Abstract
Purpose
To assess the agreement between radiologists in the detection of specific features related to T- and N-stage and evaluate accuracy in colon cancer staging.
Methods
Patients who underwent contrast-enhanced computed tomography (CECT) before surgery were enrolled and evaluated by three radiologists with different experience. Pathological data were used as the reference standard. Tumor location, presence of tumor bulging, fat stranding, lateroconal fascia invasion, enlarged vessels, axial short diameter of the biggest node, shape, enhancement pattern, intranodal necrosis, and cluster were collected. Reliability analysis was performed with κ statistics and intraclass correlation coefficients (ICCs). Logistic regressions were used to determine independent predictors associated with staging.
Results
Seventy five patients were evaluated. The reliability analysis was moderate to substantial for tumor location (κ = 0.853), T staging (κ = 0.531), tumor bulging (κ = 0.478), fat stranding (κ = 0.490), lateroconal fascia invasion (κ = 0.436), enlarged vessels (κ = 0.401), the axial short diameter of the biggest node (ICC = 0.732), shape (κ = 0.484), enhancement pattern (κ = 0.431), intranodal necrosis (κ = 0.606), and cluster (κ = 0.358). For all readers, sensitivity was higher for T3 lesions (60–69%) and specificity was higher for T2 and T4 lesions (84.5–90.0% and 82.4–85.1%). The diagnostic accuracy was acceptable for all lesions and among all readers (50.7–92.1%). The lateroconal fascia invasion and enlarged vessels resulted as independent predictor factors (OR = 3.292 and OR = 2.651) for T staging, while nodes’ cluster and dimension as independent predictor factors of N staging [OR = 3.798 and OR = 1.083].
Conclusion
Reader’s experience is one of the most important factors associated with the correct classification of colon cancer. Moreover, CECT can help depict radiological features independently associated with the T and N stages.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Colon cancer is one of the most prevalent types of neoplasm worldwide [1], and its most important prognostic marker is the tumor stage [2]. Once the diagnosis of colon cancer is established, staging should be performed using the latest version of the American Joint Committee on Cancer (AJCC) tumor, node, and metastasis (TNM) classification [3]. Loco-regional staging is based on tumor growth patterns (including surrounding structures and the presence of nodes metastases) and is provided mainly to establish management [4].
Upfront surgery is the recommended treatment for resectable, non-metastatic, colon cancer patients [5]. Nowadays, in the absence of official guidelines, some recent clinical trials are working on the use of neoadjuvant chemotherapy for stage III or high-risk stage II disease. To this last regard, patients with tumor invasion of at least 5 mm beyond the bowel wall are classified as at high-risk [6]. According to the preliminary results from the FOxTROT trial, high-risk colon cancer patients who underwent neoadjuvant chemotherapy had lower tumor stages, fewer positive nodes, and fewer positive resection margins compared with patients who underwent surgery immediately [7].
Contrast-enhanced computed tomography (CECT) is the preferred technique for determining the tumor stage before treatment planning, given its accuracy to distinguish between small and advanced colon tumors [8]. However, CT-based T and N staging are not deeply reported in the literature. Moreover, the accuracy of preoperative CECT for the assessment of T and N stages of colonic tumors is still debated [4, 5, 8]. CECT is rarely used for locoregional staging and decision management purposes, especially due to a low contrast resolution useful to directly distinguish the bowel wall layers, although indirect findings, such as the bulging or solid nodules beyond the colon profile, can be useful to assess the invasion of muscularis propria.
Many studies suggested CECT as a reliable method to select patients who can benefit from neoadjuvant chemotherapy [9]. In these settings, a meta-analysis published in 2016 demonstrated that CECT has a pooled sensitivity and specificity of 90% and 69% of CT in detecting T1–T2 vs T3–T4 respectively. However, the discrimination between T1–T3ab and T3cd–T4 remains challenging [10]. Concerning N staging, it has been reported that a higher number of nodes (especially in N1 and N2 regions), the largest short diameter, and the presence of internal heterogeneity represent important predictive factors of nodes status in colon cancer [11]. For these reasons, in order to address patients to the best management possible, it is paramount to perform an accurate pre-operative imaging evaluation and to define CT criteria able to increase the inter-observer agreement and, consequently, its diagnostic accuracy.
To date, for the best of our knowledge, no studies compared the agreement between multiple readers and their diagnostic accuracy using CECT in colon cancer patients. Therefore, this study aims to assess the agreement between different radiologists in the detection of specific features related to the T- and N-stage and to evaluate readers’ diagnostic accuracy in staging colon tumors, according to years of experience, using pathological data as the reference standard. Moreover, we aimed to evaluate conventional CT predictor factors associated with T- and N-staging, preoperatively.
Methods and materials
This retrospective study conformed to the ethical guidelines of the 1975 Declaration of Helsinki and the protocol was approved by the institutional review board with a waiver of written informed consent. This was a retrospective observational analysis based on previously collected routine care data. Consent was verbally obtained from participants; however, they were not required to sign it. All radiological and clinical data have been anonymized before being analyzed.
Patients population
All consecutive patients who underwent colorectal surgical resection between January 1st, 2020, and November 30th, 2020, were retrospectively enrolled.
Inclusion criteria were: (1) age > 18 years old, (2) contrast-enhanced computed tomography (CECT) obtained no more than 14 days before the surgical intervention in our Department of diagnostic radiology, (3) colorectal resection performed in our Institution, (4) complete pathological data of the surgical specimen.
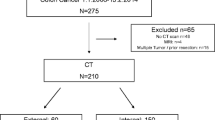
Exclusion criteria were: (1) medical contraindication to iodinated contrast agents, (2) incomplete CT protocol (i.e., unenhanced sequence only), (3) staging CECT performed in other Institution(s) and (4) CECT acquired post-operative exclusively. The flow chart in Fig. 1 summarizes the enrolling selection path.
For each patient clinical (including sex and age at diagnosis), CECT, and pathological data were retrieved from a prospectively collected database.
Image procedure and analysis
CECT images were reviewed on a PACS workstation (Enterprise AGFA Diagnostic Software—Agfa, Mortsel, Belgium) by three radiologists, the first one with a subspeciality in colorectal cancer imaging and 10 years of experience (S.G.D.—reader 1), the second one with 15 years of experience in the subspecialty of abdominal imaging (D.I.—reader 2) and the third one with 18 years of experience in general radiology (R.P.—reader 3).
Each radiologist evaluated all CECT examinations randomly and blinded to demographic, clinical and pathological data. To minimize any recall or learning bias, CECT examinations were reviewed at different time points.
For each CT the following parameters were collected: (1) tumor location, grouped in the caecum and right colon (including hepatic flexure), transverse colon, left colon (including splenic flexure), and sigmoid colon, (2) uni- or multi-focal lesions, and (3) presence of circumferential tissue or stenosis. Each radiologist was asked to determine the tumor (“T”) stage, according to the WHO staging system, the presence of bulging (defined as nodular projections of the tumor in the nearest adipose tissue) and fat stranding (defined as thin irregularities located in the nearby fat tissue), the lateroconal fascia invasion (defined as the absences of adipose cleavage plane or nodularity in the fascia), and the presence of enlarged vessels nearby the lesion. For each patient we also collected data regarding nodes, in particular: (1) the axial short diameter of the biggest node, (2) the nodal shape (oval or round), (3) the nodal enhancement pattern (homogeneous or inhomogeneous), (4) the presence of intranodal necrosis (defined as well-defined hypoattenuating area), and (5) the presence of cluster, including < or ≥ 3 related nodes (each one not further away of 5 mm).
To enhance consistency between readers’ interpretations, 10 external examples with typical imaging features were given to all readers in a group training session before performing study interpretations, as reported in Fig. 2. These training cases were then excluded from the final cohort.
Examples of imaging findings collected by the three readers regarding the tumor (a–d) and nodes (e–h). a bulging, represented by nodular projections in the nearest adipose tissue, b fat stranding, seen as thin irregularities located in the nearby fat tissue, c the lateroconal fascia invasion, represented by the absences of adipose cleavage plane between tumor and peritoneal fascia, d presence of enlarged vessels nearby the lesion. e example of round pathological node (white circle), with inhomogeneous enhancement (f white arrow), g example of intranodal necrosis and (h) example of nodes’ cluster
Images were analyzed on native axial images and for each patient, coronal and sagittal multiplanar reconstructions (MPR) were directly available on the dedicated workstation. Each reader was also able to perform personalized MPR with a specific tool available on the PACS.
In the case of multiple lesions, both radiological and pathological analyses were performed on the biggest one, defined as the target lesion. In these cases, the number of total lesions identified and histologically confirmed, was also recorded.
Pathological analysis
All surgical specimens were evaluated by a dedicated pathologist with more than 15 years of experience in abdominal pathology. For each patient, we do generally collect the grading system (G1, G2, and G3 for well, moderately, and poorly differentiated respectively), presence of perivisceral infiltration, intra- and peri-tumoral lymphocytic infiltration, vascular, lymphatic, and perineural infiltrations, resection margins (R0, R1, and R2), number of resected nodes, number of metastatic nodes, final pathological "T" and "N" stages according to the WHO staging system.
Statistical analysis
Continuous variables were expressed as mean ± standard deviation (SD), and compared using the U Mann–Whitney test. Categorical variables were expressed as median and IQR values and compared using the χ2 test or Friedman test, as appropriate. Correlations were computed with the Pearson or Spearman correlation coefficients, as appropriate.
To assess the reliability of agreement between the three radiologists, categorical variables were analyzed with weighted Fleiss’ or Cohen’s kappa (κ) statistics, as appropriate and as follows: < 0 poor, 0.01–0.20 slight, 0.21–0.40 fair, 0.41–0.60 moderate, 0.61–0.80 substantial, and 0.81–1.00 almost perfect [12].
The reliability of agreement of continuous variables was analyzed with intraclass correlation coefficients (ICC), as follows: < 0.50 poor, 0.51–0.75 moderate, 0.76–0.90 good, and > 0.91 excellent. Intraclass correlation coefficients (ICC) and their 95% CI were calculated based on a mean-rating (k = 2), absolute-agreement, 2-way mixed-effects model [13].
The sensitivity, specificity, accuracy, positive predictive (PPV), and negative predictive values (NPV) were computed using crosstabs, and 95%CIs were reported as well. Accuracy values were determined as the Area Under the Receiver Operating Characteristic curve (AUROC), and 95%CIs were computed using bootstrap. In the case of continuous variables, diagnostic values were obtained according to Youden’s J statistic. DeLong method was employed to compare accuracy between readers.
Data obtained by the most experienced reader (reader 1) were computed in backward ordinal and multinomial logistic regressions to determine independent predictor factors associated with “T” and “N” staging. B values were considered as Odd Ratio (OR), and 95%CIs were reported as well. Hosmer and Lemeshow test was computed to determine the goodness of fit.
All tests were two-sided, and the p-value ≤ of 0.05 was considered statistically significant. All the statistical analyses were performed using IBM SPSS 26.0 (SPSS Incorporated, Chicago, Illinois, USA).
Results
Patients population and pathological data
We firstly enrolled 100 patients. By applying inclusion and exclusion criteria, 25 were excluded due to: medical contraindication to iodinated contrast agents (n = 5), incomplete CT protocol (n = 10), and CT acquired post-operative exclusively (n = 10). Finally, the population was composed of a total of 75 patients (M/F = 32/43), with a mean age of 80 (± 11) years (Fig. 1).
According to final pathological data, the majority reported a G2 grading (n = 58, 77.3%), followed by G3 (n = 11, 14.7%) and G1 (n = 6, 8%). A total of 30 tumors (40%) were characterized by perivisceral infiltration, while the majority showed no tumor-infiltrating lymphocytes (n = 50, 66.6%). Lymphatic, vascular and perineural infiltrations were present in 32 (42.6%), 16 (21.3%), and 10 patients (13.3%), respectively. The tumor budding was low grade in 42 patients (56%). Finally, the T staging was grouped as follows: 4 patients (5.3%) T1, 11 (14.7%) T2, 44 (58.7%) T3, 11 (14.7%) T4a, and 5 (6.7%) T4b. The median number of resected nodes was 19 (IQR 14–25), and 30 patients (40%) showed positive nodes infiltration. Finally, the N staging was grouped as follows: N0 45 patients (60%), N1a 12 (16%), N1b 12 (16%), N2a 4 (5.3%), and N2b 2 (2.7). Most patients (n = 66, 88%) were in M0 staging. The final staging according to the WHO classification system and other pathological details are reported in Table 1.
CT findings and reliability analysis
According to the three readers, the most frequent tumor location was the right colon, followed by the sigmoid, left colon, and transverse. As reported in Table 2 the reliability analysis was almost perfect when considering the overall distribution [κ = 0.853 (95%CIs = 0.850–0.856)], and in its subcategories, especially for right, transverse, left, and sigmoid colon [κ = 0.856, κ = 0.522, κ = 0.813, and κ = 0.954, respectively]. The reliability analysis shows an almost perfect agreement also regarding the lesion focality [κ = 0.800 (0.796–0.804)], while a good agreement was found for the circumferential aspect of the tumor [κ = 0.671 (0.667–0.675)]. When the three readers were asked to identify bulging, fat stranding, and lateroconal fascia invasion, the agreement tended to be lower [κ = 0.478 (0.473–0.482), κ = 0.490 (0.486–0.494), and κ = 0.436 (0.431–0.440), respectively]. The enlarged vessels parameter was quite difficult to evaluate by the three readers, considering the low-reliability values [κ = 0.401 (0.397–0-404)]. The T staging showed an overall moderate agreement [κ = 0.531 (0.428–0.634)], and only the T3 group can be considered fair [κ = 0.402 (0.333–0.506)]. The evaluation of nodes shape was moderate [κ = 0.484 (0.379–0.589)], while in the evaluation of enhancement pattern, nodes cluster, nodes dimension and nodes necrosis the agreement was fair to substantial [κ = 0.431 (0.425–0.435), κ = 0.358 (0.354–0.363), ICC = 0.732 (0.680–0.765), and κ = 0.606 (0.601–0.611), respectively].
Interobserver reliability
Considering the different expertise between the three readers we computed a reliability analysis between the most experienced one with a subspecialty training in colorectal cancer patients (Reader 1), the abdominal radiologist (Reader 2), and the general radiologist (Reader 3).
The agreement between Reader 1 and Reader 2 was almost perfect when considering tumor location (κ = 0.867), and lesion focality (κ = 0.960), while moderate for circumferential appearance (κ = 0.551), lesion bulging (κ = 0.360), fat stranding (κ = 0.366), lateroconal fascia invasion (κ = 0.460), and enlarged vessels (κ = 0.487). Finally, the evaluation of the T stage reported an overall moderate agreement (κ = 0.498). Nodes evaluation reported moderate agreement for shape (κ = 0.562), enhancement pattern (κ = 0.592), and intranodal necrosis (κ = 0.616). On the other hand, the agreement was fair for the cluster (κ = 0.369), and almost perfect for dimension (ICC = 0.832).
When comparing CT findings between Reader 1 and Reader 3 all parameters showed reduced reliability for lesion features (κ = 0.288–0.545), except for tumor location and focality (κ = 0.825, and κ = 0.800, respectively), and nodes characteristics (κ = 0.333–0.477). Similar lower reliability values were found when comparing data collected by Reader 2 and Reader 3 for lesion features (κ = 0.301–0.510), except for tumor location and focality (κ = 0.799, and κ = 0.710, respectively), and nodes characteristics (κ = 0.287–0.423). All data regarding the interobserver reliability are reported in Table 3.
T staging: diagnostic performance
The preoperative CT diagnostic performance was divided into the three different “T” classes according to all readers. Sensitivity was higher for all readers for T3 lesions (Fig. 3), ranging from 60% (95%CIs: 43.6–74.4) for Reader 1 to 69% (95%CIs: 50.8–82.7) for Reader 2. The specificity was higher for T2 (Fig. 4) and T4 lesions (Fig. 5) in all readers, ranging from 84.5% (Reader 3, 95%CIs: 74.3–91.1) to 90.0% (Reader 2, 95%CIs: 79.9–95.3), and from 82.4% (Reader 3, 95%CIs: 71.6–89.6) to 85.1% (Reader 1, 95%CIs: 72.3–92.6), respectively. PPV was intermediate for all T staging classes for all lesion types, while NPV was higher for T2 lesions for all Readers, ranging from 84.4% (Reader 2, 95%CIs: 73.6–91.3) to 93.8% (Reader 3, 95%CIs: 85.0–97.5). Overall, the diagnostic accuracy was acceptable for all lesions and all Readers. In particular, we found a statistically significant difference only between Reader 2 and 3 for T2 lesions (p = 0.030), while not for other comparisons (Reader 1 vs Reader 2 p = 0.793 and Reader 1 vs Reader 3 p = 0.078). For T3 lesion the diagnostic accuracy did not show any statistical difference between the three Readers (Reader 1 vs Reader 2 p = 0.396, Reader 1 vs Reader 3 p = 0.431, and Reader 2 vs Reader 3 p = 0.892). Same results were found for T4 lesions (Reader 1 vs Reader 2 p = 0.663, Reader 1 vs Reader 3 p = 0.821, and Reader 2 vs Reader 3 p = 0.765). Data are summarized in Table 4 and ROC curves are reported in Fig. 6.
Case of 55-year-old men, affected by left colon cancer. Axial image (a) shows moderate wall thickening of the bowel wall nearby the splenic flexure, characterized by bulging and fat stranding, more evident in the coronal (b) and sagittal (c) planes. All the readers agreed with T3 staging, confirmed by pathological data
Case of 70-year-old men, affected by right colon cancer. Axial image (a) shows substenosis of the bowel determined by wall of the hepatic flexure, without any signs of extramural invasion. Images in the coronal (b) and sagittal (c) planes evaluated by the three radiologists agreed with the pathologically proven T2 staging
Case of 62-year-old female, affected by right colon cancer. Axial image (a) shows an important wall thickening of the bowel wall nearby the hepatic flexure, characterized by bulging, fat stranding, and enlarged vessels, more evident in the coronal (b) and sagittal (C) planes. All the readers agreed with T4 staging, confirmed by pathological data
ROC curves of the three readers (green line: Reader 1, black line: Reader 2, red line: Reader 3). The diagnostic accuracy was acceptable for all lesions and all readers. Only between reader 2 and 3 for T2 lesions the accuracy was statistically significant (p = 0.030), while not for other comparisons
Considering the abovementioned data, further analysis was performed based only on using data collected by the most experienced reader (Reader 1).
CT predictor features
Using data collected by the most experienced reader, the ordinal logistic regression between variables linked to the “T” stage identified lateroconal fascia invasion (in patients with right or left colon cancer, n = 54) and enlarged vessels as independent predictor factors [OR = 3.292 (95%CIs: 1.050–10.326), and OR = 2.651 (1.152–6.099), p = 0.035 and p = 0.020, respectively], with acceptable goodness of fit (χ2 = 60.010, p = 0.877).
The multinomial logistic regression between variables linked to the "N" stage identified nodes cluster and dimension as independent predictor factors of metastatic nodes [OR = 3.798 (95%CIs: 1.108–13.016), and OR = 1.083 (1.009–1.164), p = 0.022 and p = 0.029, respectively], with acceptable goodness of fit (χ2 = 45.270, p = 0.648). Variables included in both logistic regressions and specific results are reported in Table 5.
On these bases, we aimed to determine the diagnostic performance of independent predictor factors for "T" and "N" staging. The lateroconal invasion reported a maximum sensitivity of 57.1% (43.3–70.0) for T3 lesions and a maximum specificity [96.2% (81.1–99.3)] for T4 lesions, the latter with a very high PPV [93.8% (71.7–98.9)] and 68.1% (55.0–81.1) accuracy. Analogous results were obtained regarding enlarged vessels parameters, which showed a maximum sensitivity of 57.9% (42.2–72.1) for T3 lesions and a maximum specificity [89.2% (75.3–95.7)] for T4 lesions, the latter with an acceptable PPV [75% (50.5–89.8)] and 65.5% (50.8–80.2) accuracy. Data regarding diagnostic values of “T” staging are reported in Table 6.
On the other hand, nodes’ cluster showed a 52.6% (37.3–67.5) specificity and 32.4% (19.6–48.5) sensitivity to detect positive nodes status, with 57.8% (44.5–71.0) accuracy. Analogous results were found for nodes’ dimension, showing a 39% (25.7–54.3) sensitivity, 58.5% (42.2–73.6) specificity, and 52.1% (38.6–65.9) accuracy, maximizing Youden’s index (cut-off value of 7.5 mm). Data regarding diagnostic values of nodes’ positive status are reported in Table 7.
Discussion
The present study firstly aimed to evaluate the reliability analysis between three different radiologists in staging colon cancer patients, underling the almost perfect agreement regarding lesions’ location, focality, and circumferential aspect (κ = 0.853, κ = 0.800, and κ = 0.671, respectively). However, when the three readers were asked to deeply evaluate the primary colonic lesion, the reliability tended to be lower, for bulging (κ = 0.478), fat stranding (κ = 0.490), and lateroconal fascia invasion (κ = 0.436). Encouraging results were found for enlarged vessels (κ = 0.487), and T staging (κ = 0.498). Interestingly, the latter showed a fair agreement only for T2 lesions (κ = 0.418). Considering the different expertise between readers in the evaluation of colon cancer, a sub-analysis was performed to determine if this aspect can directly influence the overall reliability. Our results showed that the subspecialty expertise of Reader 1 and the higher years of experience in abdominal imaging of Reader 2 were more reliable when compared to Reader’s 3 experience. The presented results demonstrated that the agreement between Reader 1 and Reader 2 was of utmost acceptable regarding all tumor parameters. On the other hand, when comparing data collected by Reader 1 or Reader 2 with general radiology experience of Reader 3, the reliability analysis tended to be lower.
These results are supported and in line with a recently published paper by Hong et al. [14], who demonstrated that the overall diagnostic performance in differentiating colon cancer according to T stage, can increase along with the reader’s experience, proportionally. Elibol et al. [15] by enrolling 141 patients, showed an overall moderate agreement between two readers (κ = 0.425), in line with our results. Similar results were published in 2014 by Burton et al. [16] by enrolling 33 patients. Authors demonstrated a fair agreement between (κ = 0.206) two readers but without reporting their expertise. The latter aspect should be always profoundly evaluated, as recently demonstrated and as above-mentioned [14]. Analogous results, by evaluating more than 4000 patients, were reported by Sjövall et al. [17], with an overall fair agreement (κ = 0.440) for T staging. Also, Korbakke et al. [18] by enrolling a wide population of patients affected by colon cancer (n = 974), demonstrated that the agreement between readers was fair for T staging (κ = 0.270). CECT, even if it can be a useful tool (able also to explore the whole body and to identify patients with distant metastasis), lacks strength and reproducibility, especially with not enthusiastic reliability among readers in the evaluation of T staging.
Moreover, even if Hong et al. [14] demonstrated that the improvement in metastatic nodes detection is not strictly associated with readers’ experience, our results reported an overall acceptable agreement: in fact, the reliability analysis showed that nodes’ dimension (largest short axis) and internal necrosis were substantial (ICC = 0.432, and κ = 0.606, respectively), while the other parameters cannot be considered as reproducible. These results are encouraging, especially referring to a study by Hong et al. [19], who demonstrated that the largest short diameter and the presence of internal inhomogeneity are independent factors of malignancy, together with the number of nodes in the peritumoral area. Indeed, our study also showed similar results, considering that multinomial logistic regression among variables related to the "N" stage identifies node cluster and size as independent predictors of node metastasis. However, CT remains relatively nonspecific for N-stage determination even because of slight agreement on the critical lymph nodes’ cutoff diameter. We considered a dimensional cutoff based on a short axis diameter of 7.5 mm, based on the median value of positive nodes, demonstrating relatively low sensitivity (39%), specificity (58.5%), and accuracy (52.1%). According to a meta-analysis by Nerad et al. [20], many studies attempted to establish a critical size cutoff using long axis diameter to offer an optimal compromise between sensitivity and specificity. However, as the value increased, sensitivity decreased. This was the case of Keeney et al. [21] who raised the long axis diameter cutoff to 15 mm, obtaining an almost perfect specificity against very poor sensitivity (13%). Conversely, by reducing the value to 5 mm, as reported by Dighe et al. [22], they obtained relatively low sensitivity (64%) and specificity (53%).
Even if the agreement values between readers were not enthusiastic, especially due to the stringent k-statistic method and the lack of chance adjustment, we aimed to evaluate the analysis of each reader’s observation to evaluate the diagnostic performance of pre-operative CECT, according to the T classes. Sensitivity was higher for all readers for T3 lesion, ranging from 60 to 69%, for Readers 1 and 2, respectively. On the other hand, specificity was higher for T2 and T4 lesions for all readers, ranging from 84.5% to 90%, and from 82.4% to 85.1%, respectively. The diagnostic accuracy of our readers outperformed Elibol et al. [15] in determining the T stage of the tumor: accuracy was 55% for observer 1 and 51% for observer 2, while in our case two out of three readers reached an accuracy of at least 81.1%, 50.7%, and 61.4% for T2, T3 and T4 lesions respectively. This aspect can be strictly linked to the readers’ experience, even if we found a statistically significant difference only between readers 2 and 3 for T2 lesions, while not for other comparisons. Even if CECT is a fundamental imaging technique to stage colon cancer, especially to depict distant metastasis, to date only 13 studies were published in literature and meta-analyzed by Nerad et al. [20]. The Authors reported a pooled sensitivity, specificity and diagnostic ORs of 90%, 69%, and 20.6 for T3-T4 lesions, in line with our results.
On these bases, the final aim of our study was to evaluate if some of the collected parameters can be considered as independent factors linked to the T staging. The logistic regressions showed that lateroconal invasion and enlarged vessels can be considered independent predictor factors for the T stage (OR = 3.292 and OR = 2.651, respectively), with a 57.1% and 57.9% sensitivity for T3 lesions and 96.2% and 89.2% specificity for T4 lesions, respectively. Moreover, the lateroconal fascia invasion demonstrated a very high PPV (93.8%) for T4 lesions.
To date, extramural vascular invasion (EMVI) is recognized as a pre-surgical high-risk factor and has been incorporated into the FOxTROT trial for patient treatment stratification [7]. Despite the little agreement previously demonstrated among readers, these results prove the value of recognizing these imaging features as potentially predictive of locally advanced tumors. A lack of sufficient expertise in radiologic EMVI identification through CT imaging may have contributed to these results.
On the other hand, the multinomial backward regressions were computed to determine independent factors associated with positive nodes status, showing that nodes cluster and dimension can be considered independent of metastatic nodes (OR = 3.798 and OR = 1.083, respectively), with 52.6% and 39% sensitivity, and 57.8% and 58.5% specificity, respectively. Even if considered as independent predictor factors of positive nodes status, they demonstrated not encouraging diagnostic values, in line with studies reported above.
The present study has some limitations. First, it was retrospective and with a small sample size. Secondly, it was not possible to perform a sub-analysis according to the location due to the small numbers of each tumor, and this might add a bias. Thirdly, the lateroconal fascia invasion parameter was evaluated only in ascending and descending tumors, according to anatomy, but this aspect is explained by anatomic reasons.
To conclude, our study demonstrated that CECT can be considered a useful tool in the preoperative staging of colon cancer patients and that reader’s experience is one of the most important factors associated with the correct classification. Moreover, CECT can help depict radiological features independently associated with T and N stages with acceptable diagnostic values.
References
Binefa G, Rodriguez-Moranta F, Teule A, Medina-Hayas M. Colorectal cancer: from prevention to personalized medicine. World J Gastroenterol 2014; 20:6786–6808
Ponz de Leon M, Sant M, Micheli A, et al. Clinical and pathologic prognostic indicators in colorectal cancer. A population-based study. Cancer 1992;69:626–635.
Amin MB, Greene FL, Edge SB, Compton CC, Gershenwald JE, Brookland RK, et al. The Eighth Edition AJCC Cancer Staging Manual: continuing to build a bridge from a populationbased to a more “personalized” approach to cancer staging. CA Cancer J Clin 2017;
Tudyka V, Blomqvist L, Beets-Tan RG, et al. EURECCA consensus conference highlights about colon & rectal cancer multidisciplinary management: the radiology experts review. Eur J Surg Oncol 2014; 40:469–475
Kathryn J. Fowler, Harmeet Kaur, Brooks D. Cash, Barry W. Feig, Kenneth L. Gage, et al., ACR Appropriateness Criteria Pretreatment Staging of Colorectal Cancer Expert Panel on Gastrointestinal Imaging. Journal of The American College of Radiology, 2017.
C. Hunter, M. Siddiqui, T. Georgiou Delisle, H. Blake, N. Jeyadevan, M. Abulafi, et al., CT and 3-T MRI accurately identify T3c disease in colon cancer, which strongly predicts disease-free survival, Clin. Radiol. 72 (2017) 307–315.
Horvat N, Raj A, Liu S, et al (2019) CT Colonography in Preoperative Staging of Colon Cancer: Evaluation of FOxTROT Inclusion Criteria for Neoadjuvant Therapy. American Journal of Roentgenology 212:94–102. https://doi.org/https://doi.org/10.2214/AJR.18.19928
A. Nørgaard, C. Dam, A. Jakobsen, J. Pløen, J. Lindebjerg, S.R. Rafaelsen, Selection of colon cancer patients for neoadjuvant chemotherapy by preoperative CT scan, Scand. J. Gastroenterol. 49 (2014) 202–208.
Dighe S, Blake H, Koh MD, Swift I, Arnaout A, Temple L, Barbachano Y, Brown G, Accuracy of multidetector computed tomography in identifying poor prognostic factors in colonic cancer (2010). Br J Surg 97 (9):1407-1415.
Elias Nerad, Max J. Lahaye, Monique Maas, Patty Nelemans, Frans C. H. Bakers, Geerard L. Beets, Regina G. H. Beets-Tan, Diagnostic Accuracy of CT for Local Staging of Colon Cancer: A Systematic Review and Meta-Analysis (2016), AJR:207.
Eun Kyoung Hong, Federica Landolfi, Francesca Castagnoli, Sae Jin Park, Judith Boot, Jose Van den Berg, Jeong Min Lee, Regina Beets‐Tan, CT for lymph node staging of Colon cancer: not only size but also location and number of lymph node count (2021), Abdominal Radiology
McHugh ML (2012) Interrater reliability: the kappa statistic. Biochem Med (Zagreb) 22:276–282
Koo TK, Li MY (2016) A Guideline of Selecting and Reporting Intraclass Correlation Coefficients for Reliability Research. Journal of Chiropractic Medicine 15:155–163. https://doi.org/https://doi.org/10.1016/j.jcm.2016.02.012
Hong EK, Castagnoli F, Gennaro N, et al (2021) Locoregional CT staging of colon cancer: does a learning curve exist? Abdom Radiol 46:476–485. https://doi.org/https://doi.org/10.1007/s00261-020-02672-7
Elibol FD, Obuz F, Sokmen S, et al (2015) The role of multidetector CT in local staging and evaluation of retroperitoneal surgical margin involvement in colon cancer. Diagn Interv Radiol 22:5–12. https://doi.org/https://doi.org/10.5152/dir.2015.15089
Burton S, Brown G, Bees N, et al (2008) Accuracy of CT prediction of poor prognostic features in colonic cancer. BJR 81:10–19. https://doi.org/https://doi.org/10.1259/bjr/19492531
Sjövall A, Blomqvist L, Egenvall M, et al (2016) Accuracy of preoperative T and N staging in colon cancer - a national population-based study. Colorectal Dis 18:73–79. https://doi.org/https://doi.org/10.1111/codi.13091
Korsbakke K, Dahlbäck C, Karlsson N, et al (2019) Tumor and nodal staging of colon cancer: accuracy of preoperative computed tomography at a Swedish high-volume center. Acta Radiologica Open 8:205846011988871. https://doi.org/https://doi.org/10.1177/2058460119888713
Hong EK, Landolfi F, Castagnoli F, et al (2021) CT for lymph node staging of Colon cancer: not only size but also location and number of lymph node count. Abdom Radiol 46:4096–4105. https://doi.org/https://doi.org/10.1007/s00261-021-03057-0
Nerad E, Lahaye MJ, Maas M, et al (2016) Diagnostic Accuracy of CT for Local Staging of Colon Cancer: A Systematic Review and Meta-Analysis. American Journal of Roentgenology 207:984–995. https://doi.org/https://doi.org/10.2214/AJR.15.15785
Keeney G, Jafri SZH, Mezwa DG (1989) Computed tomographic evaluation and staging of cecal carcinoma. Gastrointest Radiol 14:65–69. https://doi.org/https://doi.org/10.1007/BF01889158
Dighe S, Purkayastha S, Swift I, et al (2010) Diagnostic precision of CT in local staging of colon cancers: a meta-analysis. Clinical Radiology 65:708–719. https://doi.org/https://doi.org/10.1016/j.crad.2010.01.024
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors of this manuscript declare no relationships with any companies, whose products or services may be related to the subject matter of the article.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Ippolito, D., Maino, C., Bianco, I. et al. The usefulness of preoperative CT in colon cancer staging: impact of radiologists’ experience. Abdom Radiol 48, 1215–1226 (2023). https://doi.org/10.1007/s00261-023-03835-y
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00261-023-03835-y