Abstract
Purpose
To compare the imaging features, pathologic characteristics, and survival outcomes between subcentimeter and 1–2 cm hepatocellular carcinoma (HCC).
Methods
This retrospective observational study evaluated the imaging features and medical records of patients with HCC smaller than 2 cm who underwent surgical resection with preoperative gadoxetic-acid-enhanced MRI (EOB-MRI) from January 2013 to December 2021. The incidence of EOB-MRI features and pathological characteristics between the subcentimeter and 1–2 cm HCC were compared. The recurrence-free survival (RFS), including early and overall tumor recurrence, and overall survival (OS) were assessed.
Results
A total of 223 patients (82 with subcentimeter HCC and 141 with 1–2 cm HCC, 179 men) were enrolled. Compared with 1–2 cm HCC, subcentimeter HCC showed fewer restricted diffusion (87.8 vs. 95.7%, P = 0.027), portal-phase washout (58.5% vs. 73.8%, P = 0.013), typical enhancement pattern (50.0% vs. 66.7%, P =0.014), and microvascular invasion (4.9% vs. 14.9%, P = 0.022). Patients with subcentimeter HCC had higher RFS (P = 0.027) and better OS (P = 0.029). The estimated RFS rates at 5 years was 83.3% for subcentimeter HCC and 67.3% for 1–2 cm HCC, respectively. The estimated OS rates at 5 years was 97.3% for subcentimeter HCC and 89.5% for 1–2 cm HCC, respectively.
Conclusion
Subcentimeter HCC showed less frequent EOB-MRI features seen typically in 1–2 cm HCC but better survival outcomes. Therefore, tailored early diagnostic criteria and immediate treatment for subcentimeter HCC may be warranted.
Graphical abstract

Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Hepatocellular carcinoma (HCC) is the most common primary liver cancer and the third leading cause of cancer-related death worldwide [1]. Due to the widespread utilization of surveillance programs for patients with high-risk factors (e.g., patients with chronic hepatitis B or cirrhosis) and advancements in magnetic resonance imaging (MRI) technique, including the widely usage of gadoxetic-acid-enhanced MRI (EOB-MRI), small HCC has become an increasingly frequent issue in clinical practice. Because patients with early-stage HCC have a better prognosis, early diagnosis and treatment for HCC larger than 1 cm were recommended by all guidelines [2].
However, the management of subcentimeter HCC varied among different guidelines. Some Asian guidelines allow diagnosing and treating such tiny tumors [3,4,5]. In contrast, the American Association for Study of Liver Disease (AASLD) only recommends an intensive imaging follow-up for subcentimeter observations [6]. There may be several reasons for this recommendation. First, it is debatable to utilize widely accepted imaging criteria to diagnose subcentimeter HCC because most of the criteria are designed for larger (≥ 1cm) HCC, and they did not consider the unique imaging and bio-pathological characteristics of subcentimeter HCC [7,8,9]. Previous research conducted by Yu et al. [10] found that the diagnostic performance of EOB-MRI for subcentimeter HCC is significantly lower than that for larger HCC, with a relatively low sensitivity of 46.0%. On the other hand, it has yet to be determined whether or not immediate treatment of subcentimeter HCC would bring an improved survival benefit. Therefore, it seems reasonable to withhold the diagnosis and treatment until it progresses to overt HCC (≥ 1cm). Although prior investigations have shown that survival outcomes were not significantly improved in patients with subcentimeter HCC who underwent early treatment strategy compared to those with larger HCC or underwent withholding strategy, these studies are limited by the various treatment strategies utilized and their insufficient sample sizes [11, 12].
For the purpose of developing appropriate diagnostic criteria and management strategies for subcentimeter HCC, it is essential to understand the distinctions between it and larger HCC. Therefore, this study aimed to compare the imaging features, pathological characteristics, and survival outcomes between subcentimeter and 1–2 cm HCC.
Materials and methods
Patients
The protocol for this observational cohort study was approved by the institutional review board of Zhongshan Hospital, Fudan University (No. B2020-372R). Written informed consent was obtained from all patients. This study was conducted in accordance with the Helsinki Declaration.
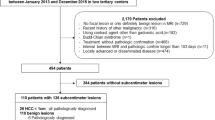
From January 2013 to December 2021, 378 patients with chronic hepatitis B who had a solitary suspected malignant ≤ 2 cm on EOB-MRI and underwent partial hepatectomy were initially identified. Patients who met the following criteria were included: (a) a definitive histopathological diagnosis of HCC; (b) Child-Pugh A liver function; and (c) no prior history of other malignancy or anti-tumor history. Of the 285 patients enrolled, 62 patients were excluded according to the following criteria: (a) those who had recurrent HCC (n = 12); (b) those who had macrovascular invasion or extrahepatic metastasis (n = 5); (c) whose time interval between MR scan and surgery was longer than one month (n = 10); (d) those who had severe respiratory-motion-related artifacts of MR images (n = 25); and (e) those died in peri-operative period or lost to follow-up (n = 10). Figure 1 illustrates the flow diagram for participant inclusion.
MR acquisitions
All MR images were acquired using a 1.5-tesla whole-body MR system (MAGNETOM Aera, Siemens Healthcare) with gadoxetate disodium (Primovist, Bayer Pharma). Our routine liver MRI protocol consisted of a breath-hold T1-weighted (T1WI) dual-echo (in-phase and opposed-phase) sequence, respiratory-triggered T2-weighted (T2WI) sequence, and diffusion-weighted sequence. The dynamic imaging sequences included three-dimensional volumetric-interpolated breath-hold T1WI (arterial phase: AP, triggered automatically when the contrast media reached the ascending aorta; portal venous phase: PVP, 60–70 s; transitional phase: TP, 180 s; hepatobiliary phase: HBP, 20 min). The detailed sequences and parameters were available in a published paper [13].
Image analysis
Two clinically experienced radiologists (H.P. and W.F., both with six years of experience in abdominal imaging) determined the location of target observation by consensus and marked them using thumbnail images with arrows through the picture archiving and communication system workstation (Centricity RA1000, General Electric). The longest diameter of observation was independently measured, and the average diameter was utilized.
Another two radiologists (Z.W.C and Y.C., with 16 and 18 years of experience in abdominal radiology, respectively) independently reviewed the MR images. They evaluated the imaging features of all observations according to the Liver Imaging Reporting and Data System version 2018 (LI-RADS v2018) [7]. Each observation was assessed for the following major features, including non-rim arterial phase hyperenhancement (non-rim APHE), non-peripheral washout, enhancing capsule, as well as other features, including T1WI hypointensity, mild-moderate T2WI hyperintensity, restricted diffusion, corona enhancement, fat deposition, TP hypointensity, HBP hypointensity, and the nodule in nodule, blood products, and mosaic appearance. The definition of these imaging features is summarized in Supplementary Table 1. The decision on discordant imaging features between two radiologists would be made by a discussion held by another senior radiologist (***BLINDED***, with 29 years of experience in abdominal imaging).
Clinicopathologic outcomes
Patients’ clinical, laboratory, and pathological data were obtained from the electronic medical records of our hospital. The initial clinical laboratory investigations included a complete blood count, serum biochemical test (including liver function), coagulation profile, tumor biomarker (including alpha-fetoprotein [AFP]), and hepatitis B virus deoxyribonucleic acid (HBV-DNA) load.
Each resected tumor specimen was assessed in consensus by two board-certificated pathologists. Histopathologic features included liver hepatitis and fibrosis stage, tumor differentiation, microvascular invasion (MVI), capsule formation, satellite foci, and serosal invasion. In addition, cytokeratin 19 (CK-19) and Ki-67 positivity were also assessed. CK-19 positivity was defined as membranous or cytoplasmic expression present in at least 5% of tumor cells, and Ki-67 positivity was indicated when at least 10% of the tumor cells showed positive [14, 15]. MVI was defined as the presence of tumor thrombi in the tiny blood vessels in the vicinity of the tumor [16].
Measurement of survival outcomes
After surgery, all patients were routinely followed up according to institutional protocol until death or until the last follow-up date (December 31, 2022). Follow-up protocol included either contrast-enhanced dynamic computed tomography or MRI and serologic tumor markers at 3–6 months intervals. Overall survival (OS) was defined as the time from surgery to death. Recurrence-free survival (RFS) was defined as the time from surgery to diagnosis of intrahepatic or extrahepatic recurrence. Early recurrence refers to recurrence within the first two years after resection.
Statistical analysis
Baseline characteristics were compared across the subcentimeter and 1–2 cm HCC using Fisher’s exact test or Chi-square test for categoric variables and Mann–Whitney U test or Student’s t test for continuous variables as appropriate. The incidence of different pathological characteristics and EOB-MRI features between subcentimeter and 1–2 cm HCC was compared by using Fisher’s exact test or Chi-square test. The weighted κ was applied to evaluate the interobserver agreement between the two reviewers assessing MRI features, with values of 0.41–0.60 indicating moderate agreement, 0.61–0.80 indicating substantial agreement, and > 0.81 indicating excellent agreement. The Kaplan–Meier analysis with log-rank test was applied to compare the RFS and OS between patients with subcentimeter HCC and 1–2 cm HCC after surgical resection. Statistical analyses were performed using SPSS Statistics version 24 (IBM Corp.). P < 0.05 was considered statistically significant.
Results
Patient and HCC characteristics
The baseline characteristics of the 223 patients (median age, 53 years, interquartile range, 46–60 years; 179 male and 44 female) are summarized in Table 1. Among the 223 patients, the study cohort comprised 83 patients with subcentimeter HCC and 141 with 1–2cm HCC. There was no significant difference between the two groups regarding baseline clinical characteristics. Most patients demonstrated histologic cirrhosis (stage F0-F2: 20.2%; stage F3: 12.1%; stage F4: 67.7%). Only two patients were classified as having albumin-bilirubin (ALBI) grade 3 disease. One hundred and eighteen (48.6%) had preoperative serum AFP levels higher than 20 ng/mL. The median time interval of the MRI exam and surgical resection was eight days (interquartile range, 4–13 days).
The mean size of all HCC assessed was 12.8 mm ± 3.7 (range, 6.1–19.9 mm). The mean size of the subcentimeter HCC group was 8.8 mm ± 0.9 (range, 6.1–9.9 mm), and that of the 1–2 cm HCC group was 15.1 mm ± 2.6 (range, 10.3–19.9 mm; P < 0.001). According to pathologic analysis, 172 (77.1%) patients were classified as having Edmondson-Steiner grade I or II HCC. Patients with subcentimeter HCC were less likely to present microvascular invasion (4 of 82 [4.9%] vs. 21 of 141 [14.9%]; P = 0.022) compared with those with 1–2 cm HCC. However, there were no significant differences in other pathological characteristics, including Edmondson-Steiner grade, capsule formation, serosal invasion, and Ki-67 and CK-19 positive status (Table 2).
Comparison of imaging features at gadoxetic-acid-enhanced MRI and interobserver agreement
Table 3 shows the comparison results of imaging features between the subcentimeter and 1–2 cm HCC. Hypointensity on HBP images (97.6% [80 of 82] of subcentimeter HCC and 99.3% [140 of 142] of 1–2 cm HCC) was the most common imaging feature in both subcentimeter and 1–2 cm HCC. Restricted diffusion (87.8% vs. 95.7%, P = 0.027) and non-peripheral washouts on PVP (58.5% vs. 73.8%, P = 0.013) were less commonly encountered in subcentimeter HCC compared to those measuring 1–2 cm (Figure 2). Subcentimeter HCC had a slightly lower prevalence of fat deposition (19.5% vs. 29.8%, P = 0.092) and an enhancing capsule (39.0% vs. 46.1%, P = 0.304), but this difference was not statistically significant.
Edmondson-Steiner grade II HCC in a 59-year-old female with HBV-related cirrhosis. a T2-weighted image shows a nodule (arrow) with mild hyperintensity. b Diffusion-weighted image shows a nodule with hyperintensity. c–e Nodule shows non-rim arterial hyperenhancement (c) without an obvious washout in the portal venous phase (d), whereas it depicts hypointensity in the transitional phase (e). f Image from the hepatobiliary phase shows that the nodule has hypointensity with a diameter of 7.6 mm. This subcentimeter HCC showed all the diagnostic hallmarks of HCC except for portal venous phase washout
The typical enhancement pattern of HCC on EOB-MRI (no-rim APHE and non-peripheral washout on PVP, according to the LI-RADS v2018) was less frequently seen in subcentimeter HCC than those measuring 1–2 cm (50.0% vs. 66.7%, P = 0.014) (Figure 3). The incidence of hypointensity on TP did not differ significantly between subcentimeter and 1–2 cm HCC (84.1% vs. 86.5, P = 0.625). Similar result was found in the comparison of the incidence of HBP hypointensity (97.6% vs. 99.3%, P = 0.280). No-rim APHE, non-peripheral washout on PVP, TP hypointensity, and nodule in nodule appearance showed excellent interobserver agreement between the two reviewers (κ = 0.833, 0.847, 0.822, and 1.000, respectively). Other evaluated imaging features showed substantial agreement between the two reviewers (Table 4).
Edmondson-Steiner grade II HCC in a 48-year-old male with chronic hepatitis B. a T2-weighted image shows a nodule (arrow) with moderated hyperintensity. b Diffusion-weighted image shows a nodule with significant hyperintensity. c–e Nodule shows non-rim arterial hyperenhancement (c), obvious washout in the portal venous phase (d), and hypointensity in the transitional phase (e). f Image from the hepatobiliary phase shows that the nodule has hypointensity with a diameter of 18.7 mm. This small HCC present all the typical imaging features of HCC
Survival outcomes
The median follow-up period was 47.4 months (range, 6.9–108.8 months). Recurrence occurred in 50 (20.6%) patients (11 in the subcentimeter HCC group and 39 in the 1–2 cm HCC group) with a median RFS of 79.4 months. Forty-three (86.0%) patients had intrahepatic recurrence, 5 (10.0%) had extrahepatic recurrence, and 3 (4.0%) had combined intrahepatic and extrahepatic recurrence. This included 26 early recurrences within the first two years. A total of 18 patients died during the follow-up, and the median OS was not reached.
According to Kaplan-Meier survival analysis, patients with subcentimeter HCC experienced less overall and early tumor recurrence (P = 0.027 and 0.046, respectively) (Fig. 4a and b). The estimated RFS rates at 1, 3, and 5 years were 98.7%, 88.5%, and 83.3% for subcentimeter HCC and 94.3%, 79.9%, and 67.3% for 1-2 cm HCC, respectively. Patients with subcentimeter HCC had a significantly longer OS than those with 1–2 cm HCC (P = 0.029) (Fig. 4c). The estimated OS rates at 1, 3, and 5 years were 98.8%, 97.3%, and 97.3% for subcentimeter HCC and 98.6%, 95.0%, and 89.5% for 1–2cm HCC, respectively.
Comparison of recurrence-free and overall survival of patients with subcentimeter and 1–2 cm HCC. Kaplan–Meier curves for overall tumor recurrence (a), early tumor recurrence (b), and overall survival (c) according to tumor size. Patients with subcentimeter HCC had a lower overall and early tumor recurrence rate and prolonged overall survival than those with HCC measuring 1–2 cm. HCC hepatocellular carcinoma
Discussion
This study showed that, compared with 1–2 cm HCC, subcentimeter HCC was characterized by a lower incidence of restricted diffusion, washout on the portal venous phase, and microvascular invasion. Patients with subcentimeter HCC had better RFS and OS. Accordingly, these results may provide a reference background for establishing management strategies for subcentimeter HCC.
Despite the guidelines of the European Association for the Study of the Liver and the AASLD stating that the diagnosis of HCC is only applicable for observations larger than 1cm, the diagnosis and management of subcentimeter HCC remain a hot topic of research [17,18,19]. Several investigations compare the imaging features between subcentimeter HCC and larger one [10, 20]. Choi et al. [20] concluded that the incidence of MRI features did not differ significantly between subcentimeter and 1–1.5 cm HCC. However, we found that subcentimeter HCC showed a significantly lower incidence of restricted diffusion and PVP washout than 1–2 cm HCC. The relatively small sample size (only 14 cases of subcentimeter HCC) in their study may be one of the possible reasons for the insignificant difference. In addition, this discrepancy can be partially attributed to the selection of 1cm and 2cm as the cutoff sizes in our study, which is aligned with LI-RADS guidelines and common clinical convention [7].
The reason why subcentimeter HCC showed less frequent washout on PVP but a similar incidence of TP hypointensity could be explained by the serval reason. First, tumors at the subcentimeter level may be in an incomplete stage of hepatocarcinogenesis. If the portal triads are not disappeared, a portion of HCC, including well-differentiated HCC, may not show washout [21]. Second, when using extracellular contrast agents that could not be absorbed by hepatocytes, the washout appearance is better shown in the delayed phase than in PVP [22]. Third, the detection of washout from small foci may be easily affected by partial volume averaging effects and low signal intensity ratio between HCC and liver parenchyma during the PVP. Our studies also revealed that subcentimeter HCC showed less frequent restricted diffusion than 1–2 cm HCC (87.8% vs. 95.7%, P = 0.027). This may be related to the inherent drawbacks of diffusion-weighted imaging, including limited spatial resolution and susceptibility to motion artifacts, especially for small observations.
It is well known that tumor size is an independent risk factor for tumor recurrence [23, 24]. Our study newly demonstrated that the correlation between tumor size and recurrence still exists in HCC smaller than 2 cm. Kaplan–Meier analysis demonstrated that patients with subcentimeter had better RFS and OS than those with 1–2 cm HCC. Being inconsistent with our study, Sun et al. [12] concluded that the OS and RFS did not differ significantly between patients with subcentimeter and 1–2 cm HCC. This discrepancy may be related to the insufficient sample size in their study (only 17 cases of subcentimeter HCC), lack of long-term follow-up, and different treatment modalities (including surgical resection and percutaneous ablation).
Our study found that the incidence of MVI in subcentimeter HCC was significantly lower than that in 1-2 cm HCC (4.9% vs. 14.9%). This may be related to the correlation between incidence of MVI and tumor burden. It has been demonstrated that MVI is one of the most important risk factors for recurrence even in patients with small HCC [25]. Considering the lower tumor aggressiveness and better survival outcomes after resection of subcentimeter HCC, early diagnosis of HCC at a subcentimeter level may be warranted. In addition, patients may not need to wait the HCC progress to larger than 1cm before initiating treatment. Our results, which are, of course, subject to the inherent drawbacks of the study design, are still meaningful for establishing the management strategy of subcentimeter HCC.
Our study has serval limitations. First, this is a retrospective-designed observational study, and selection bias was not avoidable. Second, all patients included in this study had HBV-associated chronic liver disease. Therefore, our result may be limited in its generalizability to patients with other types of etiology. Third, all patients were scanned by a 1.5-tesla scanner. A 3.0-tesla scanner may have a better resolution to describe the imaging feature of subcentimeter observations. Fourth, a median follow-up time of 47.4 months may not be sufficient to reach conclusions about OS small HCC. Therefore, studies with a long-term (more than 10 years) follow-up are needed to verify our results. Fourth, the patients suitable for surgical resection had less severity of liver cirrhosis, better liver function preservation, and younger age. Therefore, our result may need to be verified in patients with advanced cirrhosis and who received other treatment including transarterial chemoembolization and percutaneous ablation.
In conclusion, subcentimeter HCC showed less frequent restricted diffusion and portal venous phase washout seen typically in 1–2 cm HCC but better recurrence-free survival and overall survival. Therefore, it may be reasonable to propose tailored diagnostic criteria and undertake an early treatment strategy for subcentimeter HCC.
Data availability
The data used and analyzed in this study are available from the corresponding author on reasonable request.
Code availability
Not applicable.
References
Kulik L, El-Serag HB (2019) Epidemiology and Management of Hepatocellular Carcinoma. Gastroenterology 156:477-491 e471
Reig M, Forner A, Rimola J et al (2022) BCLC strategy for prognosis prediction and treatment recommendation: The 2022 update. J Hepatol 76:681-693
Omata M, Cheng AL, Kokudo N et al (2017) Asia-Pacific clinical practice guidelines on the management of hepatocellular carcinoma: a 2017 update. Hepatol Int 11:317-370
Kudo M, Kawamura Y, Hasegawa K et al (2021) Management of Hepatocellular Carcinoma in Japan: JSH Consensus Statements and Recommendations 2021 Update. Liver Cancer 10:181-223
Zhou J, Sun HC, Wang Z et al (2018) Guidelines for Diagnosis and Treatment of Primary Liver Cancer in China (2017 Edition). Liver Cancer 7:235-260
Heimbach JK, Kulik LM, Finn RS et al (2018) AASLD guidelines for the treatment of hepatocellular carcinoma. Hepatology 67:358-380
Cerny M, Bergeron C, Billiard JS et al (2018) LI-RADS for MR Imaging Diagnosis of Hepatocellular Carcinoma: Performance of Major and Ancillary Features. Radiology 288:118-128
Choi JY, Lee JM, Sirlin CB (2014) CT and MR imaging diagnosis and staging of hepatocellular carcinoma: part II. Extracellular agents, hepatobiliary agents, and ancillary imaging features. Radiology 273:30-50
Lu XY, Xi T, Lau WY et al (2011) Pathobiological features of small hepatocellular carcinoma: correlation between tumor size and biological behavior. J Cancer Res Clin Oncol 137:567-575
Yu MH, Kim JH, Yoon JH et al (2014) Small (</=1-cm) hepatocellular carcinoma: diagnostic performance and imaging features at gadoxetic acid-enhanced MR imaging. Radiology 271:748-760
Woo JH, Song KD, Kim SH (2017) Subcentimeter hypervascular nodules with typical imaging findings of hepatocellular carcinoma on gadoxetic acid-enhanced MRI: Outcomes of early treatment and watchful waiting. Eur Radiol 27:4406-4414
Sun X, Hu D, Zhang Y et al (2020) Can Immediately Treating Subcentimeter Hepatocellular Carcinoma Improve the Survival of Patients? J Hepatocell Carcinoma 7:377-384
Huang P, Zhou C, Wu F et al (2023) An improved diagnostic algorithm for subcentimeter hepatocellular carcinoma on gadoxetic acid-enhanced MRI. Eur Radiol 33:2735–2745
Durnez A, Verslype C, Nevens F et al (2006) The clinicopathological and prognostic relevance of cytokeratin 7 and 19 expression in hepatocellular carcinoma. A possible progenitor cell origin. Histopathology 49:138-151
Wu H, Han X, Wang Z et al (2020) Prediction of the Ki-67 marker index in hepatocellular carcinoma based on CT radiomics features. Phys Med Biol 65:235048
Roayaie S, Blume IN, Thung SN et al (2009) A system of classifying microvascular invasion to predict outcome after resection in patients with hepatocellular carcinoma. Gastroenterology 137:850-855
European Association for the Study of the Liver. Electronic address eee, European Association for the Study of the L (2018) EASL Clinical Practice Guidelines: Management of hepatocellular carcinoma. J Hepatol 69:182-236
Marrero JA, Kulik LM, Sirlin CB et al (2018) Diagnosis, Staging, and Management of Hepatocellular Carcinoma: 2018 Practice Guidance by the American Association for the Study of Liver Diseases. Hepatology 68:723-750
Park MJ, Kim YS, Lee WJ, Lim HK, Rhim H, Lee J (2010) Outcomes of follow-up CT for small (5-10-mm) arterially enhancing nodules in the liver and risk factors for developing hepatocellular carcinoma in a surveillance population. Eur Radiol 20:2397-2404
Choi MH, Choi JI, Lee YJ, Park MY, Rha SE, Lall C (2017) MRI of Small Hepatocellular Carcinoma: Typical Features Are Less Frequent Below a Size Cutoff of 1.5 cm. AJR Am J Roentgenol 208:544-551
Kitao A, Zen Y, Matsui O, Gabata T, Nakanuma Y (2009) Hepatocarcinogenesis: multistep changes of drainage vessels at CT during arterial portography and hepatic arteriography--radiologic-pathologic correlation. Radiology 252:605-614
Kim DH, Choi SH, Kim SY, Kim MJ, Lee SS, Byun JH (2019) Gadoxetic Acid-enhanced MRI of Hepatocellular Carcinoma: Value of Washout in Transitional and Hepatobiliary Phases. Radiology 291:651-657
Shah SA, Cleary SP, Wei AC et al (2007) Recurrence after liver resection for hepatocellular carcinoma: risk factors, treatment, and outcomes. Surgery 141:330-339
Yeh CN, Chen MF, Lee WC, Jeng LB (2002) Prognostic factors of hepatic resection for hepatocellular carcinoma with cirrhosis: univariate and multivariate analysis. J Surg Oncol 81:195-202
Wang H, Wu MC, Cong WM (2019) Microvascular invasion predicts a poor prognosis of solitary hepatocellular carcinoma up to 2 cm based on propensity score matching analysis. Hepatol Res 49:344-354
Funding
This study was supported by the Clinical Research Plan of SHDC (No. SHDC2020CR1029B), National Natural Science Foundation of China (No. 82171897), National Natural Science Foundation of China (No. 82272078), Shanghai Municipal Health Commission (No. 202240152), and Shanghai Municipal Key Clinical Specialty (No. shslczdzk03202).
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
All authors declare no conflict of interest in the study.
Ethical approval
The study protocol was complied with the principles of the Declaration of Helsinki and was approved by the institutional review board of the Zhongshan Hospital, Fudan University (No. B2020-372R).
Consent to participate
The ethics committee of Zhongshan Hospital, Fudan University agreed to the consent to participate.
Consent for publication
Not applicable.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Huang, P., Shi, Q., Ni, X. et al. Subcentimeter hepatocellular carcinoma (HCC) on gadoxetic-acid-enhanced MRI: less frequent typical imaging features compared to 1–2 cm HCC but better prognosis after surgical resection. Abdom Radiol 48, 3391–3400 (2023). https://doi.org/10.1007/s00261-023-04024-7
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00261-023-04024-7