Abstract
Objectives
Parenchymal-sparing hepatectomy (PSH) is recommended in patients with colorectal liver metastases (CRLM). Based on the principle of PSH, to investigate the impact of anatomical resection (AR) and non-anatomic resection (NAR) on the outcome of CRLM and to evaluate the potential prognostic impact of three peritumoral imaging features.
Methods
Fifty-six patients who had abdominal gadoxetic acid-enhanced magnetic resonance imaging (MRI) before CRLM surgery were included in this retrospective research. Peritumoral early enhancement, peritumoral hypointensity on hepatobiliary phase (HBP), and biliary dilatation to the CRLM at MRI were evaluated. Survival estimates were calculated using the Kaplan-Meier method, and multivariate analysis was conducted to identify independent predictors of liver recurrence-free survival (LRFS), recurrence-free survival (RFS) and overall survival (OS).
Results
NAR had a lower 3-year LRFS compared with AR (36.6% vs. 78.6%, p = 0.012). No significant differences were found in 3-year RFS (34.1% vs. 41.7%) and OS (61.7% vs. 81.3%) (p > 0.05). In NAR group, peritumoral early enhancement was associated with poor LRFS (p = < 0.001, hazard ratio [HR] = 6.260; 95% confidence interval [CI], 2.322,16.876]) and poor RFS (p = 0.035, HR =2.516; 95% CI, 1.069,5.919). No independent predictors of CRLM were identified in the AR group.
Conclusions
In patients with CRLM, peritumoral early enhancement was a predictor of LRFS and RFS after NAR according to the principle of PSH.
Graphical abstract

Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Liver is the most common metastasis site of colorectal cancer [1], with the metastasis rate of 40–50% [2]. Colorectal liver oligometastases (i.e., limited metastases confined to the liver) have been identified to benefit the survival of patients through resection and focal therapies [3, 4]. The 5-year survival rate of patients with oligostastatic colorectal liver metastases (CRLM) can be improved from 9% to 20%–50% by curative surgical resection [5,6,7,8].
Parenchymal-sparing hepatectomy (PSH) is recommended in patients with CRLM because microscopically positive surgical margins and surgical procedures have no effect on overall survival, consequently offering a high rate of repeat resection for liver recurrence [9,10,11]. In previous studies, PSH included non-anatomic resection (NAR) (including non-anatomic wedge resections) and anatomic resection (AR) (including anatomic segmentectomies and anatomic bisegmentectomies) [10,11,12,13]. Although NAR maintains functional liver remnants and reduces the risk of liver failure, it has the potential to increase the risk of intrahepatic recurrence compared to AR [14, 15]. Unfortunately, approximately half of patients have liver recurrence within 3 years after NAR [16]. Hence, following the principle of PSH, the choice of the appropriate surgical procedure for each lesion to balance between reducing intrahepatic recurrence and maintaining hepatic functional reserve demands further exploration.
Peritumoral pathological and imaging features including vascular invasion, bile duct invasion, peritumoral early enhancement, peritumoral hypointensity on hepatobiliary phase (HBP), and biliary duct dilatation probably predict a worse prognosis [17,18,19]. However, it is unclear whether these radiological risk factors can predict the long-term prognosis of different surgical procedures.
Thus, the aims of this study were to determine the impact of AR and NAR on the outcome for CRLM according to the principle of PSH and to investigate whether peritumoral imaging features to the CRLM could be used to predict long-term prognosis in different surgical procedures.
Materials and methods
Patients
This retrospective study was approved by our institutional review board. The database of our institution was reviewed from August 2014 to May 2022 inclusively to identify all patients with CRLM who had undergone preoperative (within 2 weeks before surgery) abdominal gadoxetic acid-enhanced magnetic resonance imaging (MRI) and curative surgery for CRLM, which was defined as hepatectomy with macroscopic clear resection margin[20]. Exclusion criteria: (a) patients with neoadjuvant chemotherapy or hepatic recurrence during adjuvant chemotherapy after primary site resection; (b) patients that received interventional therapy of liver before surgery, including transarterial chemoembolization or radiofrequency ablation; (c) patients that had coexistence of extrahepatic metastases; (d) patients with redo liver resection;(e) patients combined with malignancies at the other sites; (f) patients with non-PSH (≥3 segments) or combined resection (simultaneous AR and NAR)[12]; (g) patients with less than 12 months of follow-up; (h) patients with R1(microscopically positive surgical margins) surgical margin status; (i) patients with five or more CRLMs. The patient flowchart is shown in Figure 1.
Clinicopathological characteristics were collected, including age, sex, virus status, primary tumor location, synchronous or metachronous liver metastasis, preoperative liver function tests (including galanine transaminase, aspartate transaminase, gamma glutamyltransferase, lactate dehydrogenase, and phosphatase alkaline) [21], serum carcinoembryonic antigen (CEA) level, serum alpha-fetoprotein (AFP) level, stage of the primary tumor, and type of hepatectomy. The liver function tests were classified as normal or abnormally elevated, according to the laboratory ranges [21].
Preoperative assessment and surgical procedure
Hepatectomy was indicated for cases in which all tumors could be removed with clear margins, leaving future liver remnant >30% of the total liver volume. Tri-phase contrast-enhanced computed tomography (CT) and abdominal gadoxetic acid-enhanced MRI were performed for tumor-staging. The preoperative estimate whole liver volume and the postoperative estimate remnant liver volume were calculated using tri-phase contrast-enhanced CT scans. Liver function was evaluated in terms of the indocyanine green retention rate at 15 minutes and CT volumetry. All surgical procedures were designed by the multidisciplinary team (MDT) discussion depending on clinical history, physical examination, serum laboratory tests, the number and location of the tumors, the probability of achieving a negative surgical margin, the need to preserve an adequate liver remnant, and relation of the tumor to vascular structures.
All surgical procedures were performed by two hepatobiliary surgeons with more than 20 years’ experience in hepatectomy who are members of the Colorectal Cancer MDT for hepatic resection of CRLM. Following laparotomy or laparoscopy, intraoperative ultrasonography examination of the liver was routinely performed to confirm the exact location and number of CRLM, while the relationship of the CRLM to the portal vein and hepatic vein was considered to guide resection. The type of resection (AR vs. NAR) was determined by the operating surgeon using a combination of preoperative and intraoperative evaluation.
MR imaging examination
All MR images were performed with a 3-T scanner (Signa HD Excite; GE Healthcare, Milwaukee, WI). Routine in-phase and opposed phase T1-weighted images (time of repetition [TR], 260 ms; time of echo [TE], 2.3 ms and 4.6 ms, respectively; flip angle 80°; matrix, 384×160; field of view, 400×400 mm; section thickness, 5 mm; intersection gap, 2 mm) were obtained. Pre-contrast images were obtained in a transverse plane with a fat-suppressed three-dimensional (3D) T1-weighted liver acquisition with volume acceleration-extended volume (LAVA-XV) sequence (TR, 4 ms; TE, 1.9 ms; flip angle 12°; matrix, 320×224; field of view, 380×304 mm; section thickness, 4 mm; intersection gap, 0 mm). All patients were given 25 mmol/kg (0.1 mL/kg) of gadoxetic acid (Gadoxetic Acid Disodium Injection; Chiatai Tianqing Pharma, Jiang Su, China) as an intravenous bolus, using a power injector at a rate of 1 mL/s, followed by a 20-mL saline flush. After the contrast administration, the early arterial phase (20–25s), the late arterial phase (40–45s), the portal venous phase (65–70s), transition phase (3–5min), and HBP (10–20min) images were obtained using a T1-weighted 3D LAVA-XV sequence. T2-weighted fast spin echo sequences (TR, 4400 ms; TE, 85 ms; flip angle 90°; matrix, 320×224; field of view, 400×300 mm; section thickness, 5 mm; intersection gap, 1 mm) were obtained using a respiratory-triggered technique.
Image analysis
All images were retrospectively and independently reviewed by two abdominal imaging radiologists (with 10 and 15 years of experience, respectively), who were blinded to the clinical and pathologic findings. In case of disagreements, adopt the decision through consultation. The following tumoral features were evaluated: number, size (maximum diameter on HBP), tumor shape (regular or irregular such as lobulated), peritumoral early enhancement, peritumoral hypointensity on HBP, and bile duct dilatation. Peritumoral early enhancement was evaluated on early and/or late arterial phase images and excluded peritumoral rim enhancement (Fig. 2a). Bile duct dilatation was defined as a peritumoral linear or branched hyperintensity area on fat-suppressed T2-weighted images (Fig. 2b). Peritumoral hypointensity on HBP was defined as wedge-shaped hypointense area of hepatic parenchyma located outside of the tumor margin on HBP (Fig. 2c). In patients with multiple CRLMs, the patient was included in the positive group if at least one tumor showed peritumoral early enhancement, peritumoral hypointensity on HBP, and bile duct dilatation.
Images in patients with colorectal liver metastases. (a) Contrast-enhanced late arterial phase T1-weighted magnetic resonance (MR) image shows a hypointense mass with peritumoral wedge-shaped enhancement (arrow). (b) Axial fat-suppressed T2-weighted MR image shows a strong linear hyperintensity (arrow). This indicates bile duct dilatation. (c) Contrast-enhanced 20-minute hepatobiliary phase MR image shows a hypointense mass with peritumoral wedge-shaped intermediate hypointensity (arrow).
We also evaluated CRLM location (deep or surface) and distance from CRLM to vascular (<1mm or ≥1mm), given the possible impact on the choice of surgical procedure and prognosis. We identified patients who had deep-placed CRLMs whose margin was located >30 mm from the liver surface and others with surface-placed. Distance from CRLM to vascular was defined as the shortest distance from the tumor margin to the first-and second-order branches of the portal veins, hepatic veins, or hepatic arteries. In patients with multiple CRLMs, we recorded the deepest tumors and those nearest to the vascular.
Histologic analyses
All histologic specimens were analyzed by a pathologist (10 years of experience in gastrointestinal pathology), who was blinded to the original pathology reports, clinical data, imaging findings, and follow-up data. The surgical margin status and the presence or absence of portal vein, hepatic vein and bile duct invasion were re-evaluated using light microscopy for each patient. Surgical margin status was classified as R0 (microscopically negative surgical margins) or R1 (microscopically positive surgical margins) [22]. Portal vein, hepatic vein, and bile duct invasion were considered present when tumor cells were seen within the portal vein, hepatic vein, or bile duct channels in hematoxylin-eosin-stained sections [22].
Follow-up and adjuvant chemotherapy
Postoperative follow-up of patients was performed every 3-6 months during the first 2 years and every 6–12 months thereafter. The routine follow-up included contrast-enhanced CT or MR examinations and tumor marker (CEA and carbohydrate antigen 19-9) testing. Tumor recurrence was identified according to the imaging findings (CT or MRI). Liver recurrence-free survival (LRFS) is defined as the time from liver resection to intrahepatic recurrence, irrespective of the presence of additional recurrences in other organs. Recurrence-free survival (RFS) is defined as the time from liver resection to any disease recurrence (i.e., intrahepatic recurrence and extrahepatic metastases). Overall survival (OS) is defined as the interval between the operation and the date of any cause of death. All the cases without end events for each prognostic outcome were censored at the date of the last follow-up.
Postoperative chemotherapy was administrated following the standard National Comprehensive Cancer guidelines. The postoperative chemotherapy was based on FOLFOX (oxaliplatin, leucovorin, and 5-fluorouracil) and CapeOX (oxaliplatin and capecitabine).
Statistical analysis
Statistical analysis was performed with software (IBM SPSS, version 26.0. Armonk, NY: IBM Corp). If the data followed a normal distribution, mean ± standard deviation was used, whereas medians with interquartile ranges (IQRs) were reported if not. The chi-square test or the Fisher exact test were used to assess categorical variables. The unpaired 2-tailed t test or the Mann-Whitney U test were used to assess continuous variables, depending on the pattern of distribution. Interobserver agreement for peritumoral imaging features was assessed with kappa statistics. Interobserver agreement was defined as poor (κ < 0.20), fair (κ = 0.20–0.39), moderate (κ = 0.40–0.59), substantial (κ = 0.60–0.79), or almost perfect (κ = 0.80–1.00). Survival rates were estimated using the Kaplan-Meier method and were compared by using the log-rank test. Baseline variables that were considered clinically relevant or that showed a univariate relationship with outcome (p values less than or equal to 0.1 in the univariable analysis) were entered into multivariate Cox proportional-hazards regression model and used the automated backward elimination regression. Two-sided p < 0.05 was considered statistically significant.
Result
Patient characteristics
A total of 272 patients underwent hepatectomy for CRLM during the study period. One hundred and seventy patients were excluded, leaving 56 for analysis, including 16 who underwent AR and 40 NAR. The location and number of CRLMs on preoperative MR examination were consistent with those on intraoperative ultrasonography, with a total of 86 lesions, and all of them were resected. All 56 patients received postoperative chemotherapy, and the median number of completed chemotherapy cycles was 5 (range, 1–10). Baseline characteristics of the study population are summarized in Table 1. There were no statistical significances between the two groups in term of clinical-pathologic characteristics (all p > 0.05; Table 1).
Interobserver agreement between the two observers regarding peritumoral imaging features was almost perfect for bile duct dilatation (κ= 0.876; 95% confidence interval [CI] 0.707, 1.000), substantial for peritumoral early enhancement (κ = 0.709; 95% CI 0.491, 0.928) and peritumoral hypointensity on HBP (κ = 0.752; 95% CI 0.520, 0.984).
The median follow-up periods were not different between the AR (40 months, IQR: 22–81 months) and NAR groups (58 months, IQR: 41–62 months; p = 0.483). 50.0% of patients (28/56) and 66.1% of patients (37/56) developed liver recurrence and systemic recurrence, respectively. 37.5% of deaths (21/56) occurred during the entire follow-up period. Intrahepatic recurrence was significantly less common in the AR group [18.8% of patients (3/16) in the AR group and 62.5% of patients (25/40) in the NAR group (p = 0.003; Table 1)]. A similar systemic recurrence rate was observed in 62.5% of patients (10/16) in the AR group and 67.5% of patients in the NAR group (p = 0.721; Table 1). A similar mortality rate was observed in 31.3% of patients (5/16) in the AR group and 40.0% of patients (16/40) in the NAR group (p = 0.541; Table 1).
Correlation between imaging and pathologic features
The results showed that the correlation between imaging features (i.e., peritumoral early enhancement, peritumoral hypointensity on HBP, and bile duct dilatation) and pathologic invasion was not statistically significant (all p > 0.05, Table 2).
Predictors of LRFS, RFS and OS in the overall cohort
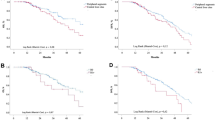
The 3-year LRFS rates were 78.6% and 36.6% in AR and NAR groups (p = 0.012), respectively. The 3-year RFS and OS rates were 41.7% and 81.3% in AR group, and 34.1% and 61.7% in NAR group (p = 0.794 and p = 0.302), respectively. Long-term prognosis of the AR group and NAR group is demonstrated in Figure 3.
On multivariable analysis, NAR (p = 0.022; hazard ratio [HR] = 4.402; 95% CI 1.240,15.633), abnormal liver function (p = 0.014; HR = 4.071; 95% CI 1.327, 12.484) poorly differentiated primary tumor (p = 0.007; HR = 4.071; 95% CI 1.327,12.484) and two or more CRLMs (p = 0.035; HR = 2.675; 95% CI 1.073,6.667) were independently associated with worse LRFS, poorly differentiated primary tumor (p = 0.003; HR = 3.332; 95% CI 1.504,7.380) , primary tumor located in colon (p = 0.006; HR = 3.828; 95% CI 1.468,9.979) and two or more CRLMs (p = 0.042; HR = 2.183; 95% CI 1.029,4.629) were independently associated with worse RFS (Table 3). There was no independent predictor for OS (Table 3).
Predictors of LRFS, RFS and OS in the AR and NAR groups
In NAR group, on multivariate analysis, two or more CRLMs (p= 0.005; HR =3.862; 95% CI 1.512,9.864) and peritumoral early enhancement (p < 0.001; HR = 6.260; 95% CI 2.322,16.876) were independently associated with worse LRFS, poorly differentiated primary tumor (p = 0.029; HR = 3.505; 95% CI 1.139,10.781), primary tumor located in colon (p = 0.040; HR = 3.386; 95% CI 1.060,10.817), the largest tumor size of 5 cm or larger (p = 0.036; HR = 8.822; 95% CI 1.152,67.570) and peritumoral early enhancement (p = 0.035, HR = 2.516; 95% CI, 1.069,5.919) were independently associated with worse RFS, poorly differentiated primary tumor (p = 0.048; HR = 3.594; 95% CI 1.014,12.739) and the largest tumor size of 5 cm or larger (p = 0.001; HR = 70.315; 95% CI 5.567,888.188) were independently associated with worse OS (Table 4). In contrast, there were no independent predictors of LRFS, RFS and OS in AR group (Table 5). In AR and NAR groups, bile duct dilatation and peritumoral hypointensity on HBP were not independent predictors of LRFS, RFS and OS.
Only 14 of 56 patients (5 of 16 and 9 of 40 in the AR and NAR group, respectively) presented with peritumoral early enhancement. All of 5 in the AR group had no intrahepatic recurrence and only 2 had systemic recurrence. However, all of 9 in the NAR group had intrahepatic recurrence. In NAR group, there was a significant difference in 3-year LRFS and RFS rates between patients with and without peritumoral early enhancement (LRFS: 11.1% vs. 44.4%, p = 0.001; RFS: 11.1% vs. 41.0%, p = 0.010; Fig. 4).
Discussion
Surgical resection can improve the survival of patients with CRLM, but the postoperative recurrence rate is high [8, 23]. On the basis of PSH principle, it remains a challenge to select the appropriate surgical procedure to reduce intrahepatic recurrence and maximize the functional liver remnant [12]. This study revealed that AR was associated with improved LRFS, although it did not correlate with either RFS or OS. Further, our analyses indicated that peritumoral early enhancement was a risk factor for LRFS and RFS in patients with NAR but not in patients with AR.
The influence of the surgical procedures on LRFS is undefined [11, 14, 15]. The underlying causes are the lack of clarity in the definition of AR, the failure to exclude patients receiving mixed AR and NAR, and the interchangeable use of the terms AR and non-PSH in the literature, which lead to confounding the results and limiting inter-study comparability [24]. We indicated that NAR was associated with an increased risk of liver recurrence compared with AR in patients undergoing PSH. Similar results have been reported by Finch et al. They also proposed that intrahepatic recurrence was significantly more common in the NAR group [25]. This could be because AR is associated with more extensive parenchymal resection, so coexisting micrometastases in the same lobe are removed [25]. Meanwhile, similar to previous studies, we also found that NAR did not present a disadvantage to the patients in terms of RFS and OS and poorly differentiated primary tumor was associated with worse RFS [25,26,27,28].
It was suggested that peritumoral imaging features with CRLM were predictors of long-term prognosis [19]. In our study, peritumoral early enhancement was associated with poor LRFS and poor RFS in the NAR group, although this predictive imaging feature was not demonstrated to be associated with a poor prognosis in the overall cohort. This is probably due to the fact that potentially ‘tumor-bearing’ portal tributaries cannot be removed by NAR [24, 29]. Thus, peritumoral early enhancement may be an important sign in surgical planning and may require resection of the potential "tumor-bearing" portal tributaries. Peritumoral early enhancement may be due to tumor compression of the surrounding hepatic parenchyma, portal veins and hepatic veins or portal vein obstruction caused by tumor cells, resulting in arterioportal shunt [30, 31]. Although there was a larger percentage of portal vein invasion in CRLM with peritumoral early enhancement, the difference did not reach statistical significance (p = 0.060). The study by Nakai et al. also did not demonstrate a correlation between peritumoral early enhancement and microvascular invasion, which was proved in hepatocellular carcinoma [19, 31, 32]. The reason may be that the imaging features and pathological features may not completely correspond, because our research is retrospective. Therefore, prospective research needs to be conducted to further explore the correlation between imaging features and pathological features.
Currently, it has been documented that biliary dilatation caused by liver metastases is usually the result of ductal invasion rather than biliary compression [33,34,35]. However, we did not find a correlation between bile duct dilatation and bile duct invasion. The reasons might lie not only in the fact that bile duct dilatation may be more often reflected in bile duct compression in our small sample study, but also in the aforementioned problem that pathological and imaging features do not correspond in one-to-one correspondence due to retrospective studies. In addition, bile duct dilatation was not an independent predictor of long-term prognosis in our study. The potential reason for this may be that no bile duct invasion was found in any of the lesions in our study where bile duct dilatation was present.
Based on the results of our study, we found that peritumoral hypointensity on HBP had no prognostic predictive effect, nor did we find it to be associated with pathologic features. Peritumoral hypointensity on HBP indicates that gadoxetic acid uptake in nontumorous liver parenchyma is reduced by decreased or dysfunctional hepatocytes due to arterioportal shunts, portal vein obstruction, bile duct obstruction, microvascular invasion of hepatocellular carcinoma, sinusoidal obstruction, focal eosinophilic infiltration, peliosis, fibrosis, inflammation, or any combination of these [36]. As many factors are capable of influencing peritumoral hypointensity on HBP, we have not found a correlation with pathologic features. What is more, we also found no association between peritumoral hypointensity on HBP and prognosis because not all of these factors were associated with prognosis.
There are some limitations in the present study. First, due to the exclusion of patients without preoperative gadoxetic acid-enhanced MRI and those who received preoperative treatment, the number of patients was small. Therefore, our results are supportive but not conclusive. Second, it is a retrospective analysis, which may have suffered from bias in selecting operative procedures and lack of accurate correspondence between imaging features and pathological features. In the future, further validation should be done through multicenter and prospective studies. Lastly, the number of patients with peritumoral early enhancement was limited in both groups, although the two groups showed significant differences in LRFS and RFS. Our data are not definitive but represent a preliminary result and further external validations are needed.
In conclusion, based on the PSH principle, AR has the potential to improve LRFS in patients with CRLM, although it did not show any improvement on RFS and OS. Meanwhile, peritumoral early enhancement with CRLM indicated poor LRFS and RFS in patients who had undergone NAR. Therefore, peritumoral early enhancement can be used as a reference indicator in the choice of surgical procedure and could further decrease the risk of postoperative recurrence.
Data availability
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Abbreviations
- CRLM:
-
Colorectal liver metastasis
- PSH:
-
Parenchymal-sparing hepatectomy
- AR:
-
Anatomic resection
- NAR:
-
Non-anatomic resection
- HBP:
-
Hepatobiliary phase
- MRI:
-
Magnetic resonance imaging
- CT:
-
Computed tomography
- MDT:
-
Multidisciplinary team
- CEA:
-
Carcinoembryonic antigen
- AFP:
-
Alpha-fetoprotein
- IQR:
-
Interquartile range
- TR:
-
Time of repetition
- TE:
-
Time of echo
- LAVA-XV:
-
Liver acquisition with volume acceleration-extended volume
- LRFS:
-
Liver recurrence-free survival
- RFS:
-
Recurrence-free survival
- OS:
-
Overall survival
- HR:
-
Hazard ratio
- CI:
-
Confidence interval
References
Hess KR, Varadhachary GR, Taylor SH et al. (2006) Metastatic patterns in adenocarcinoma. Cancer 106 (7):1624-1633. https://doi.org/10.1002/cncr.21778
Hackl C, Neumann P, Gerken M, Loss M, Klinkhammer-Schalke M, Schlitt HJ (2014) Treatment of colorectal liver metastases in Germany: a ten-year population-based analysis of 5772 cases of primary colorectal adenocarcinoma. BMC Cancer 14:810. https://doi.org/10.1186/1471-2407-14-810
Hellman S, Weichselbaum RR (1995) Oligometastases. J Clin Oncol 13 (1):8-10. https://doi.org/10.1200/jco.1995.13.1.8
Weichselbaum RR, Hellman S (2011) Oligometastases revisited. Nat Rev Clin Oncol 8 (6):378-382. https://doi.org/10.1038/nrclinonc.2011.44
Nordlinger B, Sorbye H, Glimelius B et al. (2013) Perioperative FOLFOX4 chemotherapy and surgery versus surgery alone for resectable liver metastases from colorectal cancer (EORTC 40983): long-term results of a randomised, controlled, phase 3 trial. Lancet Oncol 14 (12):1208-1215. https://doi.org/10.1016/s1470-2045(13)70447-9
Abdalla EK, Adam R, Bilchik AJ, Jaeck D, Vauthey JN, Mahvi D (2006) Improving resectability of hepatic colorectal metastases: expert consensus statement. Ann Surg Oncol 13 (10):1271-1280. https://doi.org/10.1245/s10434-006-9045-5
Poston GJ, Adam R, Alberts S et al. (2005) OncoSurge: a strategy for improving resectability with curative intent in metastatic colorectal cancer. J Clin Oncol 23 (28):7125-7134. https://doi.org/10.1200/jco.2005.08.722
Kopetz S, Chang GJ, Overman MJ et al. (2009) Improved survival in metastatic colorectal cancer is associated with adoption of hepatic resection and improved chemotherapy. J Clin Oncol 27 (22):3677-3683. https://doi.org/10.1200/jco.2008.20.5278
Sakai N, Furukawa K, Takayashiki T, Kuboki S, Takano S, Ohtsuka M (2021) Recurrence patterns and their effects on clinical outcomes after R1 resection of colorectal liver metastases: a propensity score-matched analysis. Langenbecks Arch Surg. https://doi.org/10.1007/s00423-021-02096-x
Mise Y, Aloia TA, Brudvik KW, Schwarz L, Vauthey JN, Conrad C (2016) Parenchymal-sparing Hepatectomy in Colorectal Liver Metastasis Improves Salvageability and Survival. Ann Surg 263 (1):146-152. https://doi.org/10.1097/sla.0000000000001194
Matsumura M, Mise Y, Saiura A et al. (2016) Parenchymal-Sparing Hepatectomy Does Not Increase Intrahepatic Recurrence in Patients with Advanced Colorectal Liver Metastases. Ann Surg Oncol 23 (11):3718-3726. https://doi.org/10.1245/s10434-016-5278-0
Andreou A, Gloor S, Inglin J et al. (2021) Parenchymal-sparing hepatectomy for colorectal liver metastases reduces postoperative morbidity while maintaining equivalent oncologic outcomes compared to non-parenchymal-sparing resection. Surg Oncol 38:101631. https://doi.org/10.1016/j.suronc.2021.101631
Strasberg SM (2005) Nomenclature of hepatic anatomy and resections: a review of the Brisbane 2000 system. J Hepatobiliary Pancreat Surg 12 (5):351-355. https://doi.org/10.1007/s00534-005-0999-7
DeMatteo RP, Palese C, Jarnagin WR, Sun RL, Blumgart LH, Fong Y (2000) Anatomic segmental hepatic resection is superior to wedge resection as an oncologic operation for colorectal liver metastases. J Gastrointest Surg 4 (2):178-184. https://doi.org/10.1016/s1091-255x(00)80054-2
Yasui K, Shimizu Y (2005) Surgical treatment for metastatic malignancies. Anatomical resection of liver metastasis: indications and outcomes. Int J Clin Oncol 10 (2):86-96. https://doi.org/10.1007/s10147-005-0475-z
Joechle K, Vreeland TJ, Vega EA et al. (2020) Anatomic Resection Is Not Required for Colorectal Liver Metastases with RAS Mutation. J Gastrointest Surg 24 (5):1033-1039. https://doi.org/10.1007/s11605-019-04299-6
Knijn N, de Ridder JA, Punt CJ, de Wilt JH, Nagtegaal ID (2013) Histopathological evaluation of resected colorectal cancer liver metastases: what should be done? Histopathology 63 (2):149-156. https://doi.org/10.1111/his.12124
Reijonen P, Österlund P, Isoniemi H, Arola J, Nordin A (2019) Histologically Verified Biliary Invasion was Associated with Impaired Liver Recurrence-Free Survival in Resected Colorectal Cancer Liver Metastases. Scand J Surg 108 (3):201-209. https://doi.org/10.1177/1457496918812237
Nakai Y, Gonoi W, Kurokawa R et al. (2020) MRI Findings of Liver Parenchyma Peripheral to Colorectal Liver Metastasis: A Potential Predictor of Long-term Prognosis. Radiology 297 (3):584-594. https://doi.org/10.1148/radiol.2020202367
Lok HT, Fung AKY, Chong CCN et al. (2021) Comparison of long-term survival outcome after curative hepatectomy between selected patients with non-colorectal and colorectal liver metastasis: A propensity score matching analysis. Asian J Surg 44 (2):459-464. https://doi.org/10.1016/j.asjsur.2020.10.019
Jiang Z, Li C, Zhao Z et al. (2018) Abnormal Liver Function Induced by Space-Occupying Lesions Is Associated with Unfavorable Oncologic Outcome in Patients with Colorectal Cancer Liver Metastases. Biomed Res Int 2018:9321270. https://doi.org/10.1155/2018/9321270
Fonseca GM, Herman P, Faraj SF et al. (2018) Pathological factors and prognosis of resected liver metastases of colorectal carcinoma: implications and proposal for a pathological reporting protocol. Histopathology 72 (3):377-390. https://doi.org/10.1111/his.13378
D'Angelica M, Kornprat P, Gonen M et al. (2011) Effect on outcome of recurrence patterns after hepatectomy for colorectal metastases. Ann Surg Oncol 18 (4):1096-1103. https://doi.org/10.1245/s10434-010-1409-1
Margonis GA, Buettner S, Andreatos N et al. (2017) Anatomical Resections Improve Disease-free Survival in Patients With KRAS-mutated Colorectal Liver Metastases. Ann Surg 266 (4):641-649. https://doi.org/10.1097/sla.0000000000002367
Finch RJ, Malik HZ, Hamady ZZ et al. (2007) Effect of type of resection on outcome of hepatic resection for colorectal metastases. Br J Surg 94 (10):1242-1248. https://doi.org/10.1002/bjs.5640
Lalmahomed ZS, Ayez N, van der Pool AE, Verheij J, JN IJ, Verhoef C (2011) Anatomical versus nonanatomical resection of colorectal liver metastases: is there a difference in surgical and oncological outcome? World J Surg 35 (3):656-661. https://doi.org/10.1007/s00268-010-0890-9
Wei AC, Greig PD, Grant D, Taylor B, Langer B, Gallinger S (2006) Survival after hepatic resection for colorectal metastases: a 10-year experience. Ann Surg Oncol 13 (5):668-676. https://doi.org/10.1245/aso.2006.05.039
Kato T, Yasui K, Hirai T et al. (2003) Therapeutic results for hepatic metastasis of colorectal cancer with special reference to effectiveness of hepatectomy: analysis of prognostic factors for 763 cases recorded at 18 institutions. Dis Colon Rectum 46 (10 Suppl):S22-31. https://doi.org/10.1097/01.Dcr.0000089106.71914.00
Shindoh J, Makuuchi M, Matsuyama Y et al. (2016) Complete removal of the tumor-bearing portal territory decreases local tumor recurrence and improves disease-specific survival of patients with hepatocellular carcinoma. J Hepatol 64 (3):594-600. https://doi.org/10.1016/j.jhep.2015.10.015
Terayama N, Matsui O, Ueda K et al. (2002) Peritumoral rim enhancement of liver metastasis: hemodynamics observed on single-level dynamic CT during hepatic arteriography and histopathologic correlation. J Comput Assist Tomogr 26 (6):975-980. https://doi.org/10.1097/00004728-200211000-00021
Nishie A, Yoshimitsu K, Asayama Y et al. (2008) Radiologic detectability of minute portal venous invasion in hepatocellular carcinoma. AJR Am J Roentgenol 190 (1):81-87. https://doi.org/10.2214/ajr.07.2810
Kim H, Park MS, Choi JY et al. (2009) Can microvessel invasion of hepatocellular carcinoma be predicted by pre-operative MRI? Eur Radiol 19 (7):1744-1751. https://doi.org/10.1007/s00330-009-1331-8
Zeng H, Shi G, Mai S, Liu H, Wu Z (2021) Imaging and clinical features of colorectal liver metastases with macroscopic intrabiliary growth. Eur J Radiol 137:109616. https://doi.org/10.1016/j.ejrad.2021.109616
Latorre Fragua RA, Manuel Vazquez A, Rodrigues Figueira Y et al. (2019) Intrabiliary metastases in colorectal cancer: a systematic review. J Hepatobiliary Pancreat Sci 26 (7):270-280. https://doi.org/10.1002/jhbp.635
Moon SG, Han JK, Kim TK, Kim AY, Kim TJ, Choi BI (2003) Biliary obstruction in metastatic disease: thin-section helical CT findings. Abdom Imaging 28 (1):45-52. https://doi.org/10.1007/s00261-001-0191-8
Lee Y, Kim SY, Lee SS et al. (2018) Pitfalls in Gd-EOB-DTPA-Enhanced Liver Magnetic Resonance Imaging With an Emphasis on Nontumorous Lesions. Clin Liver Dis (Hoboken) 12 (2):50-59. https://doi.org/10.1002/cld.723
Acknowledgements
Not applicable
Funding
This study was supported by the National Natural Scientific Foundation of China [Grant No. 81901909] and Program for Youth Innovation in Future Medicine, Chongqing Medical University [Grant No. W0096/03020202Y2021284].
Author information
Authors and Affiliations
Contributions
LL: collected, analyzed and interpreted the patient data regarding to survival, performed the statistical analysis and wrote the manuscript. LHX: collected the patient data and analyzed the patient data regarding to survival, performed the statistical analysis and wrote the manuscript. YZ: analyzed and interpreted the patient data. QSL: analyzed and interpreted the patient data. XL: performed the statistical analysis. HLT: was a major contributor in editing the manuscript. JMW: was a major contributor in editing the manuscript. XFZ: collected the patient data. PY: analyzed the patient data regarding to survival. YM: designed the study, interpreted the data, and was a major contributor in writing the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Conflict of interests
The authors declare that they have no competing interests.
Ethical approval
The study was reviewed and approved by the local ethics committee.
Consent for publication
Not applicable.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Liu, L., Xie, L., Zhou, Y. et al. Outcomes of different parenchymal-sparing hepatectomies in patients with colorectal liver metastases and prognostic impact of peritumoral imaging features. Abdom Radiol 48, 3728–3745 (2023). https://doi.org/10.1007/s00261-023-04044-3
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00261-023-04044-3