphamide on lymphocyte populations in peripheral blood
To exclude the possibility that cyclophosphamide had any cytotoxic influence on immune effector cells, we evaluated the effects of low-dose cyclophosphamide on lymphocyte populations in the peripheral blood of eight patients by flow cytometry. No significant changes were measured in the frequency of CD19+ B cells, CD4+ T cells, CD8+ T cells or CD56+ NK cells over the treatment course (Fig. 1). This finding suggests that it is unlikely that the dose of cyclophosphamide chosen for the study would lead to a depletion of tumour antigen-specific, immune effector cells.
Frequency of lymphocyte subpopulations in the peripheral blood of patients treated with low-dose cyclophosphamide. The frequency of CD8+ T cells (a), CD4+ T cells (b), CD19+ B cells (c) and CD56+ NK cells (d) in peripheral blood of the patients in Cohort 2 was analysed by FACS on day 0, 14, 42 and 77 of study treatment
Frequency and suppressive capacity of FoxP3+ CD4+ regulatory T cells are not altered by cyclophosphamide
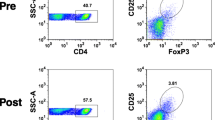
The depletion of FoxP3+ CD4+ T cells is the proposed principal mechanism for the immune-enhancing effect of low-dose cyclophosphamide. Therefore, we determined the frequency of regulatory FoxP3+ CD4+ T cells in the peripheral blood of the patients prior to treatment and 2 weeks after each cyclophosphamide administration. The percentage of nTregs relative to the total CD4+ T cell population in peripheral blood was similar to that seen in Cohort 1 and was increased compared to healthy donors, as described previously by us and other groups [16, 17]. No significant changes in nTreg frequency were observed in these patients at this time point during the treatment period (Fig. 2a, upper panel, Supplementary Figure 2). This finding was confirmed by using a different staining method for nTregs, which were defined as a population of CD3+ CD4+ CD25+ CD127 low cells (Fig. 2a, lower panel) [33].
Frequency and suppressive capacity of Tregs in the peripheral blood of patients receiving low-dose cyclophosphamide. PBMC samples were taken on day 0, 14, 42, 70 and 154 of study treatment. The frequency of Tregs among CD4+ T cells was enumerated by FACS using fluorochrome-coupled antibodies against CD3, CD4, CD25 and FoxP3 (a, upper panel) or against CD3, CD4, CD25 and CD127 (a, lower panel). Treg cells were isolated from PBMCs collected on day 0 and 70 and tested in a suppression assay at a 2:1 responder:Treg cell ratio. None of the differences between frequencies at different time points were significant. The horizontal lines represent the median values. Statistical analysis was performed by using the Wilcoxon signed-rank test (b)
In addition, the capacity of nTregs to suppress T effector cell proliferation has not been altered after three doses of cyclophosphamide compared with nTregs of the same patients isolated from pre-treatment samples (Fig. 2b).
Interaction between MDSC and regulatory T cells
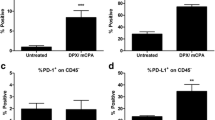
To extend the assessment of melanoma-associated immune regulation, we also determined the frequency of MDSC in the peripheral blood of patients in Cohort 2 relative to healthy donors. Together with nTregs, MDSC are a major immune-regulatory cell population that can compromise anti-tumour immunity in patients with advanced malignancies. A population of CD14+ HLA-DR−/low immature monocytes has been defined as MDSC in patients with metastatic melanoma [34, 35]. CD14+ HLA-DR-/low were detected in all patients; however, there were large differences in the frequency among individual patients (Fig. 3a). Five out of 19 patients had levels of MDSC (0.8–2.25 % per CD14+ cells) that were comparable to those seen in healthy donors, while the other patients had significantly increased levels of MDSC in blood. Although fluctuation was observed in some patients, we did not detect any consistent changes in MDSC frequency throughout the cyclophosphamide treatment (Fig. 3b). Finally, there was no correlation between the levels of MDSC and nTregs at any time point (data not shown).
Frequency of MDSC in the peripheral blood of patients receiving low-dose cyclophosphamide. The frequency of MDSC in the peripheral blood of healthy donors (HD; n = 6) and patients with metastatic melanoma (Cohort 2) prior to study treatment was determined by FACS. PBMC were stained for CD3, CD19, CD56, CD14 and HLA-DR, and MDSC were defined as CD14+ HLA-DRlow/− cells. The horizontal lines represent the median value. Statistical analysis was performed by using the Wilcoxon signed-rank test (a). The frequency of CD14+ HLA-DRlow/− MDSC in peripheral blood of patients was analysed on day 0, 14, 42, 70 and 154 of study treatment (b)
NY-ESO-1-specific immunity
Fifteen patients completed at least one cycle of vaccination and could be evaluated for NY-ESO-1-specific immunity with one patient having only samples available for serology.
Eight out of 15 patients in Cohort 2 who received cyclophosphamide developed vaccine-induced NY-ESO-1-specific antibodies during the study treatment (Table 2; Fig. 4). This was similar to Cohort 1, in which patients received the same vaccine but no cyclophosphamide and about half of the patients achieved a vaccine-induced humoral NY-ESO-1-specific immune response. Five out of 15 patients did not develop any detectable NY-ESO-1-specific antibody during the whole treatment period, and two patients had a pre-existing high NY-ESO-1-specific antibody titter that was not boosted during the study period (Table 2). The antibody titters that were seen in both cohorts were similar, so there did not appear to be any effect of cyclophosphamide on the magnitude of the serological response.
Comparison of NY-ESO-1-specific humoral and cellular immune responses between patients receiving the NY-ESO-1/ISCOMATRIX™ vaccine alone (Cohort 1) or with low-dose cyclophosphamide (Cohort 2). Graphs show the percentage of patients with vaccine-induced NY-ESO-1-specific antibodies, CD8+ T cells and CD4+ T cells for Cohort 1 and 2 at the end of study treatment. Statistical analysis was performed by paired t test (a). Flow cytometry analysis of a representative vaccine induced NY-ESO-1-specific CD4+ T cell response (patient 139, upper panel) and detailed results of the NY-ESO-1 peptide screen for the same patient (lower panel). Y-axis represents the per cent of IFN-γ-positive CD4+ T cells, and stimulating 18-mer peptide pools used for culturing T cells are shown on the x-axis, white bar = D 0, black bar = D77 (b)
The presence of NY-ESO-1-specific CD8+ and CD4+ T cells in the peripheral blood of the patients was assessed using an in vitro pre-sensitization assay with overlapping peptides covering the whole protein sequence. One patient developed a vaccine-induced NY-ESO-1-specific CD8+ T cell response, and three patients had a pre-existing response (Table 2). Although the effect on CD8+ antigen-specific T cells was modest, there was a significant increase in the percentage of patients that developed a vaccine-induced NY-ESO-1-specific CD4+ T cell response. Seven out of 14 patients developed NY-ESO-1-specific CD4+ T cell responses following the vaccine treatment; two of them had pre-existing CD4+ T cell responses to NY-ESO-1; however, these were against different epitopes (Table 2; Fig. 4). All vaccine-induced responses have already been detected after the first treatment cycle. The NY-ESO-1-specific CD4+ T cells were recognized between one and four different NY-ESO-1 epitopes. No significant difference was observed in the number or type of epitopes recognized by NY-ESO-1-specific CD4+ T cells between patients with pre-existing and vaccine-induced responses or between patients in study Cohorts 1 and 2 (Fig. 5). Recently, the frequency of MDSC has been demonstrated to be an independent prognostic factor in patients with metastatic melanoma [36]. Therefore, we investigated whether the pre-treatment levels of MDSC or Tregs would predict for a vaccine-induced CD4+ T cell response, however, could not detect such a correlation (Supplementary Figure 3).
Epitope map summarizing CD4+ T cell responses to NY-ESO-1 in both study cohorts. PBMC were collected before and at week 11 of vaccination to determine pre-existing and vaccinated NY-ESO-1-specific responses. Each bar represents a CD4+ T cell response within the indicated region of the NY-ESO-1 protein. White bars represent pre-existing, black bars vaccinated responses
Overall, inclusion of cyclophosphamide in the vaccination protocol leads to an improved immunogenicity of the NY-ESO-1/ISCOMATRIX™ vaccine with an increased number of patients developing vaccine-induced NY-ESO-1-specific CD4+ T cells.
Discussion
The phase II clinical study, LUD2002-013, evaluated the NY-ESO-1 ISCOMATRIX™ vaccine in patients with measurable Stage III or IV melanoma. Disappointing clinical and immunological outcome from the analysis of the first cohort of patients led to an enrolment of a second cohort of patients to investigate whether the administration of low-dose cyclophosphamide could improve the immunogenicity and clinical efficacy of the NY-ESO-1/ISCOMATRIX™ vaccine in patients with advanced melanoma. The inclusion of cyclophosphamide led to a significant increase in the number of patients developing a vaccine-induced NY-ESO-1-specific CD4+ T cell response without any change in NY-ESO-1-specific CD8+ T cell or antibody responses compared with a first cohort of patients treated with the vaccine only.
The main proposed mechanism for cyclophosphamide’s immune-stimulatory effect in pre-clinical models is the specific depletion of regulatory T cells [27], potentially leading to an improved priming of naïve vaccine antigen-specific T cells [37] or to the reactivation of pre-existing tumour antigen-specific T cells. Additionally, the reduction of regulatory T cells in the tumour microenvironment could lead to a more effective tumour rejection. We analysed the frequency of the FoxP3+ CD4+ regulatory T cell population in patients 14 days after the cyclophosphamide infusion and did not detect any significant changes during the study period. As previously demonstrated by us and others, patients with advanced melanoma have increased levels of nTregs in their blood compared with healthy donors or patients with fully resected disease [16, 17]. It is possible that cyclophosphamide might have had an impact on nTreg levels at an earlier time point as demonstrated by a recent clinical study using the cytotoxic agent Gemcitabine, in which Treg numbers declined shortly after administration and recovered to baseline levels within 2 days [38]. We chose to administer cyclophosphamide 24 h prior to vaccination, and this administration schedule has been demonstrated to be the most effective in increasing tumour antigen-specific T cells in a pre-clinical model [39]. In addition, the same study stressed that higher doses led to significantly reduced tumour-specific immune responses. To assess this in the clinical setting, we monitored lymphocyte subpopulations in the peripheral blood of patients in Cohort 2 of LUD2002-013 who received 300 mg/m2 cyclophosphamide. We could not detect significant alterations in lymphocyte subpopulations, making it unlikely that the dose of cyclophosphamide used here depleted immune effector cell populations. Our results contrast to a previously reported trial in patients with fully resected melanoma using a multi-peptide vaccine with the same cyclophosphamide schedule that could not demonstrate an increase in vaccine immunogenicity [40]. A strength of our study is that we undertook a detailed assessment of immune-regulatory cell populations in our patients.
Together with Tregs, MDSC are a key immune-regulatory cell population that contributes to attenuated anti-tumour immune responses in patients with advanced malignancy. Several research groups have described the presence of MDSC in patients with metastatic melanoma [34, 35, 41]. In the majority of cases, MDSC were defined as CD14+ HLA-DR−/low immature monocytes. We detected significantly elevated levels of MDSC with this phenotype in the majority of patients in Cohort 2 of LUD2002-013 at the start and throughout their treatment. The application of low-dose cyclophosphamide had no detectable impact on their numbers during the treatment course. This result differs from those of previous pre-clinical studies that demonstrated an increase in immature myeloid cells in mice upon cyclophosphamide treatment, potentially leading to further impairment of anti-tumour immunity [42, 43]. MDSC are also thought to induce or expand regulatory T cells [44]; however, we could not establish any correlation between the levels of MDSC and Tregs in the patients treated with cyclophosphamide. In addition, the frequency of MDSC in peripheral blood of metastatic melanoma patients has recently been recognized as an independent prognostic factor [36]; we could not detect any predictive value of the pre-treatment level of either MDSC or Tregs in our patient cohort to achieve a vaccine-induced CD4+ T cell response.
Our study demonstrates that cyclophosphamide can improve vaccine-induced antigen-specific CD4+ T cell responses to a whole protein vaccine in humans with metastatic melanoma. This is in keeping with findings in a mouse model where the administration of cyclophosphamide in combination with a whole tumour vaccine leads to an isolated increase in tumour-specific Th1 CD4 T cell immunity without any changes in CD8+ T cell responses [45]. The significance of an isolated increase in tumour antigen-specific CD4+ T cell immunity is unclear. Other vaccination platforms using NY-ESO-1 as a vaccination antigen in patients with advanced melanoma have shown along the induction of CD4+ T cells also an increase in NY-ESO-1-specific CD8+ T cell immunity [14]. The current paradigm in tumour immunology regards tumour antigen-specific CD8+ T cells as the main effectors of tumour rejection; however, it is increasingly recognized in pre-clinical models that tumour antigen-specific CD4+ T cells can, apart from their well-described helper cell function for CD8+ T cell and B cell immune responses, act directly as effector cells [46–48]. Clinical observation that the transfer of NY-ESO-1-specific CD4+ T cells led to major tumour regressions in a patient with metastatic melanoma supports this [8]. Therefore, interventions to improve anti-tumour CD4+ T cell immunity may be of clinical benefit.
It has been shown that the immuno-stimulatory activity of cyclophosphamide can be mediated by mechanisms other than nTreg depletion that could have accounted for the improvement in immunogenicity of our vaccine [25]. Interestingly, it has been demonstrated in mice that cyclophosphamide can selectively deplete CD8+ lymphoid dendritic cells leading to a relative increase in the number of CD8-lymphoid dendritic cells [49] that are specifically equipped to activate CD4+ T cells [50]. This could explain why low-dose cyclophosphamide increases vaccine-specific CD4+ T cell responses without any change in CD8+ T cell immunity.
Although we could not observe responses according to RECIST criteria in our patients, recent vaccine trials in patients with prostate cancer have demonstrated that survival benefits can be obtained without objective responses [51]. Survival data were not prospectively collected as part of our clinical trial, and several patients were lost to follow up. A retrospective analysis demonstrated a median overall survival of 17.2 months in Cohort 2 versus 15.3 months in Cohort 1, a difference that is statistically not significant.
With the arrival of the new class of immune-stimulatory antibodies that block immune checkpoints, more options for immunotherapy of melanoma are emerging [52]. These will include antibody combinations and combinations of immune modifying agents with cancer vaccines. The finding here that antigen-specific immunity can be enhanced with the addition of low-dose cyclophosphamide might reflect a somewhat lower-tech approach to immune modulation. Nonetheless, the measurable enhancement of vaccine-induced immunity points to another simple method that might be used in such combination studies. In conjunction with checkpoint inhibitors, it may be possible to further improve outcomes in vaccination trials [48, 53].
Abbreviations
- i.m.:
-
Intramuscularly
- i.v.:
-
Intravenously
- ICS:
-
Intracellular cytokine staining
- IHC:
-
Immunohistochemistry
- MDSC:
-
Myeloid-derived suppressor cells
- nTregs:
-
Natural regulatory T cells
- PBMC:
-
Peripheral blood mononuclear cells
- RECIST:
-
Response evaluation criteria in solid tumours
- RT-PCR:
-
Reverse transcription polymerase chain reaction
- Tregs:
-
Regulatory T cells
References
Smith FO, Downey SG, Klapper JA, Yang JC, Sherry RM, Royal RE, Kammula US, Hughes MS, Restifo NP, Levy CL, White DE, Steinberg SM, Rosenberg SA (2008) Treatment of metastatic melanoma using interleukin-2 alone or in conjunction with vaccines. Clin Cancer Res 14:5610–5618
Hodi FS, O’Day SJ, McDermott DF, Weber RW, Sosman JA, Haanen JB, Gonzalez R, Robert C, Schadendorf D, Hassel JC, Akerley W, van den Eertwegh AJ, Lutzky J, Lorigan P, Vaubel JM, Linette GP, Hogg D, Ottensmeier CH, Lebbe C, Peschel C, Quirt I, Clark JI, Wolchok JD, Weber JS, Tian J, Yellin MJ, Nichol GM, Hoos A, Urba WJ (2010) Improved survival with ipilimumab in patients with metastatic melanoma. N Engl J Med 363:711–723
Prieto PA, Yang JC, Sherry RM, Hughes MS, Kammula US, White DE, Levy CL, Rosenberg SA, Phan GQ (2012) CTLA-4 blockade with ipilimumab: long-term follow-up of 177 patients with metastatic melanoma. Clin Cancer Res 18:2039–2047
Topalian SL, Hodi FS, Brahmer JR, Gettinger SN, Smith DC, McDermott DF, Powderly JD, Carvajal RD, Sosman JA, Atkins MB, Leming PD, Spigel DR, Antonia SJ, Horn L, Drake CG, Pardoll DM, Chen L, Sharfman WH, Anders RA, Taube JM, McMiller TL, Xu H, Korman AJ, Jure-Kunkel M, Agrawal S, McDonald D, Kollia GD, Gupta A, Wigginton JM, Sznol M (2012) Safety, activity, and immune correlates of anti-PD-1 antibody in cancer. N Engl J Med 366:2443–2454
Schwartzentruber DJ, Lawson DH, Richards JM, Conry RM, Miller DM, Treisman J, Gailani F, Riley L, Conlon K, Pockaj B, Kendra KL, White RL, Gonzalez R, Kuzel TM, Curti B, Leming PD, Whitman ED, Balkissoon J, Reintgen DS, Kaufman H, Marincola FM, Merino MJ, Rosenberg SA, Choyke P, Vena D, Hwu P (2011) Gp100 peptide vaccine and interleukin-2 in patients with advanced melanoma. N Engl J Med 364:2119–2127
Barrow C, Browning J, MacGregor D, Davis ID, Sturrock S, Jungbluth AA, Cebon J (2006) Tumor antigen expression in melanoma varies according to antigen and stage. Clin Cancer Res 12:764–771
Velazquez EF, Jungbluth AA, Yancovitz M, Gnjatic S, Adams S, O’Neill D, Zavilevich K, Albukh T, Christos P, Mazumdar M, Pavlick A, Polsky D, Shapiro R, Berman R, Spira J, Busam K, Osman I, Bhardwaj N (2007) Expression of the cancer/testis antigen NY-ESO-1 in primary and metastatic malignant melanoma (MM)-correlation with prognostic factors. Cancer Immun 7:11
Hunder NN, Wallen H, Cao J, Hendricks DW, Reilly JZ, Rodmyre R, Jungbluth A, Gnjatic S, Thompson JA, Yee C (2008) Treatment of metastatic melanoma with autologous CD4+ T cells against NY-ESO-1. N Engl J Med 358:2698–2703
Robbins PF, Morgan RA, Feldman SA, Yang JC, Sherry RM, Dudley ME, Wunderlich JR, Nahvi AV, Helman LJ, Mackall CL, Kammula US, Hughes MS, Restifo NP, Raffeld M, Lee CC, Levy CL, Li YF, El-Gamil M, Schwarz SL, Laurencot C, Rosenberg SA (2011) Tumor regression in patients with metastatic synovial cell sarcoma and melanoma using genetically engineered lymphocytes reactive with NY-ESO-1. J Clin Oncol 29:917–924
Yuan J, Adamow M, Ginsberg BA, Rasalan TS, Ritter E, Gallardo HF, Xu Y, Pogoriler E, Terzulli SL, Kuk D, Panageas KS, Ritter G, Sznol M, Halaban R, Jungbluth AA, Allison JP, Old LJ, Wolchok JD, Gnjatic S (2011) Integrated NY-ESO-1 antibody and CD8+ T-cell responses correlate with clinical benefit in advanced melanoma patients treated with ipilimumab. Proc Natl Acad Sci USA 108:16723–16728
Weide B, Zelba H, Derhovanessian E, Pflugfelder A, Eigentler TK, Di Giacomo AM, Maio M, Aarntzen EH, de Vries IJ, Sucker A, Schadendorf D, Buttner P, Garbe C, Pawelec G (2012) Functional T cells targeting NY-ESO-1 or Melan-A are predictive for survival of patients with distant melanoma metastasis. J Clin Oncol 30:1835–1841
Davis ID, Chen W, Jackson H, Parente P, Shackleton M, Hopkins W, Chen Q, Dimopoulos N, Luke T, Murphy R, Scott AM, Maraskovsky E, McArthur G, MacGregor D, Sturrock S, Tai TY, Green S, Cuthbertson A, Maher D, Miloradovic L, Mitchell SV, Ritter G, Jungbluth AA, Chen YT, Gnjatic S, Hoffman EW, Old LJ, Cebon JS (2004) Recombinant NY-ESO-1 protein with ISCOMATRIX adjuvant induces broad integrated antibody and CD4(+) and CD8(+) T cell responses in humans. Proc Natl Acad Sci USA 101:10697–10702
Valmori D, Souleimanian NE, Tosello V, Bhardwaj N, Adams S, O’Neill D, Pavlick A, Escalon JB, Cruz CM, Angiulli A, Angiulli F, Mears G, Vogel SM, Pan L, Jungbluth AA, Hoffmann EW, Venhaus R, Ritter G, Old LJ, Ayyoub M (2007) Vaccination with NY-ESO-1 protein and CpG in Montanide induces integrated antibody/Th1 responses and CD8 T cells through cross-priming. Proc Natl Acad Sci USA 104:8947–8952
Odunsi K, Matsuzaki J, Karbach J, Neumann A, Mhawech-Fauceglia P, Miller A, Beck A, Morrison CD, Ritter G, Godoy H, Lele S, duPont N, Edwards R, Shrikant P, Old LJ, Gnjatic S, Jager E (2012) Efficacy of vaccination with recombinant vaccinia and fowlpox vectors expressing NY-ESO-1 antigen in ovarian cancer and melanoma patients. Proc Natl Acad Sci USA 109:5797–5802
Zitvogel L, Tesniere A, Kroemer G (2006) Cancer despite immunosurveillance: immunoselection and immunosubversion. Nat Rev Immunol 6:715–727
Nicholaou T, Ebert LM, Davis ID, McArthur GA, Jackson H, Dimopoulos N, Tan B, Maraskovsky E, Miloradovic L, Hopkins W, Pan L, Venhaus R, Hoffman EW, Chen W, Cebon J (2009) Regulatory T-cell-mediated attenuation of T-cell responses to the NY-ESO-1 ISCOMATRIX vaccine in patients with advanced malignant melanoma. Clin Cancer Res 15:2166–2173
Jandus C, Bioley G, Speiser DE, Romero P (2008) Selective accumulation of differentiated FOXP3(+) CD4(+) T cells in metastatic tumor lesions from melanoma patients compared to peripheral blood. Cancer Immunol Immunother 57:1795–1805
Klages K, Mayer CT, Lahl K, Loddenkemper C, Teng MW, Ngiow SF, Smyth MJ, Hamann A, Huehn J, Sparwasser T (2010) Selective depletion of Foxp3+ regulatory T cells improves effective therapeutic vaccination against established melanoma. Cancer Res 70:7788–7799
Gnjatic S, Altorki NK, Tang DN, Tu SM, Kundra V, Ritter G, Old LJ, Logothetis CJ, Sharma P (2009) NY-ESO-1 DNA vaccine induces T-cell responses that are suppressed by regulatory T cells. Clin Cancer Res 15:2130–2139
Yao X, Ahmadzadeh M, Lu YC, Liewehr DJ, Dudley ME, Liu F, Schrump DS, Steinberg SM, Rosenberg SA, Robbins PF (2012) Levels of peripheral CD4+ FoxP3+ regulatory T cells are negatively associated with clinical response to adoptive immunotherapy of human cancer. Blood 119:5688–5696
Zhou G, Drake CG, Levitsky HI (2006) Amplification of tumor-specific regulatory T cells following therapeutic cancer vaccines. Blood 107:628–636
Francois V, Ottaviani S, Renkvist N, Stockis J, Schuler G, Thielemans K, Colau D, Marchand M, Boon T, Lucas S, van der Bruggen P (2009) The CD4(+) T-cell response of melanoma patients to a MAGE-A3 peptide vaccine involves potential regulatory T cells. Cancer Res 69:4335–4345
Ebert LM, Macraild SE, Zanker D, Davis ID, Cebon J, Chen W (2012) A cancer vaccine induces expansion of NY-ESO-1-Specific regulatory T cells in patients with advanced melanoma. PLoS One 7:e48424
Nicholaou T, Chen W, Davis ID, Jackson HM, Dimopoulos N, Barrow C, Browning J, Macgregor D, Williams D, Hopkins W, Maraskovsky E, Venhaus R, Pan L, Hoffman EW, Old LJ, Cebon J (2011) Immunoediting and persistence of antigen-specific immunity in patients who have previously been vaccinated with NY-ESO-1 protein formulated in ISCOMATRIX. Cancer Immunol Immunother 60:1625–1637
Sistigu A, Viaud S, Chaput N, Bracci L, Proietti E, Zitvogel L (2011) Immunomodulatory effects of cyclophosphamide and implementations for vaccine design. Semin Immunopathol 33:369–383
Berd D, Maguire HC Jr, Mastrangelo MJ (1986) Induction of cell-mediated immunity to autologous melanoma cells and regression of metastases after treatment with a melanoma cell vaccine preceded by cyclophosphamide. Cancer Res 46:2572–2577
Zhao J, Cao Y, Lei Z, Yang Z, Zhang B, Huang B (2010) Selective depletion of CD4+ CD25+ Foxp3+ regulatory T cells by low-dose cyclophosphamide is explained by reduced intracellular ATP levels. Cancer Res 70:4850–4858
Walter S, Weinschenk T, Stenzl A, Zdrojowy R, Pluzanska A, Szczylik C, Staehler M, Brugger W, Dietrich PY, Mendrzyk R, Hilf N, Schoor O, Fritsche J, Mahr A, Maurer D, Vass V, Trautwein C, Lewandrowski P, Flohr C, Pohla H, Stanczak JJ, Bronte V, Mandruzzato S, Biedermann T, Pawelec G, Derhovanessian E, Yamagishi H, Miki T, Hongo F, Takaha N, Hirakawa K, Tanaka H, Stevanovic S, Frisch J, Mayer-Mokler A, Kirner A, Rammensee HG, Reinhardt C, Singh-Jasuja H (2012) Multipeptide immune response to cancer vaccine IMA901 after single-dose cyclophosphamide associates with longer patient survival. Nat Med 18:1254–1261
Laheru D, Lutz E, Burke J, Biedrzycki B, Solt S, Onners B, Tartakovsky I, Nemunaitis J, Le D, Sugar E, Hege K, Jaffee E (2008) Allogeneic granulocyte macrophage colony-stimulating factor-secreting tumor immunotherapy alone or in sequence with cyclophosphamide for metastatic pancreatic cancer: a pilot study of safety, feasibility, and immune activation. Clin Cancer Res 14:1455–1463
Therasse P, Arbuck SG, Eisenhauer EA, Wanders J, Kaplan RS, Rubinstein L, Verweij J, Van Glabbeke M, van Oosterom AT, Christian MC, Gwyther SG (2000) New guidelines to evaluate the response to treatment in solid tumors. European Organization for Research and Treatment of Cancer, National Cancer Institute of the United States, National Cancer Institute of Canada. J Natl Cancer Inst 92:205–216
Murphy R, Green S, Ritter G, Cohen L, Ryan D, Woods W, Rubira M, Cebon J, Davis ID, Sjolander A, Kypridis A, Kalnins H, McNamara M, Moloney MB, Ackland J, Cartwright G, Rood J, Dumsday G, Healey K, Maher D, Maraskovsky E, Chen YT, Hoffman EW, Old LJ, Scott AM (2005) Recombinant NY-ESO-1 cancer antigen: production and purification under cGMP conditions. Prep Biochem Biotechnol 35:119–134
Jackson HM, Dimopoulos N, Chen Q, Luke T, Yee Tai T, Maraskovsky E, Old LJ, Davis ID, Cebon J, Chen W (2004) A robust human T-cell culture method suitable for monitoring CD8+ and CD4+ T-cell responses from cancer clinical trial samples. J Immunol Methods 291:51–62
Seddiki N, Santner-Nanan B, Martinson J, Zaunders J, Sasson S, Landay A, Solomon M, Selby W, Alexander SI, Nanan R, Kelleher A, Fazekas de St Groth B (2006) Expression of interleukin (IL)-2 and IL-7 receptors discriminates between human regulatory and activated T cells. J Exp Med 203:1693–1700
Filipazzi P, Valenti R, Huber V, Pilla L, Canese P, Iero M, Castelli C, Mariani L, Parmiani G, Rivoltini L (2007) Identification of a new subset of myeloid suppressor cells in peripheral blood of melanoma patients with modulation by a granulocyte-macrophage colony-stimulation factor-based antitumor vaccine. J Clin Oncol 25:2546–2553
Poschke I, Mougiakakos D, Hansson J, Masucci GV, Kiessling R (2010) Immature immunosuppressive CD14+ HLA-DR-/low cells in melanoma patients are Stat3hi and overexpress CD80, CD83, and DC-sign. Cancer Res 70:4335–4345
Weide B, Martens A, Zelba H, Stutz C, Derhovanessian E, Di Giacomo AM, Maio M, Sucker A, Schilling B, Schadendorf D, Buttner P, Garbe C, Pawelec G (2014) Myeloid-derived suppressor cells predict survival of patients with advanced melanoma: comparison with regulatory T cells and NY-ESO-1- or melan-A-specific T cells. Clin Cancer Res 20:1601–1609
Heit A, Gebhardt F, Lahl K, Neuenhahn M, Schmitz F, Anderl F, Wagner H, Sparwasser T, Busch DH, Kastenmuller K (2008) Circumvention of regulatory CD4(+) T cell activity during cross-priming strongly enhances T cell-mediated immunity. Eur J Immunol 38:1585–1597
Rettig L, Seidenberg S, Parvanova I, Samaras P, Curioni A, Knuth A, Pascolo S (2011) Gemcitabine depletes regulatory T-cells in human and mice and enhances triggering of vaccine-specific cytotoxic T-cells. Int J Cancer 129:832–838
Wada S, Yoshimura K, Hipkiss EL, Harris TJ, Yen HR, Goldberg MV, Grosso JF, Getnet D, Demarzo AM, Netto GJ, Anders R, Pardoll DM, Drake CG (2009) Cyclophosphamide augments antitumor immunity: studies in an autochthonous prostate cancer model. Cancer Res 69:4309–4318
Slingluff CL Jr, Petroni GR, Chianese-Bullock KA, Smolkin ME, Ross MI, Haas NB, von Mehren M, Grosh WW (2011) Randomized multicenter trial of the effects of melanoma-associated helper peptides and cyclophosphamide on the immunogenicity of a multipeptide melanoma vaccine. J Clin Oncol 29:2924–2932
Lesokhin AM, Hohl TM, Kitano S, Cortez C, Hirschhorn-Cymerman D, Avogadri F, Rizzuto GA, Lazarus JJ, Pamer EG, Houghton AN, Merghoub T, Wolchok JD (2012) Monocytic CCR2(+) myeloid-derived suppressor cells promote immune escape by limiting activated CD8 T-cell infiltration into the tumor microenvironment. Cancer Res 72:876–886
Angulo I, de las Heras FG, Garcia-Bustos JF, Gargallo D, Munoz-Fernandez MA, Fresno M (2000) Nitric oxide-producing CD11b(+)Ly-6G(Gr-1)(+)CD31(ER-MP12)(+) cells in the spleen of cyclophosphamide-treated mice: implications for T-cell responses in immunosuppressed mice. Blood 95:212–220
Sevko A, Sade-Feldman M, Kanterman J, Michels T, Falk CS, Umansky L, Ramacher M, Kato M, Schadendorf D, Baniyash M, Umansky V (2013) Cyclophosphamide promotes chronic inflammation-dependent immunosuppression and prevents antitumor response in melanoma. J Invest Dermatol 133:1610–1619
Hoechst B, Ormandy LA, Ballmaier M, Lehner F, Kruger C, Manns MP, Greten TF, Korangy F (2008) A new population of myeloid-derived suppressor cells in hepatocellular carcinoma patients induces CD4(+)CD25(+)Foxp3(+) T cells. Gastroenterology 135:234–243
Machiels JP, Reilly RT, Emens LA, Ercolini AM, Lei RY, Weintraub D, Okoye FI, Jaffee EM (2001) Cyclophosphamide, doxorubicin, and paclitaxel enhance the antitumor immune response of granulocyte/macrophage-colony stimulating factor-secreting whole-cell vaccines in HER-2/neu tolerized mice. Cancer Res 61:3689–3697
Quezada SA, Simpson TR, Peggs KS, Merghoub T, Vider J, Fan X, Blasberg R, Yagita H, Muranski P, Antony PA, Restifo NP, Allison JP (2010) Tumor-reactive CD4(+) T cells develop cytotoxic activity and eradicate large established melanoma after transfer into lymphopenic hosts. J Exp Med 207:637–650
Xie Y, Akpinarli A, Maris C, Hipkiss EL, Lane M, Kwon EK, Muranski P, Restifo NP, Antony PA (2010) Naive tumor-specific CD4(+) T cells differentiated in vivo eradicate established melanoma. J Exp Med 207:651–667
Hirschhorn-Cymerman D, Budhu S, Kitano S, Liu C, Zhao F, Zhong H, Lesokhin AM, Avogadri-Connors F, Yuan J, Li Y, Houghton AN, Merghoub T, Wolchok JD (2012) Induction of tumoricidal function in CD4+ T cells is associated with concomitant memory and terminally differentiated phenotype. J Exp Med 209:2113–2126
Nakahara T, Uchi H, Lesokhin AM, Avogadri F, Rizzuto GA, Hirschhorn-Cymerman D, Panageas KS, Merghoub T, Wolchok JD, Houghton AN (2010) Cyclophosphamide enhances immunity by modulating the balance of dendritic cell subsets in lymphoid organs. Blood 115:4384–4392
Dudziak D, Kamphorst AO, Heidkamp GF, Buchholz VR, Trumpfheller C, Yamazaki S, Cheong C, Liu K, Lee HW, Park CG, Steinman RM, Nussenzweig MC (2007) Differential antigen processing by dendritic cell subsets in vivo. Science 315:107–111
Kantoff PW, Higano CS, Shore ND, Berger ER, Small EJ, Penson DF, Redfern CH, Ferrari AC, Dreicer R, Sims RB, Xu Y, Frohlich MW, Schellhammer PF (2010) Sipuleucel-T immunotherapy for castration-resistant prostate cancer. N Engl J Med 363:411–422
Pardoll DM (2012) The blockade of immune checkpoints in cancer immunotherapy. Nat Rev Cancer 12:252–264
Mkrtichyan M, Najjar YG, Raulfs EC, Abdalla MY, Samara R, Rotem-Yehudar R, Cook L, Khleif SN (2011) Anti-PD-1 synergizes with cyclophosphamide to induce potent anti-tumor vaccine effects through novel mechanisms. Eur J Immunol 41:2977–2986
Conflict of interest
I. D. Davis, W. Chen and J. Cebon are patent holders of ISCOMATRIX™ vaccine, CSL Limited. E. Maraskovsky is an employee of CSL Limited. All other authors have no conflict of interest to disclose.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Klein, O., Davis, I.D., McArthur, G.A. et al. Low-dose cyclophosphamide enhances antigen-specific CD4+ T cell responses to NY-ESO-1/ISCOMATRIX™ vaccine in patients with advanced melanoma. Cancer Immunol Immunother 64, 507–518 (2015). https://doi.org/10.1007/s00262-015-1656-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00262-015-1656-x