Abstract
Imaging evaluation of the gallbladder is a fundamental skill in the majority of radiology practice. Due to ease of accessibility, low cost, lack of ionizing radiation, and excellent spatial resolution, ultrasound is often the first imaging modality used to evaluate the gallbladder. In this invited article we review and update how ultrasound can evaluate common pathologies including gallbladder polyps, tumefactive sludge, adenomyomatosis, and acute cholecystitis. We also discuss the role of Doppler, microvascular flow imaging, and contrast enhanced ultrasound in the sonographic assessment of the gallbladder.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Gallbladder pathology remains a common and prevalent health issue, impacting up to 15% of the adult population in the Unites States [1]. Despite recent literature demonstrating non-inferior alternative imaging strategies [2], ultrasound remains the primary first line imaging assessment for right upper quadrant symptoms, and gallbladder pathology specifically, due to broad availability, dynamic acquisition, and relatively low cost.
Complete sonographic assessment of the gallbladder and biliary tree requires appropriate scanning technique including sufficient fasting to ensure adequate distension, evaluation with the highest frequency transducer possible, and assessment of the gallbladder fundus in two planes [3]. Additional techniques such as intercostal scanning, decubitus, upright, or prone positioning, and various breathing maneuvers may assist in evaluating the full extent of the gallbladder and biliary system.
Gallbladder polyps and other incidental findings
Incidental findings in the gallbladder are common, with prevalence ranging from 6 to 29% of gallbladder ultrasound examinations [4]. Most intraluminal findings are often benign, such as gallstones, adenomyomatosis, or biliary sludge, and do not require repeat evaluation when classic imaging features are present.
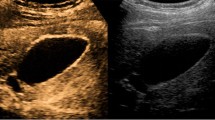
Gallbladder polyps are another common incidental finding, seen in 3–6% of the population [5]. The vast majority of gallbladder polyps are benign cholesterol polyps with no risk of malignancy. Recently the Society of Radiologists in Ultrasound (SRU) convened a consensus conference focused on the imaging and management of incidental gallbladder polyps. First, it was established that the vast majority of polyps are benign, while gallbladder carcinoma, an aggressive though rare malignancy, is less likely to be associated with polyps (in contradistinction to other common polypoid neoplasms such as adenomatous polyps of the colon). With this background, the SRU consensus conference aimed to reduce unnecessary follow up and surgery on lower risk polyps, advocating instead for follow up and surgery only for polyps with higher risk morphologic features, and with a higher size cutoff compared to prior management recommendations. Then, the SRU consensus recommendations describe the technical parameters required for a complete and high-quality ultrasound examination, recommending short interval follow up if the gallbladder is contracted, or if differentiating between tumefactive sludge and true mass proves challenges, with contrast-enhanced ultrasound (CEUS) as an alternative consideration.
The SRU consensus recommendations stratify gallbladder polyps into three categories: Extremely Low Risk, Low Risk, and Indeterminate Risk, based on polyp morphology (Fig. 1). Extremely Low Risk polyps, with a classic “ball-on-the-wall” appearance (Fig. 2) or with a thin stalk, only require follow up if ≥ 10 mm at 6, 12, and 24 months from detection. Low Risk polyps, which are sessile (Fig. 3) or have a thick measurable stalk, are followed at 1 year if 7–9 mm and at 6, 12, 24, and 36 months if ≥ 10 mm. Both Extremely Low Risk and Low Risk polyps are referred for surgical evaluation if ≥ 15 mm. Indeterminate Risk polyps show focal wall thickening under the polyp, and generally require surgical evaluation, though ultrasound follow up can be considered if ≤ 6 mm.
Summary of the 2022 SRU gallbladder polyp consensus conference recommendations. Reproduced, with permission, from Kamaya A, Fung C, Szpakowski J, et al. (2022) Management of incidentally detected gallbladder polyps: society of radiologists in ultrasound consensus conference recommendations. Radiology 305(2): 277–289
Acute cholecystitis
Acute cholecystitis comprises approximately 3–10% of all ER patient presentations related to abdominal pain at the time of hospital admission [6]. Acute cholecystitis is most frequently associated with gallstones (acute calculous cholecystitis [ACC]) and represents approximately 90–95% of cases. ACC generally results from increased intraluminal pressure from a stone obstructing the cystic duct resulting in cholesterol supersaturated bile which triggers an acute inflammatory response [6, 7]. In the remaining 5–10% of cases, patients may develop acute cholecystitis in the absence of gallstones (acute acalculous cholecystitis [AAC]). AAC is often seen in critically ill patients with multifactorial pathogenesis, relating to microvascular endothelial injury from hypoperfusion in combination with direct endothelial toxicity from inspissation of static bile [6, 8]. Regardless of etiology, the diagnosis of acute cholecystitis is often based on the Tokyo Diagnostic Criteria using a combination of signs of local and systemic inflammation, and characteristic imaging findings [9].
Ultrasound is the first-line imaging modality of choice for most patients with suspected acute cholecystitis [9, 10]. Diagnostic ultrasound in the setting of acute cholecystitis (Fig. 4) has an overall sensitivity of 71% and a specificity of 85%, with an accuracy of 0.83, indicating good discriminability [6]. Gallstones are the most sensitive sonographic finding for the diagnosis of acute cholecystitis (sensitivity 88%; positive likelihood ratio PLR 4.10), followed by positive sonographic Murphy sign (51%; 3.60), gallbladder wall thickness > 4 mm (45%; 3.29), and pericholecystic fluid (26%; 5.62) [9, 11]. Additional sonographic findings including a transverse dimension of the gallbladder greater than 40 mm, application of the tensile gallbladder fundus sign (absence of gallbladder fundus flattening by the overlying abdominal wall due to the degree of fundal distension), and/or a cystic artery velocity of greater than 40cm/s are ancillary; these findings can support the diagnosis, though currently need further investigation to support their stand-alone utility in making the diagnosis of acute cholecystitis (see detailed discussion on cystic artery waveforms below) [11, 12].
61-year-old female presenting with acute right upper quadrant pain. Diffuse gallbladder wall thickening up to 6 mm, gallbladder distension, and multiple gallstones are seen in this patient with a positive sonographic Murphy’s sign. Echogenic fat is also seen adjacent to the liver, consistent with regional inflammation (arrow). Subsequent pathology review following cholecystectomy confirmed acute calculous cholecystitis
The sonographic Murphy sign as classically described is the point of maximal tenderness elicited by direct pressure of the transducer over a sonographically localized gallbladder, as opposed to the physical exam finding of a sudden arrest of inspiration during palpation of the right upper quadrant due to pain, the latter representing a traditional clinical Murphy’s sign [6, 13]. One method for evaluating for sonographic Murphy sign is performed by starting with the probe in the left upper quadrant and, using the same transducer pressure, evaluating 4–5 positions along the upper abdomen by traversing to the right upper quadrant and back—finishing in the left upper quadrant—ensuring the gallbladder is included in one of the positions evaluated. Traversing the abdomen twice while trying to reproduce the point of maximal tenderness theoretically aids in reducing false positive examinations, particularly in the setting of previous clinical Murphy sign evaluation by the clinical care team. Although the sensitivity of the Murphy sign for acute cholecystitis is only 51% in the literature, the specificity is up to 96% (if performed correctly), making this dynamic sonographic evaluation an excellent discriminator for patients with right upper quadrant pain [6, 11].
Patient body habitus, intraabdominal gas or patient immobility can lead to technically difficult and non-diagnostic ultrasound examinations for which alternative imaging modalities may be required [14]. Computed Tomography (CT) is often the next modality of choice if ultrasound is unavailable or non-diagnostic and has an estimated pooled sensitivity of 78% with a specificity of 81% based on a recent meta-analysis [15]. Findings of acute cholecystitis on CT include distention of the gallbladder, mural thickening, pericholecystic fluid, and pericholecystic fat stranding (edema) [16]. Gallstones can be identified on CT if their intrinsic attenuation provides an attenuation difference sufficient enough to differentiate from surrounding bile; however, approximately 20% of gallstones are similar enough in attenuation to bile and therefore not well seen on routine CT [17]. Multi-spectral CT techniques may improve gallstone detection, however detailed discussion is beyond the focus of this manuscript [18]. The gold standard in the radiological diagnosis of acute cholecystitis is a nuclear medicine cholescintigraphy (colloquially known as Hepatic Iminodiacetic Acid or HIDA scan) which has a reported sensitivity of 96% and specificity of 90% [18]. Cholescintigraphy is particularly valuable, and is the preferred imaging modality, in the setting of suspected acute acalculous cholecystitis [20]. Magnetic Resonance Imaging (MRI) has an overall sensitivity and specificity of 88 and 89%, respectively [21]. Findings identifiable on MRI include gallstones, gallbladder wall thickening, gallbladder wall edema, gallbladder distention, pericholecystic and perihepatic free fluid [22]. Despite the high sensitivity and specificity, MRI is not frequently used as a first-line diagnostic test specifically for acute cholecystitis given the overall cost, limited accessibility, and length of the procedure when compared to other modalities [20]. However, MRI remains important in the evaluation for choledocholithiasis.
Importantly, no clinical finding, lab test or diagnostic imaging finding alone is sufficient to establish or exclude acute cholecystitis in isolation and the diagnosis requires the integration of clinical, biochemical, and diagnostic imaging information [9, 23].
Utility of Doppler in acute cholecystitis—latest evidence
With the ongoing challenges with definitively diagnosing various gallbladder pathologies such as acute cholecystitis, recent studies have reinvestigated older imaging signs, now with the added benefit of more advanced equipment and higher image quality. Some of these re-visited imaging techniques include assessment of the gallbladder wall vascularity using color or power Doppler, as well as assessment of the cystic artery with spectral Doppler. More advanced small-vessel and slow-flow Doppler and non-Doppler flow imaging techniques are now also available. This section will review recent evidence for the use of Doppler for the valuation of the gallbladder wall, however is by no means exhaustive of the available US techniques available for the gallbladder wall. Use of contrast-enhanced ultrasound (CEUS) and its utility in evaluating the gallbladder wall is discussed in the next section.
Color and power Doppler
Several studies investigating the use of color and power Doppler to evaluate the gallbladder wall for hyperemia were reported over 25 years ago [24,25,26,27]. Wall hyperemia has been assessed using qualitative three- or four-point scales or defined simplify by identifying color Doppler signal within the fundal one third of the gallbladder. By one report, sensitivity of 95% and specificity of 86% were reached by simply using power Doppler for acute cholecystitis, with no utility found in the resistive index (RI) of the arterial spectral waveform [24]. Areas of necrosis may fail to show color or power Doppler flow and therefore may contribute to a false negative examination [24].
Limited data exist on the reproducibility of these findings, particularly when taking into account patient body habitus. Increasing abdominal wall thickness and hepatic steatosis may impact the detection of color and even power Doppler signal, an increasingly common problem in the United States and worldwide.
Spectral Doppler
Color Doppler has been previously used to study the length of the cystic artery visualized in the gallbladder wall as a sign of hyperemia, with only moderate sensitivity achieved [28]. More recently there has been renewed attention towards spectral Doppler assessment of the hepatic and cystic arteries. In one single-site study of 229 patients, investigators evaluated velocities within the hepatic artery (HA) and found that of the 21 patients with acute cholecystitis, HA velocity ≥ 100 cm/s improved diagnostic accuracy (63–69%), and could help differentiate between acute and chronic cholecystitis (Fig. 5) [29].
44-year-old male with right upper quadrant pain and sonographic Murphy sign. A Longitudinal image through the gallbladder reveals a distended gallbladder without wall thickening. A stone is seen in the gallbladder fundus. Findings were equivocal for acute calculous cholecystitis. B Duplex image of the hepatic artery (HA) reveals a peak systolic velocity of 133 cm/s (> 100 cm/s), raising the suspicion for acute cholecystitis, which was confirmed following a subsequent cholecystectomy
Even more recently, a study from the same institution evaluated 73 patients with suspected acute cholecystitis and evaluated cystic artery (CA) velocities, and found that of those with acute cholecystitis, average CA velocity was 50 cm/s, compared to 22 cm/s for controls 30]. Using 40 cm/s alone as a cut-off, positive predictive value was nearly 95%, with overall accuracy of 81% (Fig. 6). CA velocity < 20 cm/s effectively ruled out acute cholecystitis, whereas intermediate velocities were indeterminate, though were often seen in chronic cholecystitis.
32-year-old male with right upper quadrant pain and sonographic Murphy sign. A Longitudinal grayscale image of the gallbladder shows distention without wall thickening. Echogenicity of surrounding fat is possible. No gallstones were identified. B Color Doppler image of the gallbladder shows flow related signal within branches of the cystic artery within the fundal third of the gallbladder. C Duplex image of the cystic artery reveals a peak velocity of 60 cm/s (> 40 cm/s). The diagnosis of acute cholecystitis was made. However, the clinical presentation remained unclear. A contrast-enhanced CT was performed three days later, with an image through the gallbladder D revealing wall thickening, mucosal irregularity and areas of non-enhancement, and pericholecystic edema. Subsequent cholecystectomy revealed acute necrotizing cholecystitis
Specifically in patients with cirrhosis, one study found that CA velocities were also significantly higher in those with acute cholecystitis versus those without (32 vs 16 cm/s) [31]. The same study also found that RIs were much lower in those with versus without acute cholecystitis (0.72 versus 0.84). Using a CA velocity cutoff of 40 cm/s, specificity for acute cholecystitis reached 100%, while using RI less than 0.75, sensitivity was 83%.
One benefit of spectral waveform analysis is the quantitative nature of the measurements, and therefore the decreased subjectivity. Interestingly, these authors show that spectral results appear to perform similar to, or better than, traditional signs of acute cholecystitis, such as wall thickening, Murphy’s sign, and impacted gallstone. Replicating these findings in larger, preferably multi-institutional trials will be important to verify the utility of these cutoff values. In addition, incorporating these measurements into a multi-parametric model may be important. Performance of these measurements in acalculous cholecystitis and gangrenous cholecystitis remain additional knowledge gaps.
Advanced flow imaging techniques
In recent years several new vascular imaging techniques have emerged that offer improved sensitivity to small-vessel and low velocity flow. These microvascular flow imaging (MVFI) techniques are known by various names based on the manufacturer, however in general are non-contrast techniques that leverage adaptive filtering of random motion while maintaining sensitivity to directional flow, allowing for high-resolution imaging of flow in smaller vessels than what was previously possible by traditional color, power, and spectral Doppler techniques [32]. MVFI has been shown to improve the detection of gallbladder wall blood flow compared to color and power Doppler, and offers increased confidence in the diagnosis of wall perforation (Fig. 7) [33, 34]. Unfortunately these techniques remain limited by angle and depth dependency, and general perform poorly in low signal-to-noise situations such as in obesity, similar to color and power Doppler techniques [33].
57-year-old male hospitalized following stroke, found to have gallbladder wall edema (due to volume overload). A Longitudinal ultrasound image of the gallbladder with standard Power Doppler fails to show flow-related signal in the gallbladder wall. B Advanced “slow flow” Doppler mode shows flow-related signal within a cystic artery branch in the gallbladder wall. Note tumefactive sludge layering in the gallbladder without flow in either technique
MVFI may also provide incremental sensitivity for flow when attempting to differentiate intraluminal tumefactive sludge and gallbladder neoplasm, and to differentiate large polyps and gallbladder carcinoma (Fig. 7) [33, 35]. Of note, some specular reflectors such as those from gallstones may be observed in the MVFI images and should not be misinterpreted as perfused tissue.
Contrast-enhanced ultrasound applications in the gallbladder
Contrast-enhanced ultrasound: brief review
Contrast-enhanced ultrasound (CEUS) is accomplished by the intravenous administration of ultrasound contrast agents (UCAs). UCAs are incredibly safe and non-organotoxic, with an extremely low rate of adverse events, and can therefore be administered at any level of renal function [36, 37]. These agents are composed of 3–5 µm particles containing a high-molecular weight fluorocarbon gas encased by a lipid or protein shell. When injected intravenously these microparticles remain completely intravascular, without the extra-vascular/interstitial phase of enhancement encountered in CT and MRI. These pure blood pool agents therefore provide exquisite differentiation of vascularized from non-vascularized tissues. In addition, the high temporal and spatial resolution afforded by ultrasound allows for exquisite evaluation of patterns of enhancement. Finally, the unique resonance of microbubbles in the ultrasound field allows for the reconstruction of a contrast-only image (real-time tissue subtraction), further improving the differentiation of perfused and non-perfusion structures.
Assessment of intraluminal contents
As discussed previously, detection of intraluminal material on routine ultrasound images is common, and typically includes gallstones, layering biliary precipitate (sludge), and a conglomerate of inspissated bile (tumefactive sludge) which are typically mobile. Non-mobile structures could include small adherent stones and gallbladder polyps. Gallbladder cancer also presents as a nonmobile, intraluminal mass.
Tumefactive sludge, a term first coined by Fakhry in 1982 [38], is defined as nonmobile polypoid intraluminal gallbladder contents without posterior acoustic shadowing. While tumefactive sludge is not a common occurrence on abdominal ultrasound [39], it may pose a diagnostic challenge given overlapping imaging features with those of gallbladder cancer. In one study 14% of patients with “tumefactive sludge” were found to have superimposed malignancy within the gallbladder [39]. This necessitates due diligence in completely excluding malignancy [40]. Current practice for further evaluation includes short interval follow-up ultrasound, and evaluation with a contrast-enhanced examination, with CT and MR likely the most widely used modalities [5]. However, each of these modalities can be confounded by heterogeneous internal contents that may be intrinsically hyperattenuating (CT) or hyperintense on T1-weighted imaging (MRI), impacting the interpretation of post-contrast images on these two modalities (Fig. 8).
48-year-old female presented to the Emergency Department with right upper quadrant abdominal pain. Initial ultrasound suggested a gallbladder mass (not shown). A Noncontrast CT in a patient with renal dysfunction: heterogeneous high-attenuation mass within the gallbladder lumen. B Dual-screen contrast-only (left panel) and grayscale (right) shows avascular mixed echogenicity contents within the gallbladder consistent with sludge and stones, with a few echogenic interfaces from the surface of the stones (specular reflectors). Cholelithiasis without malignant cells was confirmed at pathology
Contrast-enhanced ultrasound has shown 100% accuracy in differentiating perfused solid mass from tumefactive sludge (Fig. 9) [41]. This is attributable to UCA’s exclusively intravascular distribution and the inherent high spatial resolution of ultrasound as discussed above. This also allows differentiation of adherent stones/sludge material (no internal enhancement) from gallbladder polyps (with internal vascularity/enhancement).
37-year-old pregnant female with abdominal pain. Abdominal ultrasound performed in the emergency department showed a non-mobile, polypoid intraluminal echogenic mass at the gallbladder fundus. Contrast-enhanced ultrasound was offered as a relatively safe imaging technique to differentiate tumefactive sludge from malignancy. Dual-screen contrast-only (left panel) and grayscale (right) shows the polypoid mass at the fundus on the grayscale image, completely devoid of enhancement in the contrast image, indicating lack of vascularized tissue and confirming tumefactive sludge
Some challenges may remain and could include: reverberation artifact from the anterior abdominal wall; sidelobe artifact from adjacent bowel; incomplete subtraction from highly echogenic specular reflectors such as stones; and pseudoenhancement from phase aberrations generated from ultrasound passing through overlying enhanced liver, leading to incompletely subtracted signal from echogenic intraluminal material [42, 43].
Assessment of gallbladder wall
When signs or symptoms are attributable to the gallbladder, the initial workup usually includes a right upper quadrant or targeted gallbladder ultrasound. As described above, US offers high sensitivity in detecting stones and acute cholecystitis; a triad of gallstones, gallbladder distension, and positive sonographic Murphy’s sign is classic for acute cholecystitis. With contrast-enhanced imaging (CT or MRI), a hyperenhancing wall may be seen, and hyperemia of the adjacent liver may be present. At CEUS, the wall can similarly show hyperenhancement: a normal gallbladder wall should enhance uniformly without mucosal discontinuity. Normal gallbladder wall is expected to be smooth and thin (< 3 mm) [44].
Gangrenous cholecystitis is a complicated form of acute cholecystitis with an incidence up to 26% among urgent cholecystectomies [45]. Mortality and morbidity are higher with gangrenous cholecystitis, and better outcomes are seen with a rapid diagnosis and urgent cholecystectomy rather than medical management. The robust spatial resolution of CEUS will allow for the meticulous assessment of the gallbladder wall and detection for areas of mucosal irregularity and heterogeneity (ischemia), non-enhancement (necrosis), or frank discontinuity (perforation) (Fig. 10).
66-year-old female presented to the Emergency Department with right upper quadrant abdominal pain, nausea, and vomiting. Initial workup with right upper quadrant US (not shown) demonstrated classic findings of acute cholecystitis and questioned a gallbladder mass. Dual-screen contrast-only (left panel) and grayscale (right) shows a tensely distended gallbladder, hyper-enhancing gallbladder wall (straight arrow), with areas of non-enhancing wall (curved arrows) concerning for gangrenous acute cholecystitis. The absence of vascular internal content on CEUS excludes malignancy. Pathology confirmed gangrenous cholecystitis without malignant cells
Abnormal wall thickening can be encountered in a wide array of pathologic states including inflammation, liver disease, third spacing, adenomyomatosis, and gallbladder cancer [46]. Morphologically, the thickening can be focal or diffuse, suggesting different pathologies. Thickening in turn has been further classified based on different aspects such as stratification, symmetry, and preservation of gallbladder/liver interface which can suggest likelihood of malignancy even in cases of early gallbladder cancer [47]. These findings can be appreciated on CEUS in addition to unique features including heterogeneous enhancement, branched/irregular lesional vascularity, mural irregularity involving the inner or outer layer, and extension into the liver parenchyma (Fig. 11) [48].
74-year-old male presented to the Emergency Department with upper abdominal pain. Initial workup with CT (not shown) showed incidental gallbladder wall thickening. Dual-screen contrast-only (left panel) and grayscale (right) 17 s following contrast administration shows multifocal hyper-enhancing asymmetric gallbladder wall thickening with irregular gallbladder/liver interface suggestive of gallbladder cancer with hepatic invasion. Subsequent cholecystectomy and partial liver resection confirmed gallbladder adenocarcinoma with hepatic invasion
Adenomyomatosis is a reactive process with hyperplasia of the muscularis propria and invagination of the mucosa into the muscularis layer forming Rokitansky-Aschoff sinuses [49]. These changes may occur in different morphological patterns including diffuse, annular/segmental, and fundal [50]. Identifying the sinuses (cystic spaces) within the thickened areas of gallbladder wall, particularly in areas of classic involvement such as the fundus, has been described on MRI and referred to as the “string of pearls sign” [51]. With higher spatial resolution, CEUS can detect these cystic spaces, improving diagnostic confidence (Fig. 12) [52]. In our experience, adenomyomatosis also shows isoenhancement to the remainder of the gallbladder wall on all phases, with a smooth underlying mucosal surface.
70-year-old female with advanced fibrosis and hepatitis C infection undergoing hepatocellular carcinoma surveillance. US (not shown) demonstrated increasing gallbladder fundal nodularity, previously attributed to adenomyomatosis. A Dual-screen contrast-only (left panel) and grayscale (right) 46 s following contrast administration shows heterogenous echogenic fundal gallbladder wall thickening iso-enhancing to the gallbladder wall with small intramural cystic spaces (arrow) consistent with fundal adenomyomatosis. Shadowing gallstones are also seen. B MR fat-saturated T2-weighted image shows gallbladder fundal hyperintense cystic foci (curved arrow) consistent with fundal adenomyomatosis. Layering gallstones are also seen
Summary
Ultrasound remains the first line imaging modality in the assessment of gallbladder and biliary pathologies. Proper technique remains a key requirement in the adequate evaluation of the gallbladder. Incidental findings, though common, are often readily diagnosed and confirmed on ultrasound alone, particularly if ultrasound contrast is available. Recent publications such as the Society of Radiologists in Ultrasound Consensus Recommendations for gallbladder polyps also provide increasing guidance for clinicians and radiologists alike. Investigations on the use of various Doppler techniques and further applications of contrast enhanced ultrasound are emerging in the literature, with early evidence showing improvements in the sonographic evaluation of common and uncommon gallbladder and biliary conditions.
Data availability
No datasets were generated or analysed during the current study.
References
Stinton L and Shaffer E. Epidemiology of Gallbladder Disease: Cholelithiasis and Cancer. Gut Liver 2012; 6(2): 172-187. https://doi.org/10.5009/gnl.2012.6.2.172
Hiatt K, Ou J, Childs D. Role of Ultrasound and CT in the Workup of Right Upper Quadrant Pain in Adults in the Emergency Department: A Retrospective Review of More Than 2800 Cases. AJR. 2020; 214(6): 1305-10. https://doi.org/10.2214/AJR.19.22188
American College of Radiology. ACR–AIUM–SPR–SRU Practice Parameter for the Performance of an Ultrasound Examination of the Abdomen and/or Retroperitoneum. 2021; Available at: https://www.acr.org/-/media/ACR/Files/Practice-Parameters/US-Abd-Retro.pdf. Accessed June 16, 2024
Jenssen C, Lorentzen T, Dietrich C, et al. Incidental Findings of Gallbladder and Bile Ducts—Management Strategies: General Aspects, Gallbladder Polyps and Gallbladder Wall Thickening—A World Federation of Ultrasound in Medicine and Biology (WFUMB) Position Pape., Ultrasound in Medicine & Biology. 2022; 48(12): 2355–2378. https://doi.org/10.1016/j.ultrasmedbio.2022.06.016
Kamaya A, Fung C, Szpakowski J, et al. Management of Incidentally Detected Gallbladder Polyps: Society of Radiologists in Ultrasound Consensus Conference Recommendations. Radiology. 2022; 305(2):277-289. https://doi.org/10.1148/radiol.213079
Gallaher JR, Charles A. Acute Cholecystitis. JAMA. 2022; 327(10):965. https://doi.org/10.1001/jama.2022.2350
Indar AA. Acute cholecystitis. BMJ. 2002; 325(7365):639-643. https://doi.org/10.1136/bmj.325.7365.639
Laurila J, Syrjälä H, Laurila PA, et al. Acute acalculous cholecystitis in critically ill patients. Acta Anaesthesiol Scand. 2004; 48(8):986-991. https://doi.org/10.1111/j.0001-5172.2004.00426.x
Yokoe M, Hata J, Takada T, et al. Tokyo Guidelines 2018: diagnostic criteria and severity grading of acute cholecystitis (with videos). J Hepatobiliary Pancreat Sci. 2018; 25(1):41-54. https://doi.org/10.1002/jhbp.515
Pisano M, Allievi N, Gurusamy K, et al. 2020 World Society of Emergency Surgery updated guidelines for the diagnosis and treatment of acute calculus cholecystitis. World Journal of Emergency Surgery. 2020;15(1):61. https://doi.org/10.1186/s13017-020-00336-x
Huang SS, Lin KW, Liu KL, et al. Diagnostic performance of ultrasound in acute cholecystitis: a systematic review and meta-analysis. World Journal of Emergency Surgery. 2023;18(1):54. https://doi.org/10.1186/s13017-023-00524-5
An C, Park S, Ko S, et al. Usefulness of the tensile gallbladder fundus sign in the diagnosis of early acute cholecystitis. AJR. 2013; 201: 340-346. https://doi.org/10.2214/AJR.12.9919
Simeone J, Brink J, Mueller P, et al. The sonographic diagnosis of acute gangrenous cholecystitis: importance of the Murphy sign. AJR. 1989;152(2):289-290. https://doi.org/10.2214/ajr.152.2.289
Brook O, Kane R, Tyagi G, et al. Lessons learned from quality assurance: Errors in the diagnosis of acute cholecystitis on ultrasound and CT. AJR. 2011; 1986:597-604. https://doi.org/10.2214/AJR.10.5170
Childs DD, Lalwani N, Craven T, et al. A meta-analysis of the performance of ultrasound, hepatobiliary scintigraphy, CT and MRI in the diagnosis of acute cholecystitis. Abdominal Radiology. Published online November 20, 2023. https://doi.org/10.1007/s00261-023-04059-w
Paulson EK. Acute cholecystitis: CT findings. Seminars in Ultrasound, CT and MRI. 2000; 21(1):56-63. https://doi.org/10.1016/S0887-2171(00)90013-1
Anderson SW, Lucey BC, Varghese JC, Soto JA. Accuracy of MDCT in the Diagnosis of Choledocholithiasis. American Journal of Roentgenology. 2006; 187(1):174-180. https://doi.org/10.2214/AJR.05.0459
Soesbe T, Lewis M, Xi Y, et al. A Technique to Identify Isoattenuating Gallstones with Dual-Layer Spectral CT: An ex Vivo Phantom Study. Radiology. 2019; 292(2): 400-406. https://doi.org/10.1148/radiol.2019190083
Kiewiet J, Leeuwenburgh M, Bipat S, et al. A Systematic Review and Meta-Analysis of Diagnostic Performance of Imaging in Acute Cholecystitis. Radiology. 2012; 264(3):708-720. https://doi.org/10.1148/radiol.12111561
Huffman JL, Schenker S. Acute Acalculous Cholecystitis: A Review. Clinical Gastroenterology and Hepatology. 2010; 8(1):15-22. https://doi.org/10.1016/j.cgh.2009.08.034
Håkansson K, Leander P, Ekberg O, Håkansson HO. MR imaging in clinically suspected acute cholecystitis: A comparison with ultrasonography. Acta radiol. 2000;41(4):322-328. https://doi.org/10.1080/028418500127345587
Adusumilli S, Siegelman ES. MR imaging of the gallbladder. Magn Reson Imaging Clin N Am. 2002;10(1):165-184. https://doi.org/10.1016/S1064-9689(03)00055-2
Trowbridge RL, Rutkowski NK, Shojania KG. Does This Patient Have Acute Cholecystitis? JAMA. 2003;289(1):80. https://doi.org/10.1001/jama.289.1.80
Schiller VL, Turner RR and Sarti DA. Color doppler imaging of the gallbladder wall in acute cholecystitis: sonographic-pathologic correlation. Abdom Imaging 1996; 21: 233-237. https://doi.org/10.1007/s002619900053
Uggowitzer M, Kugler C, Schramayer G, et al. Sonography of acute cholecystitis: comparison of color and power Doppler sonography in detecting a hypervascularized gallbladder wall. AJR Am J Roentgenol 1997; 168: 707-712. https://doi.org/10.2214/ajr.168.3.9057520
Draghi F, Ferrozzi G, Calliada F, et al. Power Doppler ultrasound of gallbladder wall vascularization in inflammation: clinical implications. Eur Radiol 2000; 10: 1587-1590. https://doi.org/10.1007/s003300000371
Soyer P, Brouland JP, Boudiaf M, et al. Color velocity imaging and power Doppler sonography of the gallbladder wall: a new look at sonographic diagnosis of acute cholecystitis. AJR Am J Roentgenol 1998; 171: 183-188. https://doi.org/10.2214/ajr.171.1.9648785
Jeffrey RB, Jr., Nino-Murcia M, Ralls PW, et al. Color Doppler sonography of the cystic artery: comparison of normal controls and patients with acute cholecystitis. J Ultrasound Med 1995; 14: 33-36. https://doi.org/10.7863/jum.1995.14.1.33
Loehfelm TW, Tse JR, Jeffrey RB, et al. The utility of hepatic artery velocity in diagnosing patients with acute cholecystitis. Abdom Radiol (NY) 2018; 43: 1159-1167. https://doi.org/10.1007/s00261-017-1288-z
Perez MG, Tse JR, Bird KN, et al. Cystic artery velocity as a predictor of acute cholecystitis. Abdom Radiol (NY) 2021; 46: 4720-4728. 20210703. https://doi.org/10.1007/s00261-021-03020-z
Tochio H, Nishiuma S, Okabe Y, et al. Diagnosis of acute cholecystitis in patients with liver cirrhosis: waveform analysis of the cystic artery by color Doppler imaging. J Med Ultrason (2001) 2004; 31: 21-28. https://doi.org/10.1007/s10396-003-0001-8
Aziz MU and Robbin ML. Improved Detection of Gallbladder Perforation Using Ultrasound Small Vessel Slow Flow "Perfusion" Imaging. J Ultrasound Med 2022; 41: 511-518. 20210422. https://doi.org/10.1002/jum.15729
Aziz MU, Eisenbrey JR, Deganello A, et al. Microvascular Flow Imaging: A State-of-the-Art Review of Clinical Use and Promise. Radiology 2022; 305: 250-264. 20220927. https://doi.org/10.1148/radiol.213303
Ra JC, Lee ES, Park HJ, et al. Efficacy of Superb Microvascular Imaging for Diagnosing Acute Cholecystitis: Comparison with Conventional Ultrasonography. Ultrasound Med Biol 2018; 44: 1968-1977. 20180621. https://doi.org/10.1016/j.ultrasmedbio.2018.05.014
Kong WT, Shen HY, Qiu YD, et al. Application of contrast enhanced ultrasound in gallbladder lesion: is it helpful to improve the diagnostic capabilities? Med Ultrason 2018; 20: 420-426. https://doi.org/10.11152/mu-1626
Ranganath PG, Robbin ML, Back SJ, et al. Practical advantages of contrast-enhanced ultrasound in abdominopelvic radiology. Abdom Radiol (NY) 2018; 43: 998–1012. 2018/01/15. https://doi.org/10.1007/s00261-017-1442-7
Pepin EW, Nordeck SM and Fetzer DT. Nontraditional Uses of US Contrast Agents in Abdominal Imaging and Intervention. Radiographics 2022; 42: 1724-1741. https://doi.org/10.1148/rg.220016
Fakhry J. Sonography of tumefactive biliary sludge. AJR 1982; 139(4): 717-719. https://doi.org/10.2214/ajr.139.4.717
Kim M, Kang T, Jang KM, et al. Tumefactive gallbladder sludge at US: Prevalence and clinical importance. Radiology 2017; 283(2): 570-579. https://doi.org/10.1148/radiol.2016161042
Kamaya A. Editorial Comment: Validating Usage of the Society of Radiologists in Ultrasound Consensus Conference Recommendations for Management of Incidental Gallbladder Polyps. AJR Am J Roentgenol 2024 20240228. https://doi.org/10.2214/AJR.24.31062.
Xie X Xu H, Xie X, et al. Differential diagnosis between benign and malignant gallbladder diseases with real-time contrast-enhanced ultrasound. Eur Radiol 2010; 20: 239–248.https://doi.org/10.1007/s00330-009-1538-8
Fetzer DT, Rafailidis V, Peterson C, et al. Artifacts in contrast-enhanced ultrasound: a pictorial essay. Abdom Radiol (NY) 2018; 43: 977–997. 2017/12/05. https://doi.org/10.1007/s00261-017-1417-8
Fetzer DT, Vijay K, Caserta MP, et al. Artifacts and Technical Considerations at Contrast-enhanced US. Radiographics 2023; 43: e220093. https://doi.org/10.1148/rg.220093
Teefey S, Kimmey M, Bigler S, et al. Gallbladder wall thickening: an in vitro sonographic study with histologic correlation. Acad Radiol 1994; 1(2):121-127. https://doi.org/10.1016/s1076-6332(05)80830-x
Wilson A, Kozol R, Salwen W, et al. Gangrenous cholecystitis in an urban VA hospital. J Surg Res 1994; 56(5): 402-404. https://doi.org/10.1006/jsre.1994.1064
van Breda Vriesman A, Engelbrecht M, Smithuis R, Puylaert J. Diffuse gallbladder wall thickening: differential diagnosis. AJR 2007; 188(2): 495-501. https://doi.org/10.2214/AJR.05.1712
Joo I, Lee J, Kim J, et al. Differentiation of adenomyomatosis of the gallbladder from early-stage, wall-thickening-type gallbladder cancer using high-resolution ultrasound. Eur Radiol 2013; 23(3):730-738. https://doi.org/10.1007/s00330-012-2641-9
Xu J, Guo L, Xu H, et al. Differential Diagnosis of Gallbladder Wall Thickening: The Usefulness of Contrast-Enhanced Ultrasound. Ultrasound in Medicine & Biology 2014; 40(12): 2794-2804. https://doi.org/10.1016/j.ultrasmedbio.2014.06.015
Golse N, Lewin M, Rode A, et al. Gallbladder adenomyomatosis: Diagnosis and management. J Visc Surg 2017; 154(5):345-353. https://doi.org/10.1016/j.jviscsurg.2017.06.004
Aguirre J, Bohler R, Guraieb S. Hyperplastic cholecystoses; a new contribution to the unitarian theory. Am J Roentgenol Radium Ther Nucl Med 1969; 107(1):1-13. https://doi.org/10.2214/ajr.107.1.1
Haradome H, Ichikawa T, Sou H, et al. The pearl necklace sign: an imaging sign of adenomyomatosis of the gallbladder at MR cholangiopancreatography. Radiology 2003; 227(1):80-88. https://doi.org/10.1148/radiol.2271011378
Meacock L, Sellars M, Sidhu P. Evaluation of gallbladder and biliary duct disease using microbubble contrast-enhanced ultrasound. British Journal of Radiology 2010; 83(991): 615-627. https://doi.org/10.1259/bjr/60619911
Author information
Authors and Affiliations
Contributions
All authors wrote and reviewed the manuscript. CF and RS prepared Figs. 1–3, RN, KV, and DF prepared Figs. 4–12.
Corresponding author
Ethics declarations
Competing interest
CF: Research grant: Exact Imaging, Partner Radiologist MIC Medical Imaging, Shareholder: Mikata Health RS: None. RN: None. KV: None. DF: Research Agreements: GE HealthCare, Philips Healthcare, and Siemens Healthineers. Advisory Board: GE HealthCare, Philips Healthcare, Siemens Healthineers, and Bracco Diagnostics. Speakers Bureau: Siemens Healthineers.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Fung, C., Spychka, R., Noorelahi, R. et al. Ultrasound of the gallbladder: not the same bag of tricks. Abdom Radiol (2024). https://doi.org/10.1007/s00261-024-04530-2
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00261-024-04530-2