Abstract
Purpose
This study aimed to establish a nomogram based on preoperative magnetic resonance imaging (MRI) features to predict the very early recurrence (VER, less than 6 months) of intrahepatic mass-forming cholangiocarcinoma (IMCC) after R0 resection.
Methods
This study enrolled a group of 193 IMCC patients from our institution between March 2010 and January 2022. Patients were allocated into the development cohort (n = 137) and the validation cohort (n = 56), randomly, and the preoperative clinical and MRI features were collected. Univariate and multivariate stepwise logistic regression assessments were adopted to assess predictors of VER. Nomogram was constructed and certificated in the validation cohort. The performance of the prediction nomogram was evaluated by its discrimination, calibration, and clinical utility. The performance of the nomogram was compared with the T stage of the American Joint Committee on Cancer (AJCC) 8th edition staging system.
Results
Fifty-three patients (27.5%) experienced VER of the tumor and 140 patients (72.5%) with non-VER, during the follow-up period. After multivariate stepwise logistic regression, number of lesions, diffuse hypoenhancement on arterial phase, necorsis and suspicious lymph nodes were independently associated with VER. The nomogram demonstrated significantly higher area under the curve (AUC) of 0.813 than T stage (AUC = 0.666, P = 0.006) in the development cohort, whereas in the validation cohort, the nomogram showed better discrimination performance, with an AUC of 0.808 than T stage (0.705) with no significantly difference (P = 0.230). Decision curve analysis reflected the clinical net benefit of the nomogram.
Conclusion
The nomogram based on preoperative MRI features is a reliable tool to predict VER for patients with IMCC after R0 resection. This nomogram will be helpful to improve survival prediction and individualized treatment.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
The incidence of intrahepatic cholangiocarcinoma (iCCA) has been increasing worldwide over the last several decades [1]. Intrahepatic mass-forming cholangiocarcinoma (IMCC) subtype is the most common type of iCCA [2]. Hepatectomy is considered an effective and safe procedure for iCCA [3]. Even if a patient undergoes R0 resection, recurrence still occurs, and the 5-year survival rate ranges from 25 to 40% [4]. However, the high heterogeneity of iCCA makes it difficult to accurately identify the risk of recurrence and develop appropriate interventions for individuals. Generally, according to the time of relapse, recurrence patterns can be classified into two types: early recurrence (≤ 24 months) and late recurrence (> 24 months) [5, 6]. The prognosis of iCCA patients with early recurrence is worse, and the median overall survival time is lower than that of patients with late recurrence [5]. Very early recurrence (VER) is defined as recurrence within 6 months after the initial resection [7], and nearly 25% of iCCA patients experience it [5, 7]. Patients with VER are considered not very suitable for direct tumor operation, maybe as candidates for neoadjuvant systemic chemotherapy, or new clinical trials, alternative liver directed treatment options [8]. Consequently, accurate and early identification of patients who may develop VER is conductive to choosing the optimal treatment options which may delay the first recurrence time as much as possible [9].
Currently, the American Joint Committee on Cancer (AJCC) 8th edition staging system [10] is the most commonly used to guide treatment and predict the prognosis of patients with IMCC, but it can only be accurately evaluated after surgery. In addition, there may be significant prognostic differences between patients with the same pathological stage, indicating that other factors may also affect the prognosis of iCCA patients [11, 12]. Therefore, it is necessary to develop a non-invasive, early, and comprehensive evaluation method to estimate the possibility of a worse prognosis of IMCC before therapy.
Nowadays, magnetic resonance imaging (MRI) is the noninvasive diagnostic criterion for preoperative staging and surveillance of IMCC [13, 14]. Preliminary studies have found that some MRI features, such as the arterial phase (AP) enhancement patterns, and the area of diffusion restriction are associated with patient prognosis after R0 resection [15, 16]. Additionally, nomograms based on MRI features have been reported as useful methods in prognostic evaluation methods for iCCA [17, 18]. We hypothesised that MRI features might have a better predictive value for VER in IMCC patients after R0 resection, which might be hopeful in developing surgical strategies and follow-up plans.
In this context, this study aimed to establish a comprehensive preoperative MRI-based nomogram to predict VER more accurately and to compare its predictive ability with that of the postoperative AJCC 8th edition staging system for IMCC patients who undergo R0 resection.
Materials and methods
This retrospective study was approved by the ethics committee of our institution, and written informed consent of patients was waived.
Patients
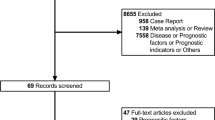
We analyzed the data from patients who consecutively underwent hepatectomy for IMCC at our institution between March 2010 and January 2022. Patients were included according to the following criteria: (1) IMCC was pathologically diagnosed after hepatectomy. (2) All patients underwent contrast-enhanced (CE) abdominal MRI examination before surgery. The exclusion criteria were: (1) Administration of preoperative neoadjuvant therapies (NT) including chemotherapy, concurrent chemoradiation therapy, etc (n = 17). (2) With a history of other malignant tumors (n = 24). (3) Loss to follow-up within 6 months (n = 16). (4) Resection margin status of specimens were non-R0 (n = 10). (5) Poor image quality (n = 4). The two groups were randomly assigned with a ratio of 7:3. Fig. 1 shows the workflow of the patients inclusion and exclusion criteria. The mean time interval between surgery and MRI examination was 15.4 days. Each patient was then categorised into the VER group or non-VER group with a 6-month interval of recurrence.
Liver MR technique
MR examinations were performed using 3.0-T scanner devices with a phased-array coil (Discovery MR 750, SIGNA Excite HDx, or SIGNA Pioneer, GE Healthcare; and SIGNA Architect, Philips Healthcare). Clinical routine MRI sequences were performed, including T2-weighted imaging (T2WI) on the coronal, T1-weighted in-phase and opposed-phase imaging, T2WI fat-suppressed (FS), diffusion-weighted imaging (DWI) with two b values (0 and 800 s/mm2), pre-contrast, and contrast-enhanced (CE) T1-weighted imaging (T1WI) on the axial. one of the following contrast agents was injected into the patient: gadoxetate disodium (Gd-EOB-DTPA, Primovist®, Bayer HealthCare) at a rate of 1 mL/s or gadopentetate dimeglumine (Gd-DTPA, Magnevist®, Bayer HealthCare) at a rate of 2 mL/s were administered, followed by 20 mL of 0.9% saline flush at a rate of 2 mL/s. AP, portal venous phase (PVP), and delayed phase (DP, or transitional phase with gadoxetate disodium) of CE T1WI were acquired at 20–35 s, 70–90 s, and 170–200 s after contrast material administration. Hepatobiliary phase (HBP) imaging using gadoxetate disodium was available in 35 patients and obtained 15–20 min after injection. For CE imaging, coronal CE T1WI was provided for clinical diagnosis at last. The MRI acquisition protocol is provided in the Supplementary Table S1.
Clinical data collection and imaging records
Preoperative factors were collected, including age, sex, body mass index (BMI), hepatitis B surface antigen (HBsAg), tumor markers (alpha-fetoprotein [AFP], carcinoembryonic antigen [CEA], and carbohydrate antigen [CA] 19-9), routine blood tests (total bilirubin, alkaline phosphatase, and aspartate aminotransferase), and MRI features. The MRI features included the tumor size (≤ 5 cm vs. > 5 cm), number of lesions (= 1 vs. > 1), tumor location (peripheral vs. perihilar), signal homogeneity (homogeneous vs. heterogeneous), AP enhancement patterns (diffuse hyperenhancement vs. rim-enhancement vs. diffuse hypoenhancement), target appearance at DWI (absent vs. present), necrosis (absent vs. present), tumor margin (well-defined vs. ill-defined), peritumoral enhancement (absent vs. present), suspicious lymph nodes (negative vs. positive), hepatic capsule retraction (absent vs. present), and bile duct dilatation (absent vs. present). Diagnostic criteria were introduced in Supplementary Table S2. All MRI features were re-evaluated by two radiologists with 6 and 11 years of liver imaging interpretation experience, respectively. The images were anonymized and randomly distributed to the radiologists. Both radiologists were unknown the demographic, clinicopathologic, and the follow-up results, but were aware that this study was regarding IMCC. The final judgment was made by a chief radiologist with 20 years of experience in case of a disagreement between the first two radiologists. In cases with multiple lesions, the features were recorded in the largest one.
Surgical management and histopathologic analysis
Liver resection of three or more segments was defined as major resection. Lymphadenectomy is not always performed in patients with IMCC, especially in those without suspicious metastatic lymph nodes based on preoperative imaging and intra-operative findings. Whether the patient needs adjuvant treatment after surgery depends on the pathological staging of the tumor, the patient's physical condition, and the patient's willingness to decide. Postoperative treatment methods include radiotherapy, chemotherapy, immunotherapy, and targeted therapy, single or multiple combinations. Hepatectomy specimens from each IMCC patient were viewed microscopically. Formalin-fixed paraffin-embedded tissue blocks were sectioned at 5 μ and stained with hematoxylin and eosin. All surgical specimens were re-staged by professional pathologists with more than 25 years of experience in the interpretation of liver lesions according to the AJCC 8th edition staging system [19].
Follow up protocol
Patients were postoperatively followed up with radiographic observations and serum tumor markers every 3–6 months for the first two years and then annually or symptomatic. The endpoint of this study was VER, which was defined as tumor recurrence occurring within 6 months after hepatectomy and determined according to the findings of pathological confirmation (surgery or biopsy) or typical imaging features.
Statistical analysis
Fisher's exact test or Chi-square and two-sample independent t-test or Mann–Whitney U test were used for analyzing the significance across the demographic, clinicopathologic and MRI features. Cohen's kappa statistics were used to evaluate the interobserver agreement for the imaging features. The agreement levels of kappa were explained as follows: almost perfect, 0.81–1.0; good, 0.61–0.80; moderate, 0.41–0.60; fair, 0.21–0.40; and slight, < 0.20 [20]. Univariate and multivariate stepwise logistic regression assessments were carried out to filtrate the predictive factors. Next, the independent predictors were subsequently incorporated into the establishment of the nomogram. Receiver operating characteristic (ROC) curve, calibration curve and decision curve analysis (DCA) were performed to assess the discrimination efficiency of the nomogram. DeLong’s test was used to compare the difference of area under ROC curves (AUCs). A 2-tailed P < 0.05 was considered statistically significant. SPSS Statistics software (version 26.0, Chicago, IL, United States) and R version 4.0.3 for Windows (32/64 bit) were used for data management and analyses.
Results
Patient characteristics and postsurgical outcome
The whole cohort included 193 patients with IMCC who underwent R0 resection. One hundred and thirty-seven patients in the development cohort (81 males and 56 females, mean age of 58.9 years, range 36–80 years) and 56 patients in the validation cohort (35 males and 21 females, mean age of 59.7 years, range 41–76 years). A total of 53 patients (27.5%) had VER at the last follow-up visit. There was no significant difference in the VER rate between the two cohorts (36/137 vs. 17/56, P = 0.925). According to the surgical findings, 67 (34.7%) patients underwent major hepatectomy. Among 193 patients, 75 (38.9%) participants received completed adjuvant therapy after surgery. Tumor stage was T1 in 91 patients, T2 in 53 patients, T3 in 32 patients, and T4 in 17 patients. The TNM stage data were missing in 61 (31.6%) patients due to the unavailability of lymph node status. The remaining 132 patients underwent lymphadenectomy along with liver resection, and 86 participants showed no evidence of lymph node metastasis (N0), 46 participants (45%) had N1. Comparisons of the demographic, clinicopathological, and MRI features between the development and validation cohorts were shown in Tables 1 and 2. No significant differences were observed between the two cohorts (all P > 0.05).
Development and validation of a VER-predicting nomogram
In the univariate analysis, CA19-9 (P = 0.042), tumor size (P = 0.026), number of lesions (P = 0.002), diffuse hypoenhancement on AP (P = 0.010), necrosis (P < 0.001), and suspicious lymph nodes (P < 0.001) were significantly related to VER. Then, these variables were adopted into the multivariate stepwise analysis, which showed that the number of lesions (odds ratio [OR] = 3.452, 95% confidence interval [CI] 1.202–9.915, P = 0.021), diffuse hypoenhancement on AP (OR = 15.042, 95% CI 1.387–163.169, P = 0.026), necrosis (OR = 2.749, 95% CI 1.120–6.745, P = 0.027), and suspicious lymph nodes (OR = 2.471, 95% CI 1.012–6.029, P = 0.047) remained as independent predictive factors for VER (Table 3). A predictive nomogram was established using the findings of multivariate stepwise logistic regression (Fig. 2).
A regression coefficient-based nomogram for predicting very early recurrence (VER) in patients with IMCC. Draw a line perpendicular from the corresponding axis of each parameter until it reaches the top line marked “Points”. Sum up the number of points for all parameters then draw a line descending from the axis marked “Total Points” until it reaches the bottom line to determine the probability of VER. For arterial phase (AP) enhancement pattern, “Pattern 1” refers to diffuse hyperenhancement, “Pattern 2” refers to rim-enhancement, and “Pattern 3” refers to is diffuse hypoenhancement. AP arterial phase, VER very early recurrence.
Comparisons of AUC values of the nomogram and the AJCC 8th edition staging system
This nomogram had a favorable diagnostic performance of the VER, with an AUC of 0.813 in the development cohort (Fig. 3a). Good performance was also observed in the validation cohort (Fig. 3b), with an AUC of 0.808. Specifically, the nomogram based on the development cohort showed improvements compared with that based on the T stage, which had the AUCs of 0.666 in the development cohort and 0.705 in the validation cohort, respectively. The other diagnostic performance results are described in Table 4. The nomogram performed significantly better than the T stage in the development cohort (P = 0.006), but with no significant difference in the validation cohort (P = 0.230) in terms of the prediction of VER using the DeLong’s test. The Hosmer–Lemeshow test demonstrated that the nomogram model had a good fit in the development and validation cohorts (P = 0.875 and 0.453, respectively) and the calibration curve showed that the probability of VER predicted by the nomogram was in good agreement with the actual probability (Fig. 4). Moreover, the DCA also showed that the nomogram added more net benefit than the T stage, with threshold rates ranging from 0 to 55%, and from 0 to 80%, respectively (Fig. 5). Two representative clinical examples of nomogram applications are shown in Fig. 6.
ROC curve of each model. Comparison of the values of area under the curve between nomogram (red line) and T stage (blue line) of American Joint Committee on Cancer (AJCC) 8th edition staging system in the development cohort (a) and validation cohort (b). TNM tumor-node-metastasis, AJCC American Joint Committee on Cancer
Decision curve for the nomogram predicting the VER in the development (a) and validation (b) cohorts. The y-axis measures the net benefit, and the x-axis is the threshold probability. The grey line is the net benefit of assuming that all patients have VER; the horizontal black line is the net benefit of assuming no patients have VER; and the red line is expected net benefit of per patient based on the predictive nomogram. The nomogram (red line) received a higher net benefit than T stage (blue line) of American Joint Committee on Cancer (AJCC) 8th edition staging system.
Two representative clinical examples of nomogram applications. Magnetic resonance imaging (MRI) findings for a representative case involving a 62-year-old man exhibiting intrahepatic mass-forming cholangiocarcinoma (IMCC) (a–c). a On T2-weighted imaging fat suppressed (T2WI-FS) imaging, there is a hyperintense mass in the left lateral section with multiple satellite nodules (arrowhead, approximately 46 points). b On the arterial phase (AP), the nodule shows diffuse hypoenhancement (100 points). Necrosis, bright signal intensity foci on T2WI-FS without enhancement, is shown in (a) and (b) (star, approximately 38 points). c Suspicious lymph nodes were observed on T2WI-FS of preoperative MRI (arrowhead, approximately 35 points). Hence, a total score of 219 points was obtained, which corresponds to a probability of very early recurrence nearly 90% according to the lower scale of the nomogram. Follow-up computed tomography 3.5 months after surgery shows intrahepatic tumor recurrence (d, arrowhead). A 47-year-old woman of IMCC without very early recurrence (VER) confirmed by postoperative pathological examination (e–f). e On T2WI-FS, there is a single hyperintense lesion in the caudate lobe of liver (arrowhead). f On the AP, the nodule shows diffuse hyperenhancement (arrowhead, 0 points). No satellite nodule, intrahepatic metastasis, necrosis or suspicious lymph nodes was observed on any sequence of preoperative MRI (all 0 points). Therefore, a total score of 0 points was obtained, which corresponds to a probability of VER around 1% according to the lower scale of the nomogram. In this patient, tumor recurrence was not detected during the 34-month follow-up from the date of surgery.
Interobserver agreement for MRI features
We analyzed the interobserver agreement (Cohen’s kappa) for each MRI feature between the two radiologists. For the variables included in the nomogram, the agreement was good to almost perfect for the number of lesions (0.865 and 0.825 for the development and validation cohorts, respectively), AP enhancement patterns (0.799 and 0.748), necrosis (0.732 and 0.755), and suspicious lymph nodes (0.700 and 0.681). The interobserver agreement for the other imaging factors is shown in Supplementary Table S3.
Discussion
In the current study, we developed and validated a nomogram based on MRI features to predict VER in individuals with IMCC in a preoperative setting. The results demonstrated that the presence of number of lesions (P = 0.021), diffuse hypoenhancement on AP (P = 0.026), necrosis (P = 0.027) and suspicious lymph nodes (P = 0.047) were independent risk factors for predicting VER after R0 resection in patients with IMCC. To obtain a better predictive ability than that of a single imaging finding, we integrated these factors into a nomogram, which is a simple multivariate visualization tool used in oncology [21]. The nomogram was able to better predicte VER among patients with IMCC than the T stage of the AJCC 8th edition staging system, and demonstrated good predictive efficiency and clinical utility.
The study of VER has been applied to a variety of malignant liver tumors, such as hepatocellular carcinoma and combined hepatocellular-cholangiocarcinoma [22, 23], and the prognosis of patients with VER is more aggressive and worse. As for iCCA patients, the median overall survival (OS) time among patients with and without VER was 13.8 months and 59.7 months, respectively (P < 0.001) [7]. As the utilization of NT has increased over time for resectable iCCA [24, 25], some scholars have proposed that NT can be provided to patients at risk of VER to prolong their survival and prognosis [8]. In a study by Tsilimigras et al. [7], a nomogram constructed based on preoperative factors, including age, race, cirrhosis, tumor size, tumor numbers, and imaging lymph node metastases, was independently associated with VER, with an AUC ranging from 0.710 to 0.750. However, the preoperative imaging features of the tumors have not been fully evaluated.
Number of lesions is an independent predictor of VER. These findings agree with previous studies in which multiple tumors were related to a higher recurrence rate and shorter survival time of iCCA [26, 27]. In a large study of 880 iCCA patients, Tsilimigras et al. [7] demonstrated that the number of lesions is a reliable predictor for VER in iCCA. In addition, the number of lesions may help to guide preoperative and postoperative treatment strategies for patients.
Variable AP enhancement patterns can be seen on postcontrast MRI images, and the most common of IMCC is rim-enhancement pattern [14]. In the current study, we demonstrated that diffuse hypoenhancement was a risk factor that increased the probability of VER. Consistent with a recent study, Min et al [15] found that the trend of prognosis improvement was increasing from the hypoenhancement group to the rim enhancement group and then to the hyperenhancement group. Besides, on histopathology, the diffuse hypoenhancement on AP may be mainly caused by abundant necrosis and stromal fibrosis [28], both of which play an important roles in promoting enhanced malignant behavior, therapeutic resistance and poor prognosis [29, 30].
Our study shows that necrosis is a predictor for VER. A common assumption for the development of tumor necrosis is that rapid growth of malignant cells, especially in more aggressive cancer types, exceeds its own blood supply and then produces hypoxia conditions, leading to tissue necrosis areas [31]. Tsilimigras et al noted that tumor necrosis was related to shorter recurrence-free survival in patients with T1 stage iCCA [32], indicating that this evaluation might be used to guide clinical decision making, routinely.
Lymph node metastasis has already been reported as an extremely poor prognostic risk factor after curative resection for iCCA [33, 34]. Besides, suspicious lymph node on imaging was associated with actual positive lymph nodes, which is commonly recognized and acceptted in AJCC TNM staging systems [35]. In additiona, our results showed that preoperative suspicious lymph node was an independent predictor of VER, which was consistent with previous study [7]. This result suggested that suspicious lymph nodes could serve as a convenient biomarker for the prediction of invasive biological behavior that provide prognostic information.
In addition, the results of univariate analysis showed that increasing CA 19-9 and tumor size are associated with the occurrence of VER as well, whereas they were not independent factors. High serum CA19-9 and large tumor size are reflected the tumor burden in some studies, and is associated with poor OS and proposed as a component of prognostic models [33, 36, 37].
Our study has some limitations that are worth noting. First, it is possible that the long-term retrospective research design in a single-institution has caused selection bias. Second, the contrast agents and MRI protocols used in the current study were different. However, the heterogeneity of the MRI protocol may strengthen the generalizability, as it reflects the actual clinical practice. Third, subsequent treatments after surgery may play a critical role in the occurrence of VER, however, because of its complexity and diversity, the detail of treatments was not included in the analysis of the study. Forth, in our study, 61 (31.6%) patients did not undergo lymphadenectomy, making it difficult to accurately evaluate lymph node status (Nx). Lymphadenectomy is not always performed, especially in those without suspicious metastatic lymph nodes based on preoperative imaging. Considering the inevitable limitation, we have adopted the variable of preoperative suspicious lymph node as a substitute for lymph node metastasis. Finally, although the proposed predictive nomogram performed well on the validation cohort in the same institution, external validation is still needed.
Conclusion
In conclusion, our study presents a predictive nomogram that incorporates number of lesions, diffuse hypoenhancement on AP, necrosis, and suspicious lymph nodes, which can be conveniently used to facilitate the preoperative individualized prediction of VER and may become a tool to guide individual management in patients with IMCC.
Abbreviations
- AFP:
-
Alpha-fetoprotein
- AJCC:
-
American Joint Committee on Cancer
- AP:
-
Arterial phase
- AUC:
-
Area under the curve
- CA 19-9:
-
Cancer antigen 19-9
- CEA:
-
Carcinoembryonic antigen
- CI:
-
Confidence interval
- IMCC:
-
Intrahepatic mass-forming cholangiocarcinoma
- NT:
-
Neoadjuvant therapy
- OR:
-
Odds ratio
- ROC:
-
Receiver operating characteristic
Reference
Bertuccio P, Malvezzi M, Carioli G, Hashim D, Boffetta P, El-Serag H B, La Vecchia C and Negri E, Global trends in mortality from intrahepatic and extrahepatic cholangiocarcinoma. J Hepatol 2019; 71: 104-114.
Kendall T, Verheij J, Gaudio E, Evert M, Guido M, Goeppert B and Carpino G, Anatomical, histomorphological and molecular classification of cholangiocarcinoma. Liver Int 2019; 39 Suppl 1: 7-18.
Littau M J, Kim P, Kulshrestha S, Bunn C, Tonelli C, Abdelsattar Z M, Luchette F A and Baker M S, Resectable intrahepatic and hilar cholangiocarcinoma: Is margin status associated with survival? Surgery 2022; 171: 703-710.
Mazzaferro V, Gorgen A, Roayaie S, Dit Busset M D and Sapisochin G, Liver resection and transplantation for intrahepatic cholangiocarcinoma. J Hepatol 2020; 72: 364-377.
Zhang X F, Beal E W, Bagante F, Chakedis J, Weiss M, Popescu I, Marques H P, Aldrighetti L, Maithel S K, Pulitano C, Bauer T W, Shen F, Poultsides G A, Soubrane O, Martel G, Koerkamp B G, Itaru E and Pawlik T M, Early versus late recurrence of intrahepatic cholangiocarcinoma after resection with curative intent. Br J Surg 2018; 105: 848-856.
Zhao L, Ma X, Liang M, Li D, Ma P, Wang S, Wu Z and Zhao X, Prediction for early recurrence of intrahepatic mass-forming cholangiocarcinoma: quantitative magnetic resonance imaging combined with prognostic immunohistochemical markers. Cancer Imaging 2019; 19: 49.
Tsilimigras D I, Sahara K, Wu L, Moris D, Bagante F, Guglielmi A et al., Very Early Recurrence After Liver Resection for Intrahepatic Cholangiocarcinoma: Considering Alternative Treatment Approaches. JAMA Surg 2020; 155: 823-831.
Tsilimigras D I and Pawlik T M, Preoperative Model and Patient Selection for Neoadjuvant Therapy for Intrahepatic Cholangiocarcinoma-Reply. JAMA Surg 2021; 156: 395-396.
Yadav S, Xie H, Bin-Riaz I, Sharma P, Durani U, Goyal G, Borah B, Borad M J, Smoot R L, Roberts L R, Go R S, McWilliams R R and Mahipal A, Neoadjuvant vs. adjuvant chemotherapy for cholangiocarcinoma: A propensity score matched analysis. Eur J Surg Oncol 2019; 45: 1432-1438.
Chun Y S, Pawlik T M and Vauthey J N, 8th Edition of the AJCC Cancer Staging Manual: Pancreas and Hepatobiliary Cancers. Ann Surg Oncol 2018; 25: 845-847.
Sasaki K, Margonis G A, Andreatos N, Chen Q, Barbon C, Bagante F et al., Serum tumor markers enhance the predictive power of the AJCC and LCSGJ staging systems in resectable intrahepatic cholangiocarcinoma. HPB (Oxford) 2018; 20: 956-965.
Buettner S, Galjart B, van Vugt J L A, Bagante F, Alexandrescu S, Marques H P et al., Performance of prognostic scores and staging systems in predicting long-term survival outcomes after surgery for intrahepatic cholangiocarcinoma. J Surg Oncol 2017; 116: 1085-1095.
Granata V, Grassi R, Fusco R, Setola S V, Belli A, Ottaiano A, Nasti G, La Porta M, Danti G, Cappabianca S, Cutolo C, Petrillo A and Izzo F, Intrahepatic cholangiocarcinoma and its differential diagnosis at MRI: how radiologist should assess MR features. Radiol Med 2021; 126: 1584-1600.
Jhaveri K S and Hosseini-Nik H, MRI of cholangiocarcinoma. J Magn Reson Imaging 2015; 42: 1165-79.
Min J H, Kim Y K, Choi S Y, Kang T W, Lee S J, Kim J M, Ahn S and Cho H, Intrahepatic Mass-forming Cholangiocarcinoma: Arterial Enhancement Patterns at MRI and Prognosis. Radiology 2019; 290: 691-699.
Promsorn J, Eurboonyanun K, Chadbunchachai P, Apivatanasiri C, Wirasorn K, Chindaprasirt J, Sookprasert A and Harisinghani M, Diffusion-weighted imaging as an imaging biomarker for assessing survival of patients with intrahepatic mass-forming cholangiocarcinoma. Abdom Radiol (NY) 2022; 47: 2811-2821.
Liang W, Xu L, Yang P, Zhang L, Wan D, Huang Q, Niu T and Chen F, Novel Nomogram for Preoperative Prediction of Early Recurrence in Intrahepatic Cholangiocarcinoma. Front Oncol 2018; 8: 360.
Sheng R, Huang X, Jin K, Gao S, Zeng M, Wu D and Shi G, Contrast-enhanced MRI could predict response of systemic therapy in advanced intrahepatic cholangiocarcinoma. Eur Radiol 2022; 32: 5156-5165.
Lee A J and Chun Y S, Intrahepatic cholangiocarcinoma: the AJCC/UICC 8th edition updates. Chin Clin Oncol 2018; 7: 52.
Hallgren K A, Computing Inter-Rater Reliability for Observational Data: An Overview and Tutorial. Tutor Quant Methods Psychol 2012; 8: 23-34.
Balachandran V P, Gonen M, Smith J J and DeMatteo R P, Nomograms in oncology: more than meets the eye. Lancet Oncol 2015; 16: e173-80.
Wu Y, Liu H, Zeng J, Chen Y, Fang G, Zhang J, Zhou W, Zeng Y and Liu J, Development and validation of nomogram to predict very early recurrence of combined hepatocellular-cholangiocarcinoma after hepatic resection: a multi-institutional study. World J Surg Oncol 2022; 20: 60.
Hirokawa F, Hayashi M, Asakuma M, Shimizu T, Inoue Y and Uchiyama K, Risk factors and patterns of early recurrence after curative hepatectomy for hepatocellular carcinoma. Surg Oncol 2016; 25: 24-9.
Mason M C, Massarweh N N, Tzeng C D, Chiang Y J, Chun Y S, Aloia T A, Javle M, Vauthey J N and Tran Cao H S, Time to Rethink Upfront Surgery for Resectable Intrahepatic Cholangiocarcinoma? Implications from the Neoadjuvant Experience. Ann Surg Oncol 2021; 28: 6725-6735.
Utuama O, Permuth J B, Dagne G, Sanchez-Anguiano A, Alman A, Kumar A, Denbo J, Kim R, Fleming J B and Anaya D A, Neoadjuvant Chemotherapy for Intrahepatic Cholangiocarcinoma: A Propensity Score Survival Analysis Supporting Use in Patients with High-Risk Disease. Ann Surg Oncol 2021; 28: 1939-1949.
Jin K P, Sheng R F, Yang C and Zeng M S, Combined arterial and delayed enhancement patterns of MRI assist in prognostic prediction for intrahepatic mass-forming cholangiocarcinoma (IMCC). Abdom Radiol (NY) 2022; 47: 640-650.
King M J, Hectors S, Lee K M, Omidele O, Babb J S, Schwartz M, Tabrizian P, Taouli B and Lewis S, Outcomes assessment in intrahepatic cholangiocarcinoma using qualitative and quantitative imaging features. Cancer Imaging 2020; 20: 43.
Baheti A D, Tirumani S H, Rosenthal M H, Shinagare A B and Ramaiya N H, Diagnosis and management of intrahepatic cholangiocarcinoma: a comprehensive update for the radiologist. Clin Radiol 2014; 69: e463-70.
Sulpice L, Rayar M, Desille M, Turlin B, Fautrel A, Boucher E, Llamas-Gutierrez F, Meunier B, Boudjema K, Clément B and Coulouarn C, Molecular profiling of stroma identifies osteopontin as an independent predictor of poor prognosis in intrahepatic cholangiocarcinoma. Hepatology 2013; 58: 1992-2000.
Atanasov G, Dietel C, Feldbrügge L, Benzing C, Krenzien F, Brandl A, Mann E, Englisch J P, Schierle K, Robson S C, Splith K, Morgul M H, Reutzel-Selke A, Jonas S, Pascher A, Bahra M, Pratschke J and Schmelzle M, Tumor necrosis and infiltrating macrophages predict survival after curative resection for cholangiocarcinoma. Oncoimmunology 2017; 6: e1331806.
Atanasov G, Schierle K, Hau H M, Dietel C, Krenzien F, Brandl A, Wiltberger G, Englisch J P, Robson S C, Reutzel-Selke A, Pascher A, Jonas S, Pratschke J, Benzing C and Schmelzle M, Prognostic Significance of Tumor Necrosis in Hilar Cholangiocarcinoma. Ann Surg Oncol 2017; 24: 518-525.
Tsilimigras D I, Ejaz A, Cloyd J, Guglielmi A, Aldrighetti L, Weiss M, Bauer T W, Alexandrescu S, Poultsides G A, Maithel S K, Marques H P, Martel G, Pulitano C, Shen F, Soubrane O, Koerkamp B G, Endo I and Pawlik T M, Tumor Necrosis Impacts Prognosis of Patients Undergoing Resection for T1 Intrahepatic Cholangiocarcinoma. Ann Surg Oncol 2022. https://doi.org/10.1245/s10434-022-11462-y
Wang Y, Li J, Xia Y, Gong R, Wang K, Yan Z, Wan X, Liu G, Wu D, Shi L, Lau W, Wu M and Shen F, Prognostic nomogram for intrahepatic cholangiocarcinoma after partial hepatectomy. J Clin Oncol 2013; 31: 1188-95.
Hyder O, Marques H, Pulitano C, Marsh J W, Alexandrescu S, Bauer T W, Gamblin T C, Sotiropoulos G C, Paul A, Barroso E, Clary B M, Aldrighetti L, Ferrone C R, Zhu A X, Popescu I, Gigot J F, Mentha G, Feng S and Pawlik T M, A nomogram to predict long-term survival after resection for intrahepatic cholangiocarcinoma: an Eastern and Western experience. JAMA Surg 2014; 149: 432-8.
Amin M B, Greene F L, Edge S B, Compton C C, Gershenwald J E, Brookland R K, Meyer L, Gress D M, Byrd D R and Winchester D P, The Eighth Edition AJCC Cancer Staging Manual: Continuing to build a bridge from a population-based to a more "personalized" approach to cancer staging. CA Cancer J Clin 2017; 67: 93-99.
Zhou S N, Lu S S, Ju D W, Yu L X, Liang X X, Xiang X, Liangpunsakul S, Roberts L R, Lu Y Y and Zhang N, A New Prognostic Model Covering All Stages of Intrahepatic Cholangiocarcinoma. J Clin Transl Hepatol 2022; 10: 254-262.
Rhee H, Choi S H, Park J H, Cho E S, Yeom S K, Park S, Han K, Lee S S and Park M S, Preoperative magnetic resonance imaging-based prognostic model for mass-forming intrahepatic cholangiocarcinoma. Liver Int 2022; 42: 930-941.
Funding
No funding was received for conducting this study.
Author information
Authors and Affiliations
Contributions
Study concepts/study design: SC, HZ; data acquisition or data analysis/interpretation: LW, RZ, WP; statistical analysis: SC, LW, RZ; drafting the article or revising it critically for important intellectual content: SC, SZ, HZ; final approval of the version to be submitted: SC, LW, RZ, WP, SZ, HZ authors.
Corresponding author
Ethics declarations
Conflict of interest
The authors have no relevant financial or non-financial interests to disclose.
Ethical approval
This study was approved by the institutional review board in view of the retrospective nature of the study.
Informed consent
The requirement for informed consent was waived
Publication
This article is not under consideration for publication elsewhere.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Chen, S., Wan, L., Zhao, R. et al. Preoperative MRI features predicting very early recurrence of intrahepatic mass-forming cholangiocarcinoma after R0 resection: a comparison with the AJCC 8th edition staging system. Abdom Radiol 49, 21–33 (2024). https://doi.org/10.1007/s00261-023-04038-1
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00261-023-04038-1