Abstract
Purpose
To evaluate the role of ancillary features (AFs) of Liver Imaging Reporting and Data System (LI-RADS) in the diagnostic performance of small HCC (≤ 20 mm) on gadoxetic acid-enhanced MRI.
Methods
A total of 154 patients with 183 hepatic observations were analysed in this retrospective study. Observations were categorized using only major features (MFs) and combined MFs and AFs. Independently significant AFs were identified through logistic regression analysis, and upgraded LR-5 criteria were developed using these as new MFs. The diagnostic performance of the modified LI-RADS (mLI-RADS) was calculated and compared with that of LI-RADS v2018 using McNemar’s test.
Results
Restricted diffusion, transitional and hepatobiliary phase hypointensity were independently significant AFs. The mLI-RADS a, c, e, g, h and i (upgraded LR-4 lesions that were categorized using only MFs to LR-5 using a certain or any one, two, three of the above AFs as new MFs) yielded a significantly greater sensitivity than that of the LI-RADS v2018 (68.0%, 69.1%, 69.1%, 69.1%, 69.1%, 68.0% vs. 61.9%, all p < 0.05), whereas the specificities were not significantly different (84.9%, 86.0%, 84.9%, 83.7%, 84.9%, 87.2% vs. 88.4% all p > 0.05). When independently significant AFs were used to upgrade the LR-4 nodules categorized by combined MFs and AFs (mLI-RADS b, d and f), the sensitivities were improved, but the specificities were decreased (all p < 0.05).
Conclusions
Independently significant AFs may be used to upgrade an observation from LR-4 (categorized only using MFs) to LR-5, which can improve diagnostic performance for small HCC.
Graphical abstract

Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
The Liver Imaging Reporting and Data System (LI-RADS) published by the American College of Radiology (ACR) is used to assign a category to focal hepatic observations obtained for high-risk hepatocellular carcinoma (HCC) patients and was recently updated in 2018 (LI-RADS v2018) [1]. It defines various major features (MFs) and ancillary features (AFs) of hepatic observations and uses a combination of MFs to assign initial categories and then adjusts the categories according to AFs. LR-5 is the category of definite HCC and is often used to evaluate the diagnostic performance of LI-RADS for HCC. AFs include AFs favoring malignancy and favoring benignity [1].
Gadoxetic acid-enhanced magnetic resonance imaging (EOB-MRI), with hepatocellular specificity, plays an important role in the diagnosis of HCC [2]. However, recent studies have shown that low sensitivities of LI-RADS are more problematic for EOB-MRI [3, 4]. This might be because LI-RADS was originally designed for extracellular contrast agent-enhanced MRI, and EOB-MRI was incorporated later into its algorithm [5].
Small HCC often poses a diagnostic challenge due to its tendency to exhibit atypical imaging features. Each AF favoring malignancy appears to vary in frequency and importance, and certain features may be more helpful in diagnosing HCC [6]. However, in the LI-RADS, the use of AFs is optional, and the diagnosis is primarily based on MFs. In addition, an observation can be upgraded by one category up to LR-4 but not to LR-5 by using AFs [7, 8]. Therefore, the weight of AFs may be somehow overlooked.
Therefore, the purpose of this study was to identify the independently significant AFs favoring malignancy and to explore whether modifications to LI-RADS using these AFs could further improve diagnostic performance for small HCC (≤ 20 mm) on EOB-MRI.
Materials and methods
This study was approved by the Ethics Committee of Tianjin Third Central Hospital. Informed consent was waived due to the retrospective study design.
Study population
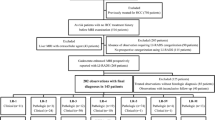
Data from patients with a high risk of HCC were retrospectively collected between June 2016 and June 2021. The inclusion criteria were as follows: (1) ≥ 18 years old; (2) received EOB-MRI; (3) nodule size ≤ 20 mm and number ≤ 3; and (4) definitive diagnosis by surgical, biopsy pathology or benign lesions confirmed by characteristic imaging and follow-up ≥ 24 months. The exclusion criteria were as follows: (1) cirrhosis due to congenital hepatic fibrosis or vascular disorder; (2) interval > 1 month between pathological diagnosis and EOB-MRI; (3) received treatment prior to the EOB-MRI; (4) liver function was Child‒Pugh C; or (5) LR-NC (cannot be categorized) or LR-TIV (tumour in vein) (Fig. 1). Patient demographic, clinical, and laboratory characteristics were extracted from the electronic medical records.
Reference standard
All HCCs and non-HCC malignancies were pathologically confirmed and benign lesions were confirmed by pathologic diagnosis or clinical diagnosis based on typical imaging features and stability or regression for at least 24 months. Histological diagnoses were all based on the formal pathologic reports of our institution made by one of two pathologists who had more than 10 years of experience in liver pathology.
MRI techniques
For all examinations, studies were performed using a 3.0-T MR system (Magnetom Verio, Siemens Healthcare, Erlangen, Germany), and an 8-channel phased-array torso coil was used for all measurements. The liver MRI protocol consisted of in- and out-of-phase T1-weighted imaging acquired with a gradient recalled echo (GRE) sequence, a respiratory-triggered axial T2-weighted turbo spin echo (TSE) sequence with fat suppression, free-breathing single-shot echo-planar diffusion-weighted imaging (DWI) with b values of 0, 50, 600, 1000 s/mm2, and pre- and postcontrast T1-weighted three-dimensional volumetric interpolated breath-hold examination (VIBE) sequences acquired with a GRE sequence in the arterial phase (25 s after aortic enhancement using the bolus tracking method), portal venous phase (60 s), transitional phase (TP) (3 min), and hepatobiliary phase (HBP) (20 min). Contrast-enhanced dynamic MRI was obtained after intravenous administration of gadoxetic acid (Primovist, Bayer Healthcare, Leverkusen, Germany) administered at 0.025 mmol/kg of body weight at a rate of 1.0 ml/s using a power injector, followed by 25 ml of 0.9% saline as a chaser at the same rate.
MRI analysis and category assignment
All MRI scans were independently reviewed by two board-certified radiologists with 10 years (W.H.) and 8 years (D.W.) of experience in abdominal MRI, respectively. All readers were blinded to the pathologic results and reviewed all imaging features of each hepatic observation. LI-RADS categorization was assigned to each observation only according to MFs and combining MFs and AFs (assigning initial categories by MFs and then adjusting according to AFs) according to LI-RADS v2018, respectively. Logistic regression analyses were performed to identify independent significant AFs for the diagnosis of small HCC, and then these AFs were used as new MFs to upgrade the LR-5 criteria and reassign the categorization. Discrepancies between the two readers were resolved by a third radiologist (R.L. with 16 years of experience in abdominal MRI) to reach a final consensus reading.
Statistical analysis
Categorical variables are summarized as counts and percentages. Continuous variables are summarized as the means and standard deviations. To determine the imaging features predictive of HCC, univariate and multivariate logistic regression analyses were performed. Variables with a p value < 0.2 in the univariable analysis were entered into the multivariable analysis to identify independently significant features for HCC diagnosis. For the multivariable analysis, a stepwise backwards elimination method was used. Diagnostic performance is reported as the sensitivity, specificity, positive predictive value (PPV), negative predictive value (NPV), accuracy, and Youden index and was compared using McNemar’s test. Unless otherwise indicated, all statistical tests were conducted at the 0.05 significance level using 2-tailed tests, and p values are reported. Statistical analyses were performed using SPSS software, version 25.0 (SPSS Inc.).
Results
Patient characteristics and pathologic findings
The study population included 154 patients with 183 hepatic observations. All lesions were confirmed by pathological diagnosis except for 30 benign lesions confirmed by typical imaging features and followed up ≥ 24 months. The clinicopathologic characteristics of the patients and hepatic observations are shown in Table 1.
Independently significant features
According to the multivariate analysis, nonrim arterial-phase hyperenhancement (APHE), nonperipheral “washout” and enhancing “capsule” were independent significant MFs for identifying small HCC. Restricted diffusion [odds ratio (OR) 15.3; 95% confidence interval (CI), 3.1–76.0; p = 0.001], TP hypointensity (OR 3.7; 95% CI 1.2–11.5; p = 0.027) and HBP hypointensity (OR 4.8; 95% CI 1.2–19.7; p = 0.028) were independently significant AFs. (Table 2).
Diagnostic performance
Based on the above results of the multivariate logistic regression analyses, the independently significant AFs, including restricted diffusion, TP hypointensity and HBP hypointensity, were used as new MFs to participate in the upgraded LR-5 criteria of LI-RADS. The mLI-RADS was as follows: mLI-RADS a: LR-4 only according to MFs (LR-4 MFs) + restricted diffusion; mLI-RADS b: LR-4 according to the MFs and adjusted by AFs (LR-4 MFs and AFs) + restricted diffusion; mLI-RADS c: LR-4 MFs + TP hypointensity; mLI-RADS d: LR-4 MFs and AFs + TP hypointensity; mLI-RADS e: LR-4 MFs + HBP hypointensity; mLI-RADS f: LR-4 MFs and AFs + HBP hypointensity; mLI-RADS g, h, i: LR-4 MFs + any one, two (Fig. 2) and all of the three independently significant AFs. The LR-5 was used to evaluate the diagnostic performance of LI-RADS for small HCC.
Axial images obtained with EOB-MRI in a 59-year-old man with HCC. T1WI a shows a 12 mm hypointense nodule (arrow) in hepatic segment VIII. The nodule exhibits APHE (b) and does not have a “washout” appearance on portal vein phase image (c). It (arrow) shows hypointensity in the TP (d) and HBP (e) and represents an enhancing “capsule” (d, e). This nodule was assessed to LR-4 only using MFs according to LI-RADS v2018. When TP and HBP hypointensity were used as new MFs, the nodule was categorized as LR-5
LI-RADS v2018 showed a sensitivity of 61.9% and a specificity of 88.4%. Compared with LI-RADS v2018, the sensitivities of all mLI-RADS were improved (all p < 0.05), while the specificity was not significantly different (all p > 0.05) except for mLI-RADS b, d and f (specificity was reduced, all p < 0.05) (Table 3).
Discussion
Our study demonstrates that restricted diffusion, TP hypointensity and HBP hypointensity, three AFs favoring malignancy, were independently significant features for the diagnosis of small HCC (≤ 20 mm) observed on EOB-MRI. When using any one, two or three of them was as new MFs to upgrade an observation from LR-4 (categorized only according to MFs) to LR-5, the sensitivity was significantly improved compared with that of LI-RADS v2018 (all p < 0.05), while the specificity was not significantly reduced (all p > 0.05).
In our study, MFs, including nonrim APHE, nonperipheral “washout” and enhancing “capsule”, were independent predictors of small HCC diagnosis. The APHE and “washout” appearance were attributed to increased arterial blood supply and decreased portal supply in HCC [9]. In addition, although the sensitivity of enhancing “capsule” was low, the specificity was higher because it reflects the pseudocapsule composed of compressed fibrous tissue and dilated sinusoids around the HCC [10]. In our study, the odds ratio (OR) of “washout” (9.8) was the highest among the MFs, and this conclusion is better than previous reports (the OR of “washout” was 4.0) [6]. This may be because the study was not only targeted at small HCC. In this previous study, the OR of APHE was the highest (7.2), while in our study, the OR of APHE was only 2.9. Therefore, it is speculated that the “washout” display may have more diagnostic significance than APHE in small HCC. In another study [11] of small HCC, the specificity of “washout” (42.3%) was lower than that in our study (76.7%), which may be due to this study only having compared HCCs and dysplastic nodules (DNs), while high-grade DN may have more “washout” due to the blood supply situation similar to that of early HCC [12].
Recent studies have shown that elevated AFs to MFs can improve diagnostic performance for the diagnosis of HCC [6, 13, 14]. However, few studies have assessed the role of AFs for the diagnosis of small HCC (≤ 20 mm) on EOB-MRI. Our study included all AFs favoring malignancy of LI-RADS v2018 to determine independent features for predicting small HCC. The results demonstrate that only restricted diffusion, TP hypointensity and HBP hypointensity were independent significant features. This implies that these AFs may be more important than others in evaluation of small lesions in addition to MFs and may have a similar strength that is comparable with MFs.
In our study, restricted diffusion was defined as an intensity on DWI higher than that of the liver and an apparent diffusion coefficient lower than that of the liver to avoid T2 shine-through according to LI-RADS v2018 [1]. Malignant tumours often show restricted diffusion due to dense cells, and in LI-RADS, restricted diffusion is also used as an AF favoring malignancy. In our study, restricted diffusion was an independent predictor of the diagnosis of small HCC, which is consistent with a previous literature conclusion [15]. However, in another study, diffusion restriction was not an independent factor, although its OR value was as high as 6.9 in univariate analysis [6]. It is speculated that the possible reason is that the study targeted large lesions (the mean size of the selected lesions was 57 mm). In addition, since DWI depends on the scanner, field strength, and acquisition technique, different DWI techniques may also have led to discordant results.
On EOB-MRI, TP hypointensity reflects the combination of early cellular enhancement of hepatic parenchyma and washout of the extracellular spaces, therefore, to pursue high specificity, the “washout” in LI-RADS does not include TP hypointensity but only as an AF favoring malignancy. However, in our study, TP hypointensity was an independent predictor for the diagnosis of small HCC. This is consistent with previous literature reports [16]. This may be due to the diminishing portal supply and organic anion transporter (OATP) expression usually during hepatocarcinogenesis. HBP hypointensity occurs in nonhepatocellular lesions due to a lack of organic anion transporting polypeptide transporter expression in combination with strong enhancement of the hepatic parenchyma. In our study, the OR of HBP hypointensity was 4.8, and HBP hypointensity was an independent predictor for the diagnosis of small HCC.
In our study, when any one of the independent features of restricted diffusion, TP or HBP hypointensity was selected as an MF for diagnosing small HCC and upgrading the nodules that were assigned the LR-4 category based only on the MFs to LR-5, the sensitivity of diagnosing HCC was improved without reducing the specificity. It is worth noting that an observation upgraded to LR-4 after adjustment of AFs according to LI-RADS v2018, when the independently significant AFs were used to upgrade to LR-5, although the diagnostic sensitivity can be improved, the specificity is significantly reduced. Therefore, we found that modifying the LR-5 criteria using independently significant AFs only applies to lesions that are categorized as LR-4 based only on MFs. In addition, when 2 or 3 independently significant AFs were used at the same time, the diagnostic sensitivity decreased slightly with the increase in the number of AFs, but both were higher than LI-RADS v2018, and the specificity gradually increased, but it was not significantly different from LI-RADS. Therefore, the simultaneous use of multiple independently significant AFs can improve diagnostic sensitivity while avoiding low specificity.
This study has several limitations. First, owing to the retrospective single-centre nature of our study design, our results could have had selection bias and might be limited in generalizability. Second, only 14 observations of non-HCC malignancies were collected, which was relatively small. However, the feasibility of non-HCC malignant tumours in patients with a high risk of HCC was relatively low, not to mention that this paper strictly stipulated that the lesions should be ≤ 20 mm. Third, the iron sparing in solid mass did not appear in any of the observations in our study, so this feature was not analysed.
In conclusion, our results show that upgrading an observation from LR-4 (categorized only according to MFs) to LR-5 using any one of independently significant AFs (restricted diffusion, TP hypointensity and HBP hypointensity) can improve sensitivity without impairing specificity in the diagnosis of small HCC (≤ 20 mm) on EOB-MRI. When two or three independently significant AFs were used simultaneously, the diagnostic performance could also be improved, and as the number of AFs used increased, its sensitivity decreased and its specificity increased.
References
American College of Radiology. CT/MRI LI-RADS® v2018.Acr.org Web site. 2020. https://www.acr.org/Clinical-Resources/Reporting-and-Data-Systems/LI-RADS/CT-MRI-LI-RADS-v2018. Accessed 6 November 2020.
Lim K, Kwon H, Cho J, et al. (2022) Added value of enhanced CT on LR-3 and LR-4 observation of Gd-EOB-DTPA MRI for the diagnosis of HCC: are CT and MR washout features interchangeable? Br J Radiol 95 (1132): 20210738. https://doi.org/10.1259/bjr.20210738.
Song JS, Choi EJ, Hwang SB, et al. (2019) LI-RADS v2014 categorization of hepatocellular carcinoma:intraindividual comparison between gadopentetate dimeglumine-enhanced MRI and gadoxetic acid-enhanced MRI. Eur Radiol 29 (1): 401-410. https://doi.org/10.1007/s00330-018-5559-z.
Lee S, Kim MJ, Kim SS, et al. (2020) Mitchell, Retrospective comparison of EASL 2018 and LI-RADS 2018 for the noninvasive diagnosis of hepatocellular carcinoma using magnetic resonance imaging. Hepatol Int 14 (1): 70-79. https://doi.org/10.1007/s12072-019-10002-3.
Hope TA, Fowler KJ, Sirlin CB, et al. (2015) Hepatobiliary agentsand their role in LI-RADS. Abdom Imaging 40(3): 613-625. https://doi.org/10.1007/s00261-014-0227-5.
Lee S, Kim SS, Bae H, et al. (2021) Application of Liver Imaging Reporting and Data System version 2018 ancillary features to upgrade from LR-4 to LR-5 on gadoxetic acid–enhanced MRI. Eur Radiol 31 (2): 855-863. https://doi.org/10.1007/s00330-020-07146-4.
Cerny M, Bergeron C, Billiard JS, et al. (2018) LI-RADS for MR imaging diagnosis of hepatocellular carcinoma: performance of major and ancillary features. Radiology 288 (1):118-128. https://doi.org/10.1148/radiol.2018171678.
Choi SH, Byun JH, Lim YS, et al. (2018) Liver Imaging Reporting and Data System: patient outcomes for category 4 and 5 nodules. Radiology 287(2):515-524. https://doi.org/10.1148/radiol.2018170748.
Shin J, Lee S, Yoon JK, et al. (2021) LI-RADS Major Features on MRI for Diagnosing Hepatocellular Carcinoma: A Systematic Review and Meta-Analysis. J Magn Reson Imaging 54(2):518-525. https://doi.org/10.1002/jmri.27570.
Lee SY, Kim MJ, Kim SS, et al. (2020) Retrospective comparison of EASL 2018 and LI-RADS 2018 for the noninvasive diagnosis of hepatocellular carcinoma using magnetic resonance imaging. Hepatol Int 14(1):70-79. https://doi.org/10.1007/s12072-019-10002-3.
Gaetano AMD, Catalano M, Pompili M, et al. (2019) Critical analysis of major and ancillary features of LI-RADS v2018 in the differentiation of small (≤ 2 cm) hepatocellular carcinoma from dysplastic nodules with gadobenate dimeglumine-enhanced magnetic resonance imaging. Eur Rev Med Pharmacol Sci 23 (18): 7786-7801. https://doi.org/10.26355/eurrev_201909_18988.
Joo I, Kim SY, Kang TW, et al. (2020) Radiologic-Pathologic Correlation of Hepatobiliary Phase Hypointense Nodules without Arterial Phase Hyperenhancement at Gadoxetic Acid-enhanced MRI: A Multicenter Study. Radiology 296(2):335-345. https://doi.org/10.1148/radiol.2020192275.
Hwang SH, Park S, Han K, et al. (2019) Optimal lexicon of gadoxetic acid-enhanced magnetic resonance imaging for the diagnosis of hepatocellular carcinoma modified from LI-RADS. Abdom Radiol (NY) 44 (9): 3078-3088. https://doi.org/10.1007/s00261-019-02077-1.
Vernuccio F, Cannella R, Meyer M, et al. (2019) LI-RADS: Diagnostic Performance of Hepatobiliary Phase Hypointensity and Major Imaging Features of LR-3 and LR-4 Lesions Measuring 10-19 mm With Arterial Phase Hyperenhancement. AJR Am J Roentgenol 213(2):W57-W65. https://doi.org/10.2214/AJR.18.20979.
Renzulli M, Biselli M, Brocchi S, et al. (2018) New hallmark of hepatocellular carcinoma, early hepatocellular carcinoma and high-grade dysplastic nodules on Gd-EOB-DTPA MRI in patients with cirrhosis: a new diagnostic algorithm. Gut 67(9):1674-1682. https://doi.org/10.1136/gutjnl-2017-315384.
Xie SD, Zhang Y, Chen JB, et al. (2022) Can modified LI‑RADS increase the sensitivity of LI‑RADS v2018 for the diagnosis of 10-19 mm hepatocellular carcinoma on gadoxetic acid‑enhanced MRI? Abdom Radiol (NY) 47 (2): 596-607. https://doi.org/10.1007/s00261-021-03339-7.
Funding
This study was supported by the grants form Tianjin Key Medical Discipline (Specialty) Construction Project (TJYXZDXK-074C), Tianjin Health Science and Technology Project (TJWJ2022QN045), and Tianjin Health Project of Science and Technology (TJWJ2022XK027).
Author information
Authors and Affiliations
Contributions
RL and WH contributed equally to this work. RL: conception, literature research, imaging analysis, manuscript drafting, final manuscript approval. WH: conception, literature research, imaging analysis, manuscript drafting, final manuscript approval. DW: literature research, imaging analysis, manuscript drafting. JW: literature research; data curation; manuscript editing. ZG: data interpretation; literature review; visual abstract. KJ: final manuscript approval.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Lyu, R., Hu, W., Wang, D. et al. LI-RADS v2018: utilizing ancillary features on gadoxetic acid-enhanced MRI to improve the diagnostic performance of small hapatocellular carcinoma (≤ 20 mm). Abdom Radiol 48, 1987–1994 (2023). https://doi.org/10.1007/s00261-023-03871-8
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00261-023-03871-8