Abstract
Purpose
To assess liver and spleen characteristics of a population with Gaucher disease (GD) using multiparametric MRI and MR elastography (MRE) for evaluation of diffuse liver and spleen disease, which includes liver fat fraction, liver and spleen volume and iron deposition, and liver and spleen stiffness correlated with DS3 Severity Scoring System for Gaucher disease (GD-DS3).
Methods
We prospectively evaluated 41 patients with type 1 Gaucher disease using a 3.0 T MRI and MRE between January 2019 and February 2020. Clinical, laboratory, and imaging data was collected. Mann–Whitney, Kruskal–Wallis, and Spearman’s correlation were applied to evaluate liver and spleen MRI and MRE, clinical and laboratory variables, and GD-DS3. ERT and SRT treatment groups were compared.
Results
Hepatomegaly was seen in 15% and splenomegaly in 42% of the population. Moderate and strong and correlations were found between liver and spleen iron overload (rho = 0.537; p = 0.002); between liver and spleen volume (rho = 0.692, p < 0.001) and between liver and spleen stiffness (rho = 0.453, p = 0.006). Moderate correlations were found between liver stiffness and GD-DS3 (rho = 0.559; p < 0.001) and between splenic volume and GD-DS3 (rho = 0.524; p = 0.001).
Conclusion
The prevalence of hepatosplenomegaly, liver fibrosis, and liver iron overload in treated patients with GD is low, which may be related to the beneficial effect of treatment. Liver MRE and splenic volume correlate with severity score and may be biomarkers of disease severity.
Graphical abstract

Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Gaucher’s disease (GD) is an autosomal recessive disease characterized by the deficiency of the lysosomal enzyme glucocerebrosidase. It results in glucosylceramide accumulation in the reticuloendothelial system, mainly in the liver, spleen, and bone marrow. Organ infiltration by Gaucher cells causes hepatosplenomegaly, anemia, thrombocytopenia, among others [1,2,3,4]. Pro-inflammatory factors contribute to organ enlargement and visceral inflammation [3, 5,6,7].
GD and its treatment are associated with many metabolic abnormalities including increased glucose and cholesterol, insulin resistance, diabetes, and metabolic syndrome [7, 8]. Many studies have demonstrated liver disorders in GD, including hepatomegaly, hemosiderosis, steatosis, increased stiffness, cirrhosis, portal hypertension, hepatocellular carcinoma, focal liver lesions (gaucheromas), and cholelithiasis [8,9,10,11]. Spleen involvement includes splenomegaly, elevated stiffness due to infiltration and inflammatory changes, gaucheromas, and splenic infarction. Elevated stiffness in the spleen may be caused by Gaucher cell infiltration, fibrosis, inflammation, or portal hypertension [1, 3, 4, 12, 13]. Splenectomy has been associated with more severe Gaucher’s disease. However, enzyme replacement therapy (ERT) and substrate reduction therapy (SRT) have changed the natural history of the disease [2, 4].
Non-invasive liver and spleen evaluation is strongly recommended for disease monitoring. Routine evaluation includes blood cell count, liver function tests, serum ferritin, and abdominal ultrasound [3, 7, 9, 13]. However, these methods have low sensitivity to detect liver and spleen involvement, such as liver steatosis, and liver and spleen iron deposition and stiffness [14]. Magnetic resonance imaging (MRI) can thoroughly evaluate the abdominal organs, measure liver and spleen volume, detect focal lesions, and quantify liver fat fraction and iron concentration. Additionally, MR elastography (MRE) can assess liver and spleen stiffness [15,16,17,18].
Original studies on GD are scarce and were performed with small populations. No study assessed MR elastography of the spleen or iron deposition on the spleen. Only 2 of these studies (7 and 31 GD patients) used magnetic resonance imaging for liver iron concentration evaluation and MR elastography for liver stiffness evaluation [10, 19]. However, they did not evaluate for liver and spleen volume, fat fraction and spleen stiffness in the context of ERT and SRT treatment.
The purpose of this study was to assess liver and spleen characteristics of a population with GD using multiparametric MRI and MRE for evaluation of diffuse liver and spleen disease, which includes liver fat fraction and liver and spleen volume, iron deposition, and stiffness, compared with GD-DS3.
Methods
Study design and population
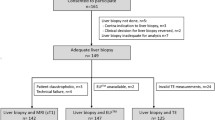
This prospective cross-sectional study consecutively included adult participants (> 18 years) with type 1 Gaucher disease from Hospital Hemorio (Rio de Janeiro, Brazil) from January 2019 to February 2020. Exclusion criteria were known chronic liver disease (CLD), such as viral and/or alcoholic CLD, primary biliary cirrhosis, primary sclerosing cholangitis, autoimmune cholangitis, primary hemochromatosis, and contraindications to MRI. Non-alcoholic fatty liver disease, secondary hemochromatosis, and GD related CLD were not exclusion criteria. This study was approved by the Ethics Committee from the D'Or Institute for Research and Education. Written informed consent was obtained from all patients.
Clinical and laboratory evaluation
Clinical records included gender, age, weight, height, body mass index (BMI), and waist circumference (WC). History of splenectomy, blood transfusions, alcohol consumption, disease duration, type of treatment and its duration were obtained. Significant alcohol consumption was defined as ≥ 20 g/day in women and ≥ 30 g/day in men. Hematocrit, hemoglobin, platelets, alanine aminotransferase (ALT), aspartate aminotransferase (AST), and ferritin were performed with a time interval up to 6 months to the MRI.
MRI
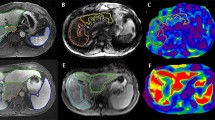
This study was conducted on 3.0 T MRI (Magneton Prisma, Siemens Healthcare) by using a combined eighteen-element phased-array abdominal coil and a thirty-two-element fixed spine coil. Axial and coronal T2-weighted imaging were obtained for anatomical reference. Screening Dixon (e-dixon) and ME Dixon (q-Dixon) sequences were performed to evaluate liver and spleen iron deposition. Patients were instructed to fast 4 h prior to the exam. Image acquisition parameters are provided in Table S1.
MR spectroscopy (MRS)
Single-voxel MRS with stimulated echo acquisition mode was placed on the right liver lobe, avoiding hepatic edges, large vessels, bile ducts, and focal lesions. HISTO provides proton density fat fraction (PDFF) that has been corrected for fat and water transverse relaxation. It is corrected for the T2 signal, at high speed, to prevent over-evaluating PDFF. To minimize the effects of T1 weighting, a TR of 3000 ms was used. The voxel was placed on the right liver lobe, avoiding hepatic edges, large vessels, bile ducts, and focal lesions. Image acquisition parameters are provided in Table S2.
MR elastography (MRE)
2D spin-echo echo-planar sequence MRE of the liver and the spleen were acquired with breath hold at end expiration. An active driver was placed on the right side of the anterior chest wall at the level of the xiphoid centered over the portal vein to measure liver stiffness and on the left side of the posterior chest wall centered at the splenic hilum to measure spleen stiffness. Image acquisition parameters are provided in Table S3.
Image analyses
MRI examination
All MR imaging results were interpreted by two radiologists (F.C.M.M. and D.B.P., both with 18 years of experience with liver imaging) blinded to clinical data.
Liver volume was automatically calculated by the LiverLab package (Siemens Healthineers). Spleen volume was calculated using OsiriX® (OsiriX Foundation).
Body surface area (BSA) was calculated by using Mosteller’s formula: BSA (m2) = \(\surd\) height [cm] × weight [kg]/3600 [20]. Predicted total liver volume (TLV) was calculated according to Vauthey’s formula for western adults: TLV = 1267.28 × BSA – 794.41 [21]. Hepatomegaly was defined as liver volume greater than 1.25 times the predicted liver volume [9]. Splenomegaly was defined as spleen volume greater than 322 cm3, based on the 97th percentile of the population-based study that established reference for individual for spleen volume [22].
MRI-PDFF maps and R2* maps and reports of screening and ME Dixon were acquired automatically using whole-liver evaluation. Liver iron concentration (LIC) was calculated using the formula used for 3 T: LIC (μmol) = 0.314 R2* − 0.96 [23, 24]. For this study, we considered LIC values higher than 36 μmol/g as iron overload. Spleen iron overload was considered when R2* of the spleen > 70 Hz [24, 25].
MRS
MRS-PDFF report was acquired automatically. We categorized liver steatosis based on PDFF-MRS measurements according to cut-offs previously described in literature as follows: no steatosis (PDFF < 5%), mild steatosis (PDFF ≥ 5% to 15%), moderate steatosis (PDFF ≥ 15% to 25%), severe steatosis (PDFF ≥ 25%)[15].
MRE
The stiffness maps were automatically provided. As large as possible free-hand ROIs were drawn in each slice of the liver and spleen, avoiding voxels with confidence index less than 95%, large vessels, edges, and regions of ambiguous wave propagation. Mean liver and spleen stiffness were calculated as the mean value of the four slices. The liver stiffness threshold values were defined as previously described in literature: normal (< 2.5 kPa), normal or inflammation (≥ 2.5–2.9 kPa), stage 1–2 fibrosis (3.0–3.5 kPa), stage 2–3 fibrosis (3.5–4.0 kPa), stage 3–4 fibrosis (4.0–5.0 kPa), stage 4 fibrosis (> 5.0 kPa) [18, 26].
Statistical analysis
The statistical analysis of data was done by using SPSS 24.0 software (IBM Corporation). Due to non-normally distributed variables, medians and interquartile ranges were used. Exact Fisher test was used to test differences between categorical variables; Mann–Whitney to compare difference between two treatment groups; and Spearman’s correlation coefficient to assess correlation between quantitative variables.
Results
Forty-one patients with type 1 Gaucher disease were prospectively enrolled in this study (34% male, 66% female; mean age 42 ± 16 years). No patient had to be excluded. Ninety-five percent of patients were treated with enzyme replacement therapy (ERT) or substrate reduction therapy (SRT) and only 5% did not receive treatment (Table S4). Patients’ clinical and laboratory characteristics are shown in Tables 1, 2 and patients’ MRI and MRE data in Table 3.
Patients that received ERT had more steatosis than patients that received SRT (53.8% vs 15.4%; = 0.037). No significant difference was found between liver fat fraction and the BMI groups and between liver fat fraction and duration of ERT/SRT. Patients that used SRT had significantly lower liver fat fraction than patients that used ERT (p = 0.015) (Fig. 1). Patients that received ERT had more liver iron overload than patients that received SRT (30.8% vs 7.7%; p = 0.006).
A moderate correlation was found between liver and spleen iron overload (rho = 0.537; p = 0.002) (Fig. 2). No significant correlation was found between spleen iron overload and spleen volume or stiffness. Patients with liver iron overload had significantly higher serum ferritin levels (p < 0.001) and liver fat fraction (p = 0.001). Liver iron concentration and blood transfusion (p > 0.05) presented no significant difference.
Most patients (87%) had normal liver stiffness. No patient with increased liver stiffness had stage 3 or 4 liver fibrosis. One missing value was seen in a patient with mild iron overload due to the black hole artifact. A strong correlation was found between liver and spleen volume (rho = 0.692, p < 0.001) (Fig. 3) and a moderate correlation was found between liver and spleen stiffness (rho = 0.453, p = 0.006) (Fig. 4). A moderate correlation was found between liver stiffness and liver fat fraction (rho = 0.420, p = 0.007) (Fig. 5). No significant difference was observed for liver volume and steatosis grades (p > 0.05). Spleen stiffness and splenomegaly (p > 0.05) also did not present significant differences.
A weak correlation between liver stiffness and volume (rho = 0.348; p = 0.03) (Fig. 6) and a moderate correlation between liver stiffness and spleen volume (rho = 0.558; p < 0.001) (Fig. 7) were found. Moderate correlations were found between liver stiffness and GD-DS3 (rho = 0.559; p < 0.001) (Fig. 8) and between splenic volume and GD-DS3 (rho = 0.524; p = 0.001) (Fig. 9).
Discussion
Gaucher disease is rare and lack original studies, especially on post-enzyme replacement and substrate reduction therapies, which have changed the natural history of disease. This study focused on the multiparametric MRI and MRE assessment of the liver and spleen of 41 adults with type 1 Gaucher disease compared to severity score (GD-DS3)[27]. Ninety-five percent were under treatment with enzyme replacement therapy (ERT) or substrate reduction therapy (SRT). Positive correlations were found between liver and spleen: iron overload; volume; and stiffness. Severity score also correlated with liver stiffness and splenic volume. The prevalence of hepatosplenomegaly, liver fibrosis, and liver iron overload in treated patients with GD is low, which may be related to a beneficial effect of treatment.
Liver stiffness may be a biomarker of disease severity. In this population of treated patients, most had normal liver stiffness, which may reflect the good response to ERT/SRT. Strong and moderate correlations were found between liver and spleen volume and between liver and spleen stiffness, respectively, showing that the disease affects both organs simultaneously. Liver stiffness in GD can be increased by a combination of factors, like Gaucher cell infiltration, fibrosis and inflammation related to GD or to non-alcoholic fatty liver disease (NAFLD) due to GD and its treatment.
This is the first study to evaluate spleen stiffness by MRE in GD. No significant correlation was found between spleen stiffness and spleen iron overload. This contributes to the finding that increased stiffness on the spleen is not caused by iron deposition, which is probably the result of Gaucher cell infiltration on the spleen, inflammation and fibrosis related to GD. Portal hypertension was not present in our population and did not contribute to the increased spleen stiffness. Only one study evaluated spleen stiffness by ultrasound in GD patients and found higher median spleen stiffness than in the control population [28]. Iron deposition on the liver and spleen is common in patients with GD, found in 22% and 37% of our population, respectively. A moderate correlation was found between spleen and liver iron overload, which once more indicates that the disease affects both organs simultaneously.
NAFLD needs further evaluation in GD. NAFLD was seen in 41.4% of the population, mostly mild steatosis. No previous study evaluated liver steatosis by MRI in GD. A study that used ultrasound reported liver steatosis in 37% of GD patients [28]. Liver stiffness may be increased in patients with NAFLD due to inflammation and fibrosis. No significant difference was found between liver fat fraction and the different BMI groups. However, ERT and SRT may influence liver fat deposition. Patients treated with SRT had significantly lower liver fat fraction than patients treated with ERT (2.3% vs 5.6%; p = 0.015). This is probably related to the mechanism of action of SRT, an oral therapy that promotes weight loss and diarrhea. On the other hand, ERT is an intravenous treatment that induces weight gain due to the reduction of the basal metabolism. Patients with no treatment have accelerated metabolism due to lack of enzyme. After ERT, there is a reduction of the basal metabolism and patients gain weight.
Hepatomegaly was seen in 15% and splenomegaly in 42% of the population. Hepatomegaly is common in GD and usually accompanies splenomegaly. Before ERT and SRT, the prevalence of hepatomegaly has been described from 87 to 100% [29,30,31,32,33] and splenomegaly in 100% [29, 30]. Hepatosplenomegaly decreases mainly during the first two years of ERT. Responses are influenced by pretreatment volume [29, 34]. When organomegaly is severe, the presence of fibrosis precludes volume normalization [29, 34]. The lower prevalence of hepatosplenomegaly may be explained by the long-term treatment with SRT/ERT in our population. Hepatosplenomegaly may be caused by infiltration of Gaucher cells but fibrosis and inflammation due to GD may also contribute to liver and spleen enlargement.
We acknowledge that the higher cost of MRI compared to US (for volume and stiffness) and CT scan (for volume and focal lesions) would make this assessment difficult to do except at large centers with highly trained and sub-specialized services. Further studies are necessary to assess the value of ultrasound elastography as a biomarker of disease severity in the Gaucher disease population.
Our study had limitations and strengths. The population is small. However, GD is rare and all studies on GD have small populations. This prospective study has the largest sample size of GD patients that underwent a thorough multiparametric MRI and MRE correlated to the severity score (GD-DS3). Additionally, 2D spin-echo echo-planar sequence was used to overcome blackhole artifact due to iron deposition. No study has performed all these MR evaluations in GD patients. A complete laboratory metabolic evaluation was not performed and the influence of metabolic syndrome on GD patients could not be evaluated. At last, liver biopsy was not performed due to lack of clinical indication and ethical considerations.
In conclusion, in this population of mostly treated adults with type 1 GD, we found that liver and spleen disease occur simultaneously. There was low prevalence of hepatosplenomegaly, liver fibrosis, and liver iron overload that could be related to a beneficial effect of treatment. Severity score (GD-DS3) correlated with liver stiffness and splenic volume, which suggests that these imaging parameters may be biomarkers of disease severity.
References
Grabowski GA. Phenotype, diagnosis, and treatment of Gaucher's disease. Lancet 2008; 372:1263-1271
Dandana A, Ben Khelifa S, Chahed H, Miled A, Ferchichi S. Gaucher Disease: Clinical, Biological and Therapeutic Aspects. Pathobiology 2016; 83:13-23
Stirnemann J, Belmatoug N, Camou F, et al. A Review of Gaucher Disease Pathophysiology, Clinical Presentation and Treatments. Int J Mol Sci 2017; 18
Andrade-Campos MM, de Frutos LL, Cebolla JJ, et al. Identification of risk features for complication in Gaucher's disease patients: a machine learning analysis of the Spanish registry of Gaucher disease. Orphanet J Rare Dis 2020; 15:256
Pandey MK, Burrow TA, Rani R, et al. Complement drives glucosylceramide accumulation and tissue inflammation in Gaucher disease. Nature 2017; 543:108-112
Pandey MK, Grabowski GA, Kohl J. An unexpected player in Gaucher disease: The multiple roles of complement in disease development. Semin Immunol 2018; 37:30-42
Kaluzna M, Trzeciak I, Ziemnicka K, Machaczka M, Ruchala M. Endocrine and metabolic disorders in patients with Gaucher disease type 1: a review. Orphanet J Rare Dis 2019; 14:275
Nascimbeni F, Dalla Salda A, Carubbi F. Energy balance, glucose and lipid metabolism, cardiovascular risk and liver disease burden in adult patients with type 1 Gaucher disease. Blood Cells Mol Dis 2018; 68:74-80
Adar T, Ilan Y, Elstein D, Zimran A. Liver involvement in Gaucher disease - Review and clinical approach. Blood Cells Mol Dis 2018; 68:66-73
Serai SD, Naidu AP, Andrew Burrow T, Prada CE, Xanthakos S, Towbin AJ. Correlating liver stiffness with disease severity scoring system (DS3) values in Gaucher disease type 1 (GD1) patients. Mol Genet Metab 2018; 123:357-363
Starosta RT, Vairo FPE, Dornelles AD, et al. Liver involvement in patients with Gaucher disease types I and III. Mol Genet Metab Rep 2020; 22:100564
Regenboog M, Bohte AE, Somers I, van Delden OM, Maas M, Hollak CE. Imaging characteristics of focal splenic and hepatic lesions in type 1 Gaucher disease. Blood Cells Mol Dis 2016; 60:49-57
Degnan AJ, Ho-Fung VM, Ahrens-Nicklas RC, et al. Imaging of non-neuronopathic Gaucher disease: recent advances in quantitative imaging and comprehensive assessment of disease involvement. Insights Imaging 2019; 10:70
Ma X, Holalkere NS, Kambadakone RA, Mino-Kenudson M, Hahn PF, Sahani DV. Imaging-based quantification of hepatic fat: methods and clinical applications. Radiographics 2009; 29:1253-1277
Starekova J, Hernando D, Pickhardt PJ, Reeder SB. Quantification of Liver Fat Content with CT and MRI: State of the Art. Radiology 2021; 301:250-262
Franca M, Alberich-Bayarri A, Marti-Bonmati L, et al. Accurate simultaneous quantification of liver steatosis and iron overload in diffuse liver diseases with MRI. Abdom Radiol (NY) 2017; 42:1434-1443
Garteiser P, Doblas S, Van Beers BE. Magnetic resonance elastography of liver and spleen: Methods and applications. NMR Biomed 2018; 31:e3891
Idilman IS, Li J, Yin M, Venkatesh SK. MR elastography of liver: current status and future perspectives. Abdom Radiol (NY) 2020; 45:3444-3462
Bohte AE, van Dussen L, Akkerman EM, et al. Liver fibrosis in type I Gaucher disease: magnetic resonance imaging, transient elastography and parameters of iron storage. PLoS One 2013; 8:e57507
Mosteller RD. Simplified calculation of body-surface area. N Engl J Med 1987; 317:1098
Vauthey JN, Abdalla EK, Doherty DA, et al. Body surface area and body weight predict total liver volume in Western adults. Liver Transpl 2002; 8:233-240
Kim DW, Ha J, Lee SS, et al. Population-based and Personalized Reference Intervals for Liver and Spleen Volumes in Healthy Individuals and Those with Viral Hepatitis. Radiology 2021; 301:339-347
d'Assignies G, Paisant A, Bardou-Jacquet E, et al. Non-invasive measurement of liver iron concentration using 3-Tesla magnetic resonance imaging: validation against biopsy. Eur Radiol 2018; 28:2022-2030
Henninger B, Alustiza J, Garbowski M, Gandon Y. Practical guide to quantification of hepatic iron with MRI. Eur Radiol 2020; 30:383-393
Alustiza JM, Artetxe J, Castiella A, et al. MR quantification of hepatic iron concentration. Radiology 2004; 230:479-484
Srinivasa Babu A, Wells ML, Teytelboym OM, et al. Elastography in Chronic Liver Disease: Modalities, Techniques, Limitations, and Future Directions. Radiographics 2016; 36:1987-2006
Weinreb NJ, Finegold DN, Feingold E, et al. Evaluation of disease burden and response to treatment in adults with type 1 Gaucher disease using a validated disease severity scoring system (DS3). Orphanet J Rare Dis 2015; 10:64
Webb M, Zimran A, Dinur T, et al. Are transient and shear wave elastography useful tools in Gaucher disease? Blood Cells Mol Dis 2018; 68:143-147
Patlas M, Hadas-Halpern I, Abrahamov A, Elstein D, Zimran A. Spectrum of abdominal sonographic findings in 103 pediatric patients with Gaucher disease. Eur Radiol 2002; 12:397-400
Hill SC, Damaska BM, Ling A, et al. Gaucher disease: abdominal MR imaging findings in 46 patients. Radiology 1992; 184:561-566
James SP, Stromeyer FW, Chang C, Barranger JA. LIver abnormalities in patients with Gaucher's disease. Gastroenterology 1981; 80:126-133
Hill SC, Reinig JW, Barranger JA, Fink J, Shawker TH. Gaucher disease: sonographic appearance of the spleen. Radiology 1986; 160:631-634
Neudorfer O, Hadas-Halpern I, Elstein D, Abrahamov A, Zimran A. Abdominal ultrasound findings mimicking hematological malignancies in a study of 218 Gaucher patients. Am J Hematol 1997; 55:28-34
Pastores GM, Weinreb NJ, Aerts H, et al. Therapeutic goals in the treatment of Gaucher disease. Semin Hematol 2004; 41:4-14
Acknowledgements
We thank Carolina Braz Parente for English revision.
Funding
This work was supported by D’Or Institute for Research and Education (IDOR) and Fundação de Amparo à Pesquisa do Estado do Rio de Janeiro (FAPERJ) (E_32/2014 – Apoio à realização de pesquisas clínicas e translacionais no RJ – FAPERJ/IDOR – 2014).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
All authors of this manuscript declare no relationships with any companies, whose products or services may be related to the subject matter of the article.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Parente, D.B., de Melo Malta, F.C.M., de Souza Cravo, R. et al. Multiparametric magnetic resonance imaging of the liver and spleen in Gaucher disease. Abdom Radiol 49, 3069–3077 (2024). https://doi.org/10.1007/s00261-024-04293-w
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00261-024-04293-w