Abstract
Patients with human immunodeficiency virus (HIV) can present with a wide range of different acute and chronic pathologies. Anorectal conditions are particularly common in this unique patient population, including pathologies, such as proctitis, anorectal abscess, anorectal fistula, and anal squamous cell carcinoma. The radiologist plays a critical role in the assessment of these common forms of anorectal disease, as these conditions can present with various findings on imaging assessment. Pelvic CT, MRI, and FDG-PET/CT are among the most common modalities used for assessment of anorectal disease in the HIV patient population. Knowledge of the fundamental clinical and imaging findings associated with these pathologies in HIV patients is critical for radiologists.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Human immunodeficiency virus (HIV) infection is a common infectious disease, with recent estimates by the Centers for Disease Control (CDC) reporting nearly 1.2 million people aged 13 or older living with HIV infection in the USA at the end of 2019 [1]. Furthermore, the number of patients who died from complications of acquired immunodeficiency syndrome (AIDS) in 2020 was nearly 700,000, indicating the importance of proper management of HIV/AIDS [2].
The manifestations of HIV infection, related to both the virus itself and its effects on the immune system, are highly variable. Within the gastrointestinal system, esophageal disorders, gastric disorders, enteritis, diarrhea, and colitis represent some of the most common findings found in such patients [3]. Furthermore, diseases of the anus and rectum are also common in patients with HIV, attributed to HIV-related infections and other risk factors. Studies of anorectal disease in HIV patients from the pre-active retroviral therapy (ART) era have well demonstrated the high prevalence of anorectal disease in this patient population, while a study examining the prevalence of anal lesions in HIV patients during the current ART era found that 44% of patients involved had at least one anal macroscopic lesion [4,5,6,7]. Although studies that have compared the prevalence of anorectal disease between these two eras have found differences in the incidence of various conditions, it is clear that anorectal pathology has not been substantially altered with the advent of such therapy [8, 9].
Studies examining sexually transmitted infections of the rectum and anus, as well as other pathologies of this region have revealed that symptoms are often non-specific, which often limits their usefulness as markers of pathology [10, 11]. Given the prevalence of anorectal disease in patients with HIV, even in the current ART era, as well as the often non-specific presenting complaints, there is a clear role for imaging in the proper diagnosis and management of such conditions (Table 1). As such, we aim to provide an imaging-based review of some of the most common manifestations of HIV within the anorectum, highlighting the relevant pathophysiology, relationship to HIV, as well as management of these processes. Special attention will be given to the role of imaging in the diagnosis and management of these HIV-related anorectal conditions.
Proctitis
Proctitis refers to an inflammatory change of the anal canal and rectal mucosa, most often seen as a side effect of radiation therapy, antibiotic use, or infection. Proctitis of infectious etiology is of particular interest given the high rates of HIV-infected individuals with a concurrent history of STD infection [12]. Patients with infectious proctitis often present with pruritis, rectal, and anal pain, which all may lead to avoidance of defecation. If left unresolved, fibrosis and endarteritis of the arterioles may ensue, ultimately leading to rectal tissue ischemia and other anorectal pathologies, including mucosal friability, bleeding, ulcers, strictures, and fistula formation [13]. The most common causes of infectious proctitis are Neisseria gonorrhoeae, Chlamydia trachomatis, and herpes simplex virus (HSV) types 1 and 2, all of which can be transmitted through unprotected anal intercourse [14].
Proctitis can present with various imaging findings on evaluation with cross-sectional imaging. Although proctitis may be self-limited and frequently managed conservatively, multidetector computed tomography (MDCT) is often the modality of choice in the case of persistent or severe symptoms. On CT assessment, the rectal wall will typically show circumferential thickening, with other potential findings, including perirectal inflammatory changes and enlarged perirectal and pelvic side wall lymph nodes (Fig. 1) [15]. Mucosal hyperenhancement is also commonly seen following intravenous contrast administration. Prior case reports of Chlamydia trachomatis proctitis have demonstrated findings on other modalities, including nodular rectal lesions on barium fluoroscopic studies and numerous small and nodular hypoechoic mucosal lesions on endoscopic ultrasound [16].
Patients with HIV presenting with clinical concern for proctitis. a 39-year-old male with history of HIV and previous perirectal abscess worsening rectal pain. A contrast-enhanced CT study of the pelvis was obtained. Axial CT images of the pelvis demonstrate diffuse circumferential rectal wall thickening with associated perirectal fat stranding (arrow). Imaging and clinical findings in this patient were consistent with a diagnosis of proctitis. b 17-year-old male presenting with severe rectal pain. Axial contrast-enhanced CT image of the pelvis demonstrates marked rectal wall thickening (arrow), perirectal stranding, and multiple enlarged perirectal lymph nodes (arrowhead)
MRI represents an additional commonly utilized modality for the assessment of suspected proctitis. Compared to CT, MRI offers relatively superior soft tissue contrast and anatomic definition [17]. Additional benefits of MRI include lack of ionizing radiation, which often makes MRI the modality of choice in patients who are pregnant, younger, or have contraindications to iodinated intravenous contrast agents [18]. While protocols may vary by institution, typical pelvic MR sequences obtained for proctitis assessment include high-resolution three-plane T2-weighted sequences, fat-suppressed T2-weighted sequences, and fat-suppressed T1-weightged sequences following administration of gadolinium-based intravenous contrast [19].
Findings suspicious for proctitis on MRI assessment may include abnormal mural thickening, mucosal hyperemia and enhancement, and reactive lymphadenopathy. One study by Tutein Nolthenius et al. found that perianal MRI findings of wall thickening, presence of perimural fat, creeping fat, and mesorectal lymphadenopathy demonstrated significant correlations with endoscopic findings of proctitis [19]. This same study also found that increased perimural signal intensity on T2-weighted sequences and increased perimural enhancement on post-contrast sequences correlated with endoscopic findings with high reproducibility among observers. Pelvic MRI also serves as an especially useful modality in the assessment of proctitis-related complications, including perirectal or perianal fistula formation and abscesses [15].
Notably, findings of irregular rectal wall thickening and pelvic lymphadenopathy in the setting of infectious proctitis can also potentially mimic rectal malignancy. Clinical, endoscopic, and histologic evaluation therefore may be required to distinguish infectious processes from HIV-related malignancy [20]. Clinical correlation is also often required for differentiation between subtypes of proctitis, such as infectious, ischemic, or radiation-induced etiologies. In addition to its confirmatory role in the diagnosis of proctitis, evaluation with pelvic CT or MRI is often needed to rule out related complications, such as perirectal abscesses.
HIV-positive patients who have progressed to acquired immunodeficiency syndrome (AIDS) may develop radiation-induced proctitis secondary to radiotherapy for AIDS-defining malignancies [21]. Patients with AIDS-defining malignancies are at increased risk of iatrogenic proctitis due to radiation-induced mucosal injury and subsequent tissue remodeling and ischemia [22]. Given that HIV and associated HPV infection increase susceptibility for anal cancer, which may be treated with radiation therapy, it is not uncommon for HIV patients to suffer from chronic radiation proctitis. Patients with radiation-induced proctitis will typically exhibit symptoms between 6 weeks of beginning radiation and 9 months after radiation completion; as such, response and tolerance to radiotherapy during this time period should be closely monitored [21]. The first line of therapy often involves an anti-inflammatory agent. Based on the American Society of Colon and Rectal Surgeons Clinical Practice Guidelines for the treatment of chronic radiation proctitis, bleeding patients with chronic radiation proctitis may be treated medically with formalin application or sucralfate retention enemas, while short-chain fatty acid enemas are not recommended [23]. Interventional treatments for this disease include hyperbaric oxygen or endoscopic argon beam plasma coagulation.
Anorectal abscess and fistula
Anorectal fistulas and abscesses are often considered as representing different phases of the same disease spectrum. Abscess development typically occurs in the early phase of disease, with the fistula representing a late phase of disease. The inciting incident responsible for the development of anorectal abscess is most commonly an infection in the anal glands, which can result in anal sepsis if not treated appropriately [24]. Most commonly, fistulas will develop from pre-existing abscess, particularly when the abscess recurs in the same location or remains unhealed. In a retrospective cohort study examining the factors associated with chronic anal fistula or recurrent sepsis following perianal abscess, researchers found that the cumulative incidence of chronic anal fistula or recurrent sepsis was 36.5%, with risk factors, including age < 40 years and diabetes [25].
While anorectal abscess and fistula are not uncommon in the general population, those with HIV exhibit a higher prevalence of this condition [26]. Although the advent of HAART has resulted in a decreased incidence in the prevalence of many forms of perianal disease in HIV patients, including fistulas, this condition merits discussion due to its unique relationship with HIV [9]. One such component of this relationship is the notion that HIV status may influence the anatomy of fistula formation. For example, one study by Manookian et al. found that incomplete anal fistulas and incomplete fistulas ending in blind sinuses were more likely to occur in HIV-positive patients [27]. This same study found that HIV-positive and HIV-negative patients demonstrated no significant differences in terms of fistula location relative to the dentate line. Given the risk factors associated with HIV infection, most importantly anal intercourse, it is unsurprising that HIV is associated with an increased propensity toward abscess development. Importantly, many of the STI’s that may co-infect with HIV, such as chlamydia, are also associated with anal abscess and fistula formation, even in those unaffected by HIV [10, 28].
The most common presentation of anorectal abscess is non-specific, encompassing symptoms such as erythema, tenderness, and pain with defecation [29]. However, the presence of fluctuant swelling adjacent to the anus is a key physical exam finding that may point toward the diagnosis of abscess. According to the American Society of Colon and Rectal Surgeons recent guidelines on the management of anorectal abscess and fistula, the initial evaluation of such patients requires inspection of the anoperineum, with digital rectal examination and anoproctoscopy being used as necessary to confirm the diagnosis [30]. Importantly, careful examination of the perineum should also include attempts to visualize the presence of the external opening of a fistula. As the treatment of anorectal fistulas necessitates a detailed understanding of the course of the fistula, the use of imaging, such as CT, US, MRI, or fistulography, may be necessary in both diagnosis and in planning for treatment [30, 31].
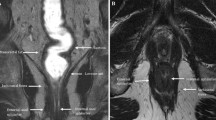
Pelvic MRI plays a critical diagnostic role in the assessment of patients with suspected anorectal fistulas and abscesses (Fig. 2). Prior studies have demonstrated high sensitivity and specificity for MRI in the detection of both anorectal fistulas (sensitivity 100%, specificity 86%) and abscesses (sensitivity 96%, specificity 97%) [32]. In patients with suspected anorectal fistulas, pelvic MRI is often employed to grade and characterize the precise course of fistulous tracts. MRI protocols for fistula evaluation vary among institutions but typically rely upon high-resolution T2-weighted sequences and T1-weighted post-contrast gradient echo sequences [33]. Active fistulas will appear as linear tracts with hypointense signal on T1-weighted images, hyperintense signal on T2-weighted images, and enhancement on post-contrast sequences. In contrast, inactive fistulas typically demonstrate hypointense signal on T1-weighted images, lack of hyperintense signal on T2-weighted images, and no significant enhancement.
32-year-old male with HIV presenting with concern for perirectal abscess or fistula. A fistula protocol pelvic MRI study with gadolinium contrast was ordered for further evaluation. Axial T2-weighted images reveal branching bilateral perianal fistulous tracts (arrows in a and b). A serpiginous fluid-filled blind ending sinus tract vs. fluid collection is also seen between the anal canal and urethra (arrow in c), which was concerning for a small abscess. Axial post-contrast T1-weighted images demonstrate mild enhancement associated with the patient’s left-sided fistulous tract (arrow in d)
MRI plays a particularly important role in establishing the relationship of the fistula to the anal sphincter complex. Surgical classification of perianal fistulas is performed utilizing the Parks classification, which divides perianal fistulas into one of four categories: intersphincteric, transsphincteric, suprasphincteric, and extrasphincteric [34]. The relationship of the fistulous tract to the internal and external sphincters on a coronal plane is the key feature which distinguishes these different subtypes. Intersphincteric fistulas traverse the intersphincteric space but do not extend across the external sphincter. In contrast, transsphincteric fistulas pass through the external sphincter after traversing the intersphincteric space, ultimately coursing into the ischiorectal fossa. Suprasphincteric fistulas will extend superiorly through the intersphincteric space and over the puborectalis muscle before descending inferiorly through the levator muscles into the ischiorectal fossa. Extrasphincteric fistulas do not cross the intersphincteric space but rather track from the rectum directly through the levator muscles and into the ischiorectal fossa. These subtypes will then typically terminate in the perianal skin, which can often be identified on physical examination.
Radiologists have developed the St James’s University Hospital classification to classify perianal fistulas based on MRI findings [35]. This classification divides fistulas in 5 different grades based on anatomic position in axial plane and the presence of secondary tracts or abscesses. These MR-based categories include grade 1 (simple linear intersphincteric), grade 2 (intersphincteric with abscess or secondary tract), grade 3 (transsphincteric), grade 4 (transsphincteric with abscess or secondary tract within the ischiorectal fossa), and grade 5 (supralevator and translevator extension) [34].
Abscess development remains one of the key complications of perianal and perirectal fistulas. Prompt diagnosis of anorectal abscesses is essential, as surgical evaluation and drainage are often required. In the acute setting, CT is often the initial modality of choice in the setting of suspected perianal abscess (Fig. 3). Anorectal abscesses typically present on pelvic CT as thick-walled fluid collections which may contain air with surrounding inflammatory changes [36]. CT can be utilized to classify anorectal abscesses into one of several key subtypes based on location, including perianal or superficial, ischioanal, intersphincteric, and supralevator [37]. On evaluation with MRI, anorectal abscesses will appear as perirectal collections classically with hyperintense signal on T2-weighted images, diffusion restriction, and peripheral enhancement [38].
42-year-old male with HIV presenting with left buttock cellulitis and concern for possible abscess. a, b Contrast-enhanced axial CT images of the pelvis demonstrate an irregular fluid collection adjacent to the lower rectum (arrow in a), consistent with a perirectal abscess. Extensive stranding and foci of subcutaneous air are seen in the soft tissues of the left buttock compatible with cellulitis and deep soft tissue infection (arrow in b). The patient subsequently underwent treatment with long-term antibiotics, perirectal abscess drainage, and seton placement. c Follow-up contrast-enhanced CT study obtained several months following initial diagnosis demonstrates significant interval improvement in inflammatory changes in the left buttock with a seton in place (arrow)
Image-guided drainage of anorectal abscesses can be performed via several techniques, such as percutaneous approaches under CT guidance or transrectal approaches under ultrasound guidance. Such techniques allow for prompt drainage of anorectal fluid collections, which could otherwise lead to sepsis and other complications if not drained in the appropriate timeline. More recently, endoscopic ultrasound has been presented as an alternative means of image-guided drainage. Endoscopic ultrasound-guided drainage may be particularly useful if the abscess location precludes a percutaneous approach. Prior case series utilizing this technique have also reported benefits, including the ability to deploy transrectal stents without requirement for drainage catheters [39].
The mainstay of treatment for acute anorectal abscess is incision and drainage, with concomitant fistulotomy being recommended in those patients who have concurrent simple anal fistulas [30]. Complex abscesses may require examination under anesthesia for proper drainage, both for appropriate identification and to minimize patient discomfort [24]. The most common complication of incision and drainage is recurrence of abscess, often associated with inadequate or delayed drainage [30]. The proper treatment for anorectal fistulas has been widely debated. However, it is agreed upon that simple fistulas may be easily treated with fistulotomy, while more complex fistulas often require more intricate methods [31]. One of the most important considerations in the treatment of anorectal fistulas is the degree of involvement of the sphincters, with preservation of sphincter function being a key treatment goal. Given this, the guidelines of the American Society of Colon and Rectal Surgeons recommend the use of endoanal advancement flaps and the ligation of intersphincteric fistula tract (LIFT) procedure while advising against the use of fistula plug or fibrin glue [30]. Despite the efficacy of the LIFT procedure and other aforementioned treatment modalities, complications of fistula management are important to recognize. The most common complication to be aware of is incontinence, due to disruption of sphincteric function, most often seen with fistulotomy performed for complex fistulas [30].
Squamous cell carcinoma of the anus
Squamous cell carcinoma of the anus (SCCA) represents a rare but clinically important consideration in patients with HIV. A multicenter cohort study by Silverberg et al. examined the risk of SCCA in HIV-infected and HIV-uninfected patients in North America, finding that HIV-infected men who have sex with men (MSM) and other HIV-infected men had incidence rates of 131 and 46 per 100,000 person years, respectively, compared to 2 per 100,000 person years for HIV-uninfected males [40]. Further studies examining the incidence of cancers in HIV-infected patients have supported this increased incidence of SCCA [41,42,43]. The predominant risk factor for the development of SCCA involves infection with human papillomavirus (HPV), in particular HPV-16 and HPV-18 [44,45,46]. Other important risk factors associated with the development of SCCA in HIV-infected patients are low CD4 count, smoking history, and history of AIDS [42].
Diagnosis of SCCA is often delayed due to the generally non-specific symptoms, which may appear in combination with a mass, non-healing ulcer, pain, itching, discharge or fecal incontinence, and fistula. The imaging workup of anal cancer includes both locoregional staging and evaluation for sites of distant metastasis (Fig. 4). Pelvic MRI remains the primary imaging modality for initial local evaluation and staging of anal malignancy [47]. Transanal endoscopic ultrasound can also play a complementary role in initial local staging. Whole-body staging with 18F-fluorodeoxyglucose positron emission tomography computed tomography (PET/CT) scans or computed tomography (CT) scans are typically obtained to assess for distant metastases.
45-year-old male with AIDS and Kaposi sarcoma who presented for evaluation of abdominal pain and distension. a, b A contrast-enhanced CT study of the chest, abdomen, and pelvis was obtained. Axial contrast-enhanced CT images demonstrate extensive retroperitoneal, mesenteric, and thoracic lymphadenopathy (arrows in a). Irregular mass-like thickening of the anal canal is also visualized (arrow in b). c–e A contrast-enhanced rectal protocol MRI study of the pelvis was subsequently obtained. A partially necrotic soft tissue mass measuring 4.2 × 3.4 cm is visualized within the anal canal with extension into the distal rectum with associated invasion into the anal sphincter musculature and perirectal fat. This mass demonstrates predominantly intermediate signal intensity on T2-weighted images (arrow in c), heterogeneous enhancement (arrow in d), and marked diffusion restriction on DWI sequences (arrow in e). f A full-body FDG-PET/CT study was subsequently performed. Axial-fused FDG-PET/CT image of the pelvis reveals marked FDG uptake associated with the patient’s anal soft tissue mass with a maximum SUV of 14.5 (arrow). Subsequent biopsy of the patient’s anal mass confirmed a diagnosis of anal squamous cell carcinoma
The updated Eighth Edition of the AJCC TNM staging criteria established in 2016 utilizes tumor size as the primary factor or the T stage of anal cancers [48]. The greatest dimension of the tumor is used to grade tumors as T1 (< 2 cm), T2 (2–5 cm), T3 (> 5 cm), or T4 (any size with invasion of adjacent organs). In the case of T4 disease, tumors will demonstrate invasion of nearby organs such as the bladder, urethra, or vagina. Notably, however, involvement of the rectal wall, subcutaneous tissues, perianal skin, and muscles of the sphincter complex are not considered to be T4 disease. Regional lymph node status (N) can be divided into N0 (no regional lymph node metastasis) and N1 (regional lymph node metastasis), with subdivisions of N1a, N1b, and N1c based on specific lymph nodes involved. Metastasis (M) can be categorized as M0 (no distant metastasis) and M1 (distant metastasis).
Anal cancer protocol MRI studies typically employ high-resolution T2-weighted sequences obtained with a small field of view and multidirectional planes through the axis of the tumor [49]. In the setting of newly diagnosed anal carcinoma, pelvic MRI plays a critical role in determining the T and N staging of anal malignancies. This initial staging assists in clinical management by helping to define tumor margins, guide surgical and systemic treatments and identify high-risk tumor features with prognostic implications [50]. MRI also plays an important role in assessing for local recurrence following surgical resection or systemic therapy.
Anal cancer typically presents as semi-circumferential or circumferential anal wall thickening or a lobulated mass involving the anal canal. The majority of anal cancers are classified as T1 or T2 at initial staging and are typically located within 1.5 cm of the anal verge [50]. Most commonly anal malignancies will demonstrate intermediate to hyperintense signal on T2-weighted or STIR sequences and intermediate to hypointense signal on T1-weighted sequences. MRI provides an accurate assessment of maximal tumor length, which is the key factor defining the T stage of anal cancer. This modality also serves a critical role in assessing extramural extension of disease, such as involvement of the sphincter complex or adjacent organs, such as the bladder, urethra, or vagina [51].
In addition to evaluating size and local extent of the primary tumor, MRI is useful for nodal staging. Tumors located superior to the dentate line will typically spread to perirectal, internal iliac, presacral, and retroperitoneal lymph nodes. In contrast, tumors located inferior to the dentate line will classically spread to inguinal and femoral lymph node [52]. Suggested short-axis diameter cutoffs for pathologic lymphadenopathy have included 8 mm for pelvic lymph nodes, 5 mm for perirectal lymph nodes, and 10 mm for inguinal lymph nodes [51].
In patients with anal squamous cell carcinoma, evaluation for distant metastatic disease is typically performed via whole-body FDG-PET/CT imaging. PET/CT is currently recommended by the National Comprehensive Cancer Network (NCCN) for the purpose of verifying staging prior to treatment, including evaluation of pathologic lymph node involvement [53]. A prior meta-analysis performed by Mahmud et al. found that PET or PET/CT studies obtained for anal cancer evaluation led to upstaging in 5.1–37.5% of patients and downstaging in 8.2–26.7% of patients, with resulting treatment modifications occurring in 12.5–59.3% of patients [54]. Given the impact PET/CT has on altering initial clinical staging, this modality is routinely performed in patients with anal squamous cell carcinoma. More recent studies have also suggested that FDG-PET may play a clinically valuable role in assessment of disease recurrence. For example, one study by Houard et al. found that post-treatment FDG-PET/CT had a sensitivity for detection of residual tumor of 92% and a specificity of 85% [55]. In this same study, patients with complete metabolic response (CMR) on FDG-PET/CT demonstrated a significantly higher 2-year progression-free survival of 96% compared to 28% for non-CMR patients.
According to the most recent NCCN guidelines, the mainstay of treatment for patients with SCCA is concurrent chemotherapy and radiation, with surgery remaining a possible therapy in select patients [56]. Anal margin tumors can be treated by surgery, as opposed to anal canal tumors, which have a worse prognosis and require treatment with definitive chemoradiotherapy [57]. For localized disease, concurrent chemoradiotherapy (CRT) with 5-fluorouracil infusion and mitomycin has been established as the standard treatment for non-metastatic anal cancer [56, 58]. Pelvic MRI and FDG-PET/CT are among the most common modalities employed to assess for potential recurrence of disease following initial treatment [50, 54] (Fig. 5).
62-year-old with history of HIV and recurrent anal squamous cell carcinoma with ulceration. The patient presented with concern for recurrent disease 16 years following initial radiation treatment. a–c An FDG-PET/CT study was obtained for further evaluation. Axial CT image of the pelvis demonstrates irregular mass-like thickening of the anal canal (arrow in a). Axial-fused FDG-PET/CT images reveal marked hypermetabolic activity along the right aspect of the anal canal with a maximum SUV of 9.1 (arrow in b). Inferior to this area, a large necrotic component of this mass with lack of FDG uptake is identified (arrow in c). d–f A pelvic MRI study with gadolinium contrast was acquired for further evaluation. Axial T2-weighted images demonstrate a large infiltrating anal mass replacing the anal sphincter complex (arrow in d) with an associated large area of cavitation (arrow in e). This mass was found to diffusely infiltrate the right levator muscle, obturator muscle, lower rectum, prostate, seminal vesicles, and bladder neck. Axial post-contrast T1-weighted image reveals heterogeneous enhancement associated with this partially necrotic mass (arrow in f). Biopsy of the patient’s anal mass confirmed a diagnosis of recurrent anal squamous cell carcinoma
Finally, several studies have examined whether special precautions are necessary in HIV-infected patients receiving chemotherapy and radiation therapy. When CRT was first applied to HIV-infected patients, reduced doses of radiotherapy and chemotherapy were given as a precaution against the compromised immunologic status and the increase of hematologic and mucosal toxicity. However, recent studies have demonstrated that HIV-infected patients receiving concomitant effective HAART and CRT do not have significantly higher rates of associated toxicities compared to HIV-uninfected patients [59,60,61].
Miscellaneous conditions
Anal ulcers and fissures
In patients with HIV, anal ulcers are a common pathology most often caused by intercourse-related tears and subsequent viral infections [12]. Wide-based anal ulcers are the most prevalent non-condylomatous lesions occurring in individuals with HIV [12]. An anal ulcer refers to a lesion that disrupts the muscularis mucosae, involving the submucosa and possibly deeper layers of tissue. An ulcer in the squamous epithelium of the distal anal canal is termed an anal fissure [62]. Primary anal fissures are due to local trauma, typically anal intercourse, while secondary anal fissures are due to underlying disease, such as sexually transmitted infections (STI), HIV, or malignancy.
Patients with anal fissures will typically present with severe pain during defecation, pruritus, and rectal bleeding. These patients may also avoid defecation because of the pain, leading to chronic constipation and increased distention of the anal mucosa [63]. On clinical examination, a laceration may be identified with possible fibrotic changes. Imaging plays a complementary role with physical and clinical evaluation in the assessment of anal fissures and ulcerations. Such anal and perianal pathologies are most commonly evaluated with pelvic MRI. On T2-weighted images, anal fissures typically appear as small areas of hyperintense signal classically located at the posterior wall of the anal canal [38]. While ulcerations and fissures are often diagnosed on clinical exam, MRI can be critical for ruling out additional entities, including perianal abscesses or fistulas.
The clinical management of anal fissures involves aggressively treating any underlying infections first. While HSV and CMV are major causes of infection-mediated anal ulcers in HIV-positive individuals, most ulcers are idiopathic, with negative cultures and biopsies [12]. Subsequently, treatment is geared toward symptom resolution, with first-line treatment typically consisting of dietary changes, stool softeners, and analgesic creams. If symptoms continue to persist, more invasive procedures including botulinum toxin A (BTX) injection may need to be considered [6].
Kaposi sarcoma
HIV represents one of the most important risk factors for the development of Kaposi sarcoma (KS). A recent retrospective study examining the temporal trends in KS incidence found that rates of this malignancy were elevated 521-fold in HIV patients compared to the general population, even with consistent decline in KS incidence during the current ART era [64]. KS is known to be caused by the Kaposi sarcoma herpes virus (KSHV), also known as human gammaherpesvirus 8 (HHV-8), which primarily targets epithelial cells and induces spindle cell formation [65,66,67].
The most common presentation of KS is cutaneous disease, presenting with elliptical lesions with a wide range of associated colors. However, KS may also manifest with visceral involvement, including the gastrointestinal tract [68]. Case reports have also demonstrated non-cutaneous KS presenting as recurrent rectal abscess, further emphasizing the possible heterogeneity in presentation of this condition [69]. In addition, anorectal KS may be easily confused with hemorrhoids or other benign lesions [10]. Given this wide range of presentation, the NCCN recommends pathology and immunophenotyping as the gold standard of diagnosis for KS [70].
Imaging findings of Kaposi sarcoma can vary widely based on the involved sites of extracutaneous disease, including possible involvement of the gastrointestinal, cutaneous, musculoskeletal, and pulmonary systems. Gastrointestinal KS may involve any level from the stomach to the anorectal region, with the duodenum representing the most common site of involvement [71]. CT or MRI may demonstrate a range of potential findings, including submucosal thickening, polypoid mucosal lesions, and hyperattenuating abdominopelvic lymphadenopathy. Submucosal masses may demonstrate a “target” or “bull’s eye” appearance, while smaller plaque-like lesions may only be well visualized on barium fluoroscopic studies [72].
The treatment of KS, according to the NCCN, is dependent on the severity of the disease and the patients’ preferences [70]. For limited disease that is asymptomatic and cosmetically acceptable, ART alone is recommended as monotherapy, while patients with advanced disease that is symptomatic or cosmetically unacceptable should be treated with a combination of ART and systemic therapy.
Lymphoma
HIV infection is associated with an increased risk of developing both Hodgkin and non-Hodgkin lymphomas, although the advent of ART has reduced the incidence of AIDS-related lymphoma [73, 74]. Of these broad classifications of lymphoma, non-Hodgkin lymphomas are more prevalent in those with HIV compared to Hodgkin lymphoma [75]. The pathophysiologic mechanism by which HIV leads to an increased risk of lymphoma development is typically attributed to the immunosuppression and subsequent reactivation of latent viral infection, most notably Epstein–Barr virus.
Extranodal involvement of lymphoma in patients with HIV is common, frequently involving the GI tract. Plasmablastic lymphoma, a very aggressive variant of diffuse large B-cell lymphoma which predominantly affects the oral cavity of HIV-infected individuals, has been demonstrated to involve the anorectum in a small subset of patients [76,77,78,79]. While not a common manifestation of lymphoma in HIV patients, it bears mention due to its potential to present with non-specific anorectal symptoms.
CT, MRI, and FDG-PET/CT are all commonly employed modalities for the imaging assessment of anorectal lymphoma (Fig. 6). On MR assessment, anorectal lymphoma typically presents as a solid mass or wall thickening with isointense signal on T1-weighted sequences, isointense to hyperintense signal on T2-weighted images, and mild enhancement [80]. Treatment and prognosis of lymphoma in HIV patients are largely dependent on the particular subtype. While ART has improved prognosis, HIV infection is still associated with increased mortality in patients with lymphoma [75]. The initial treatment of lymphoma in HIV patients consists of ART and growth factor support, along with full-dose chemotherapy with or without rituximab [81]. The specific chemotherapy regimen is dependent on the subtype and beyond the scope of this discussion.
65-year-old female with history of HIV and recently diagnosed anal lymphoma. Axial MR images demonstrated a solid homogeneous mass in the region of the anal canal, which demonstrates faint intermediate signal intensity on T2-weighted images (arrow in a) and mildly hyperintense signal on fat-suppressed pre-contrast T1-weightred images (arrow in b). Post-contrast T1-weighted image demonstrates mild enhancement associated with this mass (arrow in c). Note is also made of a necrotic left inguinal lymph node consistent with metastatic involvement (arrowhead in c). Subsequent biopsy confirmed a diagnosis of anal lymphoma
Conclusion
HIV is a complex disease, with manifestations that may potentially encompass each organ system. The anorectum is no exception, representing an important region of disease in HIV-infected patients. The spectrum of disease which may affect the anorectum is equally broad, ranging from relatively superficial ulcerations to complex fistulas and various malignancies. Given this wide range of presentation, in combination with the relatively non-specific presenting complaints in patients with such disease, it is imperative that the radiologist to understand the unique findings of these conditions. In particular, the radiologist should be aware of the unique relationship that these conditions have to HIV infection and how these conditions manifest on imaging. Imaging plays a vital role in the diagnosis and management of several such conditions affecting the anorectum in HIV patients. Knowledge of the imaging and associated clinical features of these conditions is critical for radiologists.
References
Estimated HIV Incidence and Prevalence in the United States, 2015 – 2019. Vol. 26, HIV Surveillance Supplemental Report. 2021. p. 1–81.
UNAIDS. Global HIV and AIDS Stastistics - Fact Sheet. 2021. p. 1–3.
Serlin MH, Dieterich D. Gastrointestinal Disorders in HIV. In: Global HIV/AIDS Medicine. Elsevier; 2008. p. 251–60.
Abramowitz L, Benabderrahmane D, Baron G, Walker F, Yeni P, Duval X. Systematic Evaluation and Description of Anal Pathology in HIV-Infected Patients During the HAART Era. Dis Colon Rectum. 2009 Jun;52(6):1130–6.
Goldberg GS, Orkin BA, Smith LE. Microbiology of human immunodeficiency virus anorectal disease. Dis Colon Rectum. 1994 May;37(5):439–43.
Barrett WL, Callahan TD, Orkin BA. Perianal manifestations of human immunodeficiency virus infection. Dis Colon Rectum. 1998 May;41(5):606–11.
Orkin BA, Smith LE. Perineal manifestations of HIV infection. Dis Colon Rectum. 1992 Apr;35(4):310–4.
Gonzalez-Ruiz C, Heartfield W, Briggs B, Vukasin P, Beart RW. Anorectal Pathology in HIV/AIDS-Infected Patients Has Not Been Impacted by Highly Active Antiretroviral Therapy. Dis Colon Rectum. 2004 Sep;47(9):1483–6.
Nadal SR, Manzione CR, Horta SHC. Comparison of Perianal Diseases in HIV-Positive Patients During Periods Before and After Protease Inhibitors Use. Dis Colon Rectum. 2008 Oct;51(10):1491–4.
Assi R. Sexually transmitted infections of the anus and rectum. World J Gastroenterol. 2014;20(41):15262.
Goddard SL, Poynten IM, Petoumenos K, Jin F, Hillman RJ, Law C, et al. Self-reported anal symptoms and their association with anal pathology among gay and bisexual men: a cross-sectional observational analysis. Sex Health. 2021 May;18(2):123–9.
Puy-Montbrun T, Denis J, Ganansia R, Mathoniere F, Lemarchand N, Arnous-Dubois N. Anorectal lesions in human immunodeficiency virus-infected patients. Int J Colorectal Dis. 1992 Mar;7(1):26–30.
Steed JM, Henry-Okafor Q, Pitts CJ. Proctitis in Men Who Have Sex with Men. Nurs Clin North Am. 2020 Sep;55(3):325–35.
Sigle G, Kim R. Sexually Transmitted Proctitis. Clin Colon Rectal Surg. 2015 May;28(02):070–8.
Maddu KK, Mittal P, Shuaib W, Tewari A, Ibraheem O, Khosa F. Colorectal Emergencies and Related Complications: A Comprehensive Imaging Review—Imaging of Colitis and Complications. Am J Roentgenol. 2014 Dec;203(6):1205–16.
Ootani A, Mizuguchi M, Tsunada S, Sakata H, Iwakiri R, Toda S, et al. Chlamydia trachomatis proctitis. Gastrointest Endosc. 2004 Jul;60(1):161–2.
Gee MS, Harisinghani MG. MRI in patients with inflammatory bowel disease. J Magn Reson Imaging [Internet]. 2011 Mar 1;33(3):527–34. Available from: https://doi.org/10.1002/jmri.22504
Heverhagen JT, Klose KJ. MR Imaging for Acute Lower Abdominal and Pelvic Pain. RadioGraphics [Internet]. 2009 Oct 1;29(6):1781–96. Available from: https://doi.org/10.1148/rg.296095518
Tutein Nolthenius CJ, Bipat S, Mearadji B, Spijkerboer AM, Ponsioen CY, Montauban van Swijndregt AD, et al. MRI characteristics of proctitis in Crohn’s disease on perianal MRI. Abdom Radiol [Internet]. 2016;41(10):1918–30. Available from: https://doi.org/10.1007/s00261-016-0802-z
Sullivan B, Glaab J, Gupta RT, Wood R, Leiman DA. Lymphogranuloma venereum (LGV) proctocolitis mimicking rectal lymphoma. Radiol Case Reports. 2018 Dec;13(6):1119–22.
Mallik S, Talapatra K, Goswami J. AIDS: A radiation oncologist′s perspective. J Cancer Res Ther. 2010;6(4):432–41.
Grodsky M, Sidani S. Radiation Proctopathy. Clin Colon Rectal Surg. 2015 May;28(02):103–11.
Paquette IM, Vogel JD, Abbas MA, Feingold DL, Steele SR. The American Society of Colon and Rectal Surgeons Clinical Practice Guidelines for the Treatment of Chronic Radiation Proctitis. Dis Colon Rectum. 2018 Oct;61(10):1135–40.
Abcarian H. Anorectal Infection: Abscess-Fistula. Clin Colon Rectal Surg. 2011 Mar;24(01):014–21.
Hamadani A, Haigh PI, Liu IA, Abbas MA. Who Is At Risk for Developing Chronic Anal Fistula or Recurrent Anal Sepsis After Initial Perianal Abscess? Dis Colon Rectum. 2009 Feb;52(2):217–21.
Nadal SR, Manzione CR, Galvão VM, Salim VRBM, Speranzini MB. Perianal diseases in HIV-positive patients compared with a seronegative population. Dis Colon Rectum. 1999 May;42(5):649–54.
Manookian CM, Sokol TP, Headrick C, Fleshner PR. Does HIV status influence the anatomy of anal fistulas? Dis Colon Rectum. 1998 Dec;41(12):1529–33.
Kalichman SC, Pellowski J, Turner C. Prevalence of sexually transmitted co-infections in people living with HIV/AIDS: systematic review with implications for using HIV treatments for prevention. Sex Transm Infect. 2011 Apr;87(3):183–90.
Mansour M, Weston LA. Perianal Infections: A Primer for Nonsurgeons. Curr Gastroenterol Rep. 2010 Aug;12(4):270–9.
Vogel JD, Johnson EK, Morris AM, Paquette IM, Saclarides TJ, Feingold DL, et al. Clinical Practice Guideline for the Management of Anorectal Abscess, Fistula-in-Ano, and Rectovaginal Fistula. Dis Colon Rectum. 2016 Dec;59(12):1117–33.
Rizzo JA, Naig AL, Johnson EK. Anorectal Abscess and Fistula-in-Ano: Evidence-Based Management. Surg Clin North Am. 2010 Feb;90(1):45–68.
Villa C, Pompili G, Franceschelli G, Munari A, Radaelli G, Maconi G, et al. Role of magnetic resonance imaging in evaluation of the activity of perianal Crohn’s disease. Eur J Radiol. 2012 Apr;81(4):616–22.
Gage KL, Deshmukh S, Macura KJ, Kamel IR, Zaheer A. MRI of perianal fistulas: bridging the radiological–surgical divide. Abdom Imaging. 2013 Oct;38(5):1033–42.
Criado J de M, del Salto LG, Rivas PF, del Hoyo LFA, Velasco LG, Isabel Díez Pérez de las Vacas M, et al. MR imaging evaluation of perianal fistulas: Spectrum of imaging features. Radiographics. 2012;32(1):175–94.
Morris J, Spencer JA, Ambrose NS. MR Imaging Classification of Perianal Fistulas and Its Implications for Patient Management. RadioGraphics. 2000 May;20(3):623–35.
Khati NJ, Sondel Lewis N, Frazier AA, Obias V, Zeman RK, Hill MC. CT of acute perianal abscesses and infected fistulae: a pictorial essay. Emerg Radiol. 2015 Jun;22(3):329–35.
Choe J, Wortman JR, Sodickson AD, Khurana B, Uyeda JW. Imaging of Acute Conditions of the Perineum. RadioGraphics. 2018 Jul;38(4):1111–30.
Balci S, Onur MR, Karaosmanoglu AD, Karcaaltincaba M, Akata D, Konan A, et al. MRI evaluation of anal and perianal diseases. Diagnostic Interv Radiol. 2019 Jan;25(1):21–7.
Choi EK, Kim JH, Jeong SU, Na S-Y, Boo S-J, Kim HU, et al. Endoscopic Ultrasound-Guided Perirectal Abscess Drainage without Drainage Catheter: A Case Series. Clin Endosc. 2017 May;50(3):297–300.
Silverberg MJ, Lau B, Justice AC, Engels E, Gill MJ, Goedert JJ, et al. Risk of Anal Cancer in HIV-Infected and HIV-Uninfected Individuals in North America. Clin Infect Dis. 2012 Apr;54(7):1026–34.
Grulich AE, van Leeuwen MT, Falster MO, Vajdic CM. Incidence of cancers in people with HIV/AIDS compared with immunosuppressed transplant recipients: a meta-analysis. Lancet. 2007 Jul;370(9581):59–67.
Bertisch B, Franceschi S, Lise M, Vernazza P, Keiser O, Schoni-Affolter F, et al. Risk Factors for Anal Cancer in Persons Infected With HIV: A Nested Case-Control Study in the Swiss HIV Cohort Study. Am J Epidemiol. 2013 Sep;178(6):877–84.
Hernández-Ramírez RU, Shiels MS, Dubrow R, Engels EA. Spectrum of cancer risk among HIV-infected people in the United States during the modern antiretroviral therapy era: a population-based registry linkage study. Lancet HIV. 2017;4(11):e495–504.
Machalek DA, Poynten M, Jin F, Fairley CK, Farnsworth A, Garland SM, et al. Anal human papillomavirus infection and associated neoplastic lesions in men who have sex with men: a systematic review and meta-analysis. Lancet Oncol. 2012 May;13(5):487–500.
Lin C, Franceschi S, Clifford GM. Human papillomavirus types from infection to cancer in the anus, according to sex and HIV status: a systematic review and meta-analysis. Lancet Infect Dis. 2018 Feb;18(2):198–206.
Salati SA. Anal Cancer : A Review. Int J Health Sci (Qassim). 2012;6(2):206–30.
Rao S, Guren MG, Khan K, Brown G, Renehan AG, Steigen SE, et al. Anal cancer : ESMO Clinical Practice Guidelines for diagnosis , treatment and. Ann Oncol. 2021;xxx(xxx).
Amin MB, Greene FL, Edge SB, Compton CC, Gershenwald JE, Brookland RK, et al. The Eighth Edition AJCC Cancer Staging Manual: Continuing to build a bridge from a population-based to a more “personalized” approach to cancer staging. CA Cancer J Clin. 2017 Mar;67(2):93–9.
Horvat N, Carlos Tavares Rocha C, Clemente Oliveira B, Petkovska I, Gollub MJ. MRI of Rectal Cancer: Tumor Staging, Imaging Techniques, and Management. RadioGraphics. 2019 Mar;39(2):367–87.
Kochhar R, Plumb AA, Carrington BM, Saunders M. Imaging of Anal Carcinoma. Am J Roentgenol. 2012 Sep;199(3):W335–44.
Tonolini M, Bianco R. MRI and CT of anal carcinoma: a pictorial review. Insights Imaging. 2013 Feb;4(1):53–62.
Matalon SA, Mamon HJ, Fuchs CS, Doyle LA, Tirumani SH, Ramaiya NH, et al. Anorectal cancer: Critical anatomic and staging distinctions that affect use of radiation therapy. Radiographics. 2015;35(7):2090–107.
Benson AB, Venook AP, Al-Hawary MM, Cederquist L, Chen Y-J, Ciombor KK, et al. Anal Carcinoma, Version 2.2018, NCCN Clinical Practice Guidelines in Oncology. J Natl Compr Cancer Netw. 2018 Jul;16(7):852–71.
Mahmud A, Poon R, Jonker D. PET imaging in anal canal cancer: a systematic review and meta-analysis. Br J Radiol. 2017 Dec;90(1080):20170370.
Houard C, Pinaquy J-B, Mesguich C, Henriques de Figueiredo B, Cazeau A-L, Allard J-B, et al. Role of 18 F-FDG PET/CT in Posttreatment Evaluation of Anal Carcinoma. J Nucl Med. 2017 Sep;58(9):1414–20.
Anal Carcinoma (Version 2.2021). National Comprehensive Cancer Network. 2021.
Durot C, Dohan A, Boudiaf M, Servois V, Soyer P, Hoeffel C. Cancer of the Anal Canal: Diagnosis, Staging and Follow-Up with MRI. Korean J Radiol. 2017;18(6):946.
Wang CJ, Sparano J, Palefsky JM. Human Immunodeficiency Virus/AIDS, Human Papillomavirus, and Anal Cancer. Surg Oncol Clin N Am. 2017 Jan;26(1):17–31.
Hoffman R, Welton ML, Klencke B, Weinberg V, Krieg R. The significance of pretreatment CD4 count on the outcome and treatment tolerance of HIV-positive patients with anal cancer. Int J Radiat Oncol. 1999 Apr;44(1):127–31.
Fraunholz IB, Haberl A, Klauke S, Gute P, Rödel CM. Long-term Effects of Chemoradiotherapy for Anal Cancer in Patients With HIV Infection. Dis Colon Rectum. 2014 Apr;57(4):423–31.
Housri N, Yarchoan R, Kaushal A. Radiotherapy for patients with the human immunodeficiency virus: Are special precautions necessary? Cancer. 2010 Jan;116(2):273–83.
Nelson RL. Anal fissure (chronic). BMJ Clin Evid. 2014 Nov;2014(January):1–18.
Herzig DO, Lu KC. Anal Fissure. Surg Clin North Am. 2010 Feb;90(1):33–44.
Peprah S, Engels EA, Horner M-J, Monterosso A, Hall HI, Johnson AS, et al. Kaposi Sarcoma Incidence, Burden, and Prevalence in United States People with HIV, 2000–2015. Cancer Epidemiol Biomarkers Prev. 2021 Jun;(240).
Gramolelli S, Schulz TF. The role of Kaposi sarcoma-associated herpesvirus in the pathogenesis of Kaposi sarcoma. J Pathol. 2015;235(2):368–80.
Ganem D. KSHV and the pathogenesis of Kaposi sarcoma: Listening to human biology and medicine. J Clin Invest. 2010;120(4):939–49.
Antman K, Chang Y. Kaposi’s Sarcoma. N Engl J Med. 2000 Apr;342(14):1027–38.
Arora M, Goldberg EM. Kaposi Sarcoma Involving the Gastrointestinal Tract. Gastroenterol Hepatol (N Y). 2010 Jul;6(7):459–62.
Schulberg S, Al-Feghali V, Bain K, Shehebar J. Non-cutaneous AIDS-associated Kaposi’s sarcoma presenting as recurrent rectal abscesses. BMJ Case Rep. 2018 Aug;bcr-2018–225749.
Kaposi Sarcoma (Version 2.2021). National Comprehensive Cancer Network. 2021.
Restrepo CS, Martínez S, Lemos JA, Carrillo J a, Lemos DF, Ojeda P, et al. Imaging Manifestations of Kaposi Sarcoma. RadioGraphics. 2006 Jul;26(4):1169–85.
Pantongrag-Brown L, Nelson AM, Brown AE, Buetow PC, Buck JL. Gastrointestinal manifestations of acquired immunodeficiency syndrome: radiologic-pathologic correlation. RadioGraphics. 1995 Sep;15(5):1155–78.
Engels EA, Biggar RJ, Hall HI, Cross H, Crutchfield A, Finch JL, et al. Cancer risk in people infected with human immunodeficiency virus in the United States. Int J cancer. 2008 Jul;123(1):187–94.
Besson C, Goubar A, Gabarre J, Rozenbaum W, Pialoux G, Châtelet FP, et al. Changes in AIDS-related lymphoma since the era of highly active antiretroviral therapy. Blood. 2001;98(8):2339–44.
Han X, Jemal A, Hulland E, Simard EP, Nastoupil L, Ward E, et al. HIV infection and survival of lymphoma patients in the era of highly active antiretroviral therapy. Cancer Epidemiol Biomarkers Prev. 2017;26(3):303–11.
Castillo JJ, Reagan JL. Plasmablastic lymphoma: a systematic review. ScientificWorldJournal. 2011 Mar;11:687–96.
Lopez-Iniguez. Rectal Plasmablastic Lymphoma in HIV/AIDS: Two Cases. World J Oncol. 2013;4(1):54–7.
Chagas LA, Camilo GB, Machado DC, Vidal DR, de Oliveira CE, Toledo GC, et al. Plasmablastic lymphoma of the anal canal in an HIV-infected patient. Am J Case Rep. 2014;15:543–9.
Lim JH, Lee MH, Lee MJ, Kim CS, Lee JS, Choi SJ, et al. Plasmablastic Lymphoma in the Anal Canal. Cancer Res Treat. 2009;41(3):182.
Surabhi VR, Menias CO, Amer AM, Elshikh M, Katabathina VS, Hara AK, et al. Tumors and Tumorlike Conditions of the Anal Canal and Perianal Region: MR Imaging Findings. RadioGraphics. 2016 Sep;36(5):1339–53.
B-Cell Lymphomas (Version 5.2021). National Comprehensive Cancer Network. 2021.
Funding
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
None.
Ethical approval
All authors are responsible for the contents and have read and approved the manuscript for submission.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Vos, D., Wang, M., Ramaiya, S. et al. Anorectal pathology in the HIV population: a guide for radiologists. Abdom Radiol 47, 1762–1774 (2022). https://doi.org/10.1007/s00261-022-03470-z
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00261-022-03470-z