Abstract
Purpose
To explore the diagnostic value of American College of Radiology Contrast-Enhanced Ultrasound-Liver Imaging Reporting and Data System (ACR-CEUS-LI-RADS) for hepatocellular carcinoma (HCC) in patients with cirrhosis and chronic hepatitis B.
Methods
A total of 205 patients at high risk of HCC with solitary hepatic nodule were enrolled and retrospectively analyzed. All patients were over 18 years old and had a single lesion with a diameter < 50 mm. Lesions were categorized according to size and contrast enhancement patterns in the arterial, portal venous and late phases. Diagnostic efficacy of CEUS LI-RADS for HCC, and the rate of non-HCC malignancies in the LR-M class were compared between patients with cirrhosis and chronic hepatitis B.
Results
Of all 205 nodules (median nodule size was 34 mm), 142 (69.3%) were HCC. Of the 127 (61.9%) LR-5 category nodules, 95.8% (92/96) nodules were corresponded to HCC in cirrhosis, while 61.3% (19/31) nodules were corresponded to HCC in chronic hepatitis B (P = 0.000). Positive predictive value (PPV) of LR-5 category for HCC was 95.8% in cirrhosis and 61.3% in chronic hepatitis B (P = 0.000). More category of LR-4 nodules were proved to be HCC in patients with cirrhosis than chronic hepatitis B (80.0% vs 8.3%, P = 0.000). Of 41 LR-M category nodules, more non-HCC malignancies were found in chronic hepatitis B (76.0%) than that in cirrhosis (25.0%, P = 0.001).
Conclusions
The LR-5 category is highly specific for the diagnosis of HCC in patients with cirrhosis. However, LR-5 category nodules require further CT or MRI examination or histological confirmation in patients with chronic hepatitis B for its unsatisfactory PPV for HCC.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Liver cancer is the fifth malignant tumor and the third leading cause of cancer death in the world as well as the second most common cause of cancer-related death in men in China [1]. Hepatocellular carcinoma (HCC) is the most common pathological type of primary liver cancers, accounting for about 90%, which is seriously detrimental to human health in the world [2]. High risk factors of HCC mainly include liver cirrhosis, viral hepatitis (including hepatitis B virus infection and hepatitis C virus infection), chronic alcoholism, non-alcoholic fatty hepatitis and food contaminated with aflatoxin [3]. There is no typical clinical symptom in the early stage of HCC, about 60% of the patients have progressed to the advanced stage when they are diagnosed, and lose the chance of operation [4]. Early diagnosis of HCC is extremely important to improve the prognosis of the patients. Contrast enhanced ultrasound (CEUS) contrast-enhanced computed tomography (CT) and magnetic resonance imaging (MRI) were recommended the main diagnostic methods of focal liver lesions. The reported sensitivity of CEUS in diagnosis of hepatic nodule was more than 95%, and the specificity was close to 90% [5]. CEUS possesses comparable diagnostic capacity for HCC compared with contrast-enhanced CT and MRI [6]. The clinical value of using CEUS to establish the diagnosis of HCC was recognized by the Japanese, Canadian, and Italian association of liver diseases and Asia Pacific hepatology or liver cancer society, EASL [7,8,9,10,11]. On the contrary, CEUS was excluded from the American Association for the Study of Liver Diseases (AASLD) [11]. The main reason was the potential risk of misdiagnosis of intrahepatic cholangiocarcinoma (ICC) for HCC [12, 13]. The differential diagnosis between them was necessary because clinical management and prognosis were widely divergent. Since then, details about the temporal enhancement of HCC and ICC on CEUS were compared and analyzed. These studies showed that peripheral enhancement, early (within 60 s after contrast injection) and marked washout were much more common in ICC than in HCC on CEUS [14, 15]. Based on these reports describing CEUS features of ICC and HCC, the American College of Radiology (ACR) released the diagnostic algorithm (CEUS Liver Imaging Reporting and Data System [LI-RADS]) for the lesions in patients at high risk of HCC in 2016 [16]. And the working group made a revision in 2017 [17]. The aim of the scheme was to evaluate the possibility of HCC in liver focal lesions in patients at high risk of HCC. The typical enhancement pattern of HCC was classified as LR-5, corresponding to hyperenhancement (rim and peripheral nodular enhancement excluded) in arterial phase followed by late (onset ≥ 60 s after contrast injection) and mild washout [16]. Classifications of LR-3 and LR-4 respectively represented an immediate or high probability of HCC. In addition, LR-M referred to the malignancy not specific for HCC, but rather suggestive of the possibility of ICC, mixed ICC-HCC or other non-hepatocellular malignancies. Terzi et al. have performed a study on diagnostic efficiency of CEUS LI-RADS algorithm for HCC in Italian patients with cirrhosis, indicating that LR-5 CEUS class was an optimal diagnostic tool for HCC [18]. However, the algorithm of CEUS LI-RADS was needed to be validated in other population with high risk of HCC, because this algorithm was applied not only in cirrhosis but also in other high-risk patients according to official version by ACR in 2016 and 2017. Nevertheless, diagnostic efficacy of CEUS LI-RADS algorithm for HCC in patients with chronic hepatitis remains unknown.
The main purposes of our study are: (1) to explore the diagnostic value of CEUS LI-RADS for HCC in high-risk patients; (2) to compare the diagnostic efficacy of CEUS LI-RADS for HCC in patients with cirrhosis and chronic hepatitis B; (3)to quantify the risk of nodules being HCC in the LR-3 and LR-4 classes in cirrhosis and chronic hepatitis B; (4) to compare the rate of non-HCC malignancies in the LR-M class in cirrhosis and chronic hepatitis B.
Materials and methods
Patients
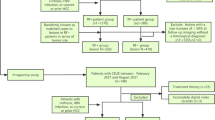
This retrospective study was approved, and the requirement for informed consent was waived, by the institutional review board of Southwest Hospital and the Third Affiliated Hospital of Chongqing Medical University. A total of 668 patients who had liver CEUS examination and pathological diagnosis after surgical resection or ultrasound guided biopsy in Southwest hospital Affiliated to Army Medical University (between January 2016 and December 2016) and the Third Affiliated Hospital of Chongqing Medical University (between May 2016 and December 2019) were retrospectively analyzed. A review of the HIS system and hospital database, laboratory examination (including hepatitis B virus marker, anti-HCV, autoimmune antibody, AFP value, liver function) and clinical information of each patient were collected. We performed a per-patient analysis. The inclusion criteria were as follows:
-
(1)
Patients with high risk factors of HCC, including cirrhosis, and chronic hepatitis B;
-
(2)
Patients with single solid nodule ≤ 50 mm;
-
(3)
The final diagnosis of hepatic nodule had been verified pathologically;
-
(4)
Real-time CEUS of hepatic nodule was performed within less than a month
-
(5)
before resection or biopsy;
The exclusion criteria were as follows:
-
(1)
Patients without above risk factors of HCC,
-
(2)
Tumor size > 50 mm which often have intra-tumoural necrosis that unpredictably influences the contrast enhancement pattern on CEUS6;
-
(3)
Nodule number ≥ 2 because CEUS could not scan multiple nodules simultaneously after one injection of contrast agent if the nodules are not at the same scan plane. In addition it is difficult to correspond the pathology of each tumor exactly to the imaging of ultrasound in patients with multiple hepatic lesions;
-
(4)
Patients with hepatic vascular thrombosis which may influence the hepatic dynamic circulation;
-
(5)
Clinical intervention (such as systemic chemotherapy, transhepatic arterial chemotherapy and embolization, radiofrequency ablation or liver resection) prior to CEUS;
-
(6)
Invisible at pre-contrast ultrasound;
-
(7)
Patients < 18 years old or other contraindication to CEUS.
According to the inclusion and exclusion criteria mentioned above, a total of 205 patients with risk factors of HCC were enrolled in the study. The patients were excluded including 323 cases with tumour diameter > 50 mm, 70 cases with tumor number ≥ 2, 29 cases with clinical intervention prior to CEUS, 6 cases with hepatic vascular thrombosis, 9 cases with invisible nodules at pre-contrast ultrasound and 26 cases without the high risk factors of HCC. All patients with chronic hepatitis B were diagnosed according to the criteria updated by American Association for the Study of Liver Diseases (AASLD) in 2018 [19].
Ultrasound examination
The Siemens Acuson Sequoia 512 ultrasound unit (Siemens Medical Solutions, Santa Clara, Calif) and Siemens Acuson S2000 ultrasound unit (Siemens Medical Solutions, Erlangen, Germany) were applied for CEUS examination. Before CEUS examination, the characteristics of each patient, including location, shape, size, echogenicity, and color Doppler feature were recorded at baseline US examination by using 4C1 convex array probe and 6C1 convex array probe respectively. CEUS was performed by using contrast pulse sequencing (CPS) imaging. Dual imaging mode and real time imaging were used with a low mechanical index of less than 0.3 to avoid the bubbles disruption. The contrast agent SonoVue (Bracco Imaging B.V, Geneva, Switzerland) was used throughout the study period. A volume of 2.4-ml agent was injected into cubital vein in bolus via a 20 G catheter over 2–3 s followed by a 5-ml saline flush at the speed about 2 ml/s. Hepatic lesion was scanned continuously within 1 min after SonoVue injection and scanned intermittently up to 4–6 min until minimize microbubble destruction. And the enhancement procedure was consisted of the arterial phase (0–30 s from the SonoVue bolus injection), portal phase (31–120 s after the injection), and delayed phase (121–240 s after the injection) according to European Federation of Societies for Ultrasound in Medicine and Biology (EFSUMB) recommendations [6]. All images of CUES were digitally stored.
In each case, the enhancement patters were analyzed who were blind to the pathological diagnosis and the CT or MRI findings. We defined the enhancement patters according to the predominant pattern occupying more than 50% of area of the lesion while compared to adjacent hepatic parenchyma on CEUS. The enhancement patterns of HCC at arterial phase were classified as follows:
-
(1)
Homogeneous enhancement—the whole nodule shows hyperechoic homogeneously compared with the surrounding liver parenchyma.
-
(2)
Heterogeneous enhancement—when the lesion displays mixed hyper-enhancement at the periphery and the central part of the lesion, enhancement area involves more than half of the lesion.
-
(3)
Peripheral enhancement—irregular ring-like hyper-enhancement at the peripheral part of the lesion with sparse filiform and punctiform internal enhancement.
-
(4)
Iso-enhancement—enhancement degree of the lesion is similar to the surrounding liver parenchyma.
-
(5)
Hypo-enhancement—the lesion enhances in the less degree than that of surrounding liver tissue.
-
(6)
Non-enhancement—there is no enhancement (microbubbles do not appear) at both the periphery and the central part of the lesion.
Washout was defined as the reduction in enhancement in whole or part relative to liver beginning in or after arterial phase and resulting in hypoenhancement. Early washout is a temporally defined subtype of washout in which oneset is within 60 s from contrast injection. Marked washout is degree-defined subtype in which the degree of washout is marked within 2 min after contrast injection. Observation appears black or punched out [17].
CEUS LI-RADS
The ACR developed a CEUS-based algorithm called CEUS-LI-RADS (Fig. 1) [16]. CEUS-LI-RADS contains five categories named LR-1, LR-2, etc., with LR-1 designating definitely benign lesions, LR-2 designating probably benign lesions, LR-3 designating intermediate probability for HCC, LR-4 designating probably HCC (Fig. 2), and LR-5 designating definite HCC (Fig. 3). LR-M is used for lesions definitely or probably malignant, not specific for HCC (Fig. 4).
Enhancement features of LR-4 category nodule. FNH in a 41-year-old man with chronic hepatitis B. The lesion with a diameter of 42 mm showed hypoechoic on conventional ultrasound (A), intense arterial hyperenhancement (12 s) (B) and showed slightly hyperehnacement in the portal (1 m 46 s) (C)and the delayed phase (4 m) (D)
Enhancement features of LR-5 category nodule. Moderately differentiated HCC with a diameter of 22 mm in a 43-year-old man with cirrhosis. The tumour appears hypoechoic on conventional ultrasound (A), global arterial hyperenhancement (17 s) (B) followed by slight washout in the portal (2 m) (C) and the late phase (3 m10 s) (D)
Enhancement features of LR-M category nodule. ICC with a diameter of 35 mm in a 42-year-old man with cirrhosis. The tumour appears hypoechoic on conventional ultrasound (A), intense arterial hyperenhancement (18 s) (B) followed by washout in the early portal phase (38 s) (C) and marked washout in the late phase (2 m 47 s) (D)
The sensitivity, specificity, positive predictive value (PPV), negative predictive value (NPV) and accuracy of HCC were calculated by comparing with the histological diagnosis.
Histological examination
All patients obtained histological diagnosis by analyzing surgical section specimen (193 cases) or biopsy specimen (12 cases). US-guided biopsy was done at both within the nodule and the adjacent liver tissue using gauge 18 needle (Bard Peripherals Vascular Inc, Tempe, Arizona 85281, USA). Liver sections or biopsy specimens were fixed with 10% formalin, stained with haematoxylin and eosin (HE) followed by immunohistochemical staining. Final pathological diagnosis was made by pathologist who was blind to the clinical information and CEUS findings.
Statistical analysis
SPSS 18.0 statistical software package (SPSS Inc, Chicago, IL) was used to perform data analysis. A P value smaller than 0.05 was considered as significant for each test. Data was expressed as mean ± standard deviation. The sensitivity, specificity, PPV, NPV, and accuracy between patients with cirrhosis and patients with chronic hepatitis were compared by chi-square test.
Results
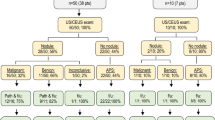
A total of 205 patients with high risks for HCC were included (mean age 53.1 ± 12.0 years). The characteristics of the patients are summarized in Table 1. One hundred thirty two (64.4%) cases with cirrhosis were included and almost all of them were associated with chronic hepatitis B. The remaining seventy-three cases (36.7%) were diagnosed as chronic hepatitis B according to the diagnostic criteria of chronic hepatitis B updated by AALSD in 2018. The mean size of the lesions was 32.53 ± 9.91 mm (median 34 mm, range 10–50 mm). One hundred and forty two (69.3%) cases were diagnosed as HCC (well/moderately/poorly differentiated: 18/95/29), whereas twenty-six (12.7%) were diagnosed as malignancy but not HCC (including thirteen ICCs, eight metastases, two lymphoepithelioma-like carcinomas, one hepatic neuroendocrine tumor, one spindle cell tumor and one follicular dendritic cell sarcoma). Thirty-seven cases (18.0%) were diagnosed as benign lesions (including ten focal nodular hyperplasias (FNH), seven cirrhotic regenerative nodules, seven inflammatory lesions, five hepatic angioleiomyolipomas, four hemangiomas, three cholangiogenic benign tumors and one hepatic adenoma). Most benign nodules (87.5%) in patients with cirrhosis are cirrhotic nodules. HCC was significantly more common in patients with cirrhosis than patients with chronic hepatitis B (90.1% vs 31.5%, P = 0.000), while non-HCC malignancy such as ICC and metastasis, was more common in patients with chronic hepatitis B than with cirrhosis (28.8% vs 3.8%, P = 0.000).
CEUS LI-RADS classification in patients with liver cirrhosis was showed in Table 2.The LR-5 pattern was present in 96 of all 132 nodules (72.7%) and corresponded to HCC in 92 (95.8%) of cases (well/moderately/poorly differentiated: 11/66/15). Of the four remaining LR-5 cases, three were cirrhotic regenerative nodules, and one lymphoepithelioma-like carcinoma. The LR-M category was displayed in 16 of all 132 nodules (12.1%), 11 out of the 16 LR-M category nodules (68.7%) were HCC (well/moderately/poorly differentiated: 0/5/6). Of the five remaining LR-M nodules, four were non-HCC malignancies (including two ICCs, one metastasis and one follicular dendritic cell sarcoma) and one inflammatory lesion. LR-4 category was found in 20 of all 132 nodules (15.2%), and 16 of 20 LR-4 category lesions (80%) were HCCs (well/moderately/poorly differentiated: 4/8/4). The remaining four nodules were cirrhotic regenerative nodules.
CEUS LI-RADS classification in patients with chronic hepatitis B was showed in Table 2. The LR-5 pattern was present in 31 of all 73 nodules (42.5%) and corresponded to HCC in 19 cases (61.3%) (well/moderately/poorly differentiated: 3/14/2). Of the 12 remaining nodules, 10 were benign nodules (four FNHs, two angioleiomyolipomas, two inflammatory lesions, one bile duct adenoma and one hemangioma) and two were ICCs. The LR-M category was displayed in 25 of all 73 nodules (34.2%), and 19 (76.0%) nodules were non-HCC malignancies (nine ICCs, seven metastases, one lymphoepithelioma-like carcinoma, one hepatic neuroendocrine tumor and one Spindle cell tumor). For the six remaining nodules, three were moderately-poorly differentiated HCCs and three were inflammatory lesions. The LR-4 category was displayed in 12 nodules of all 73 nodules (16.4%), and 11 of 12 LR-4 category lesions (91.7%) were benign, including six FNHs, three angioleiomyolipomas, one hepatic adenoma, and one hemangioma. Only one moderately differentiated HCC was classified as LR-4. Two LR-3 nodules were biliary cystadenoma and biliary hamartoma without APHE and washout. The remaining two benign hemangiomas, with typical peripheral discontinuous globular enhancement, were categorized as LR-1.
Of the total 205 nodules, 127(61.9%) nodules were classified as LR-5. 95.8% (92/96) LR-5 nodules were corresponded to HCC in cirrhosis,while 61.3% (19/31) were corresponded to HCC in chronic hepatitis B (P = 0.000). The LR-M category was displayed by 41 (20.0%) of all 205 nodules, and 68.8% (11/16) nodules were HCC in cirrhosis and 76.0% (19/25) were non-HCC malignancies in chronic hepatitis B. In cirrhosis, most (11/16, 68.7%) LR-M category nodules were moderately-poorly differentiated HCC. A total of 32/205 (15.6%) lesions were categorized as LR-4, and most of the LR-4 nodules (80.0%,16/20) in cirrhosis were HCCs while most LR-4 nodules in chronic hepatitis B were benign lesions (11/12, 91.7%). The rate of HCC in LR-4 pattern nodules and LR-5 pattern nodules was 53.1% and 87.4%, respectively. The diagnostic efficiency of CEUS LI-RADS for HCC between cirrhosis and chronic hepatitis B was showed in Table 3. The sensitivity, specificity and accuracy showed no significant difference between the two groups (P > 0.05). The PPV in patients with cirrhosis was significantly higher than that in patients with chronic hepatitis (P = 0.000). The sensitivity, specificity, accuracy, and PPV were recorded as 78.1%, 74.6%, 77.1%, 87.4% respectively.in all patients at high risk of HCC.
Discussion
CEUS, presenting a unique real-time visualization of vascular contrast enhancement, especially facilitating sensitivity in detection of arterial hypervascularization, has an excellent diagnostic accuracy in the differential diagnosis of focal liver lesions [20]. The goal of CEUS LI-RADS was to probe the possibility of HCC in focal liver lesions in patients at high risk of HCC and help to make an accurate diagnosis. All 142 HCC nodules showed AHPE in this study, with only 15 lesions of 10–20 mm in size, more likely showed isoenhanced or hypoenhanced in arterial phase, which is similar to Forner’s study [21]. The LR-5 was the most frequent pattern in HCC nodules in our study, occupying 78.2% of HCC, followed by LR-4 (11.9%) and LR-M (9.9%), indicating the enhancement patterns of HCC are various.. The proportion of LR-5 pattern in HCC was not significantly different in cirrhosis (77.3%) and chronic hepatitis (82.6%) (P = 0.573). Seventeen HCC cases were categorized as LR-4, including sixteen HCCs (well/moderately/poorly differentiated:4/8/4) in patients with cirrhosis and one moderately differentiated HCC in patients with chronic hepatitis B (P = 0.379). Fourteen moderately-poorly differentiated HCC were categorized as LR-M, eleven of which were in cirrhosis, while three were in chronic hepatitis B (P = 0.859). The diversity of HCC enhancement pattern would reduce the sensitivity of CEUS LI-RADS algorithm to diagnose HCC. Because only the nodule with arterial phase hyperenhancement followed by late (> 60 s after injection) and mild washout, namely LR-5, can be classified as HCC. The LR-M nodules, particularly manifesting early (within 60 s after rejection) and marked washout, could not be diagnosed as HCC according to the new CEUS LI-RADS. Therefore, the sensitivity was decreased from 88.0 to 78.1% when applying the LR-5 category to diagnose HCC (P = 0.027) while the specificity and PPV was increased statistically from 42.8% and 77.6% to 74.6% and 87.4% (P = 0.000; P = 0.032, respectively). Some researchers considered that the presence and extent of washout closely related to the degree of cellular differentiation and tumour size [22,23,24]. Lesions showing hyperenhancement in arterial phase without washout were more likely to be better differentiated HCC, whereas the lesions with hyperenhancement followed by early washout in portal phase or marked washout were more likely to be poorly differentiated HCC. In addition, HCCs with larger size are tend to enhance inhomogeneously in the arterial phase and washed out earlier in portal-venous phase [25, 26]. We included only the nodule ≤ 50 mm to avoid the influence the tumour size as possible. There were no LR-5 nodules proven to be ICC in patients with cirrhosis in the present study. Our study indicated that the CEUS LR-5 pattern provided an excellent PPV (95.8%) for HCC in patients with cirrhosis. This result was similar to the study of Terzi et al. [18]. (PPV = 98%). Only three cirrhotic regenerative nodule and one lymphoepithelioma-like carcinoma showing LR-5 pattern were misdiagnosed as HCC in cirrhotic patients. Therefore, the risk of false positive diagnosis of ICC for HCC was neglectable in cirrhosis when applying LR-5 pattern to diagnose HCC by CEUS.
However, the PPV in patients with chronic hepatitis (61.3%) was significantly lower than that in cirrhosis (P = 0.000). Ten LR-5 nodules were proven pathologically benign in chronic hepatitis in our study, leading to misdiagnosis of HCC. These false positive diagnosis would reduce the PPV in patients with chronic hepatitis. According to CEUS LI-RADS algorithm, this false positive diagnosis of HCC is not neglectable in our daily work. It is reasonable to consider that if a nodule displayed LR-5 pattern in patients with chronic hepatitis B, a further CT or MRI, if equivocal, biopsy was still needed to confirm the diagnosis of HCC.
LR-M pattern was considered as malignant feature, but not specific for HCC. Most (90.2%) LR-M nodules in our study were malignancy, including 23 cases (56.1%) of non-HCC malignant nodules and 14 cases (34.1%) of moderately-poorly differentiated HCC. The proportion of non-HCC malignancy of in chronic hepatitis was significantly higher than that in cirrhosis (76.0% vs 25.0%, P = 0.001). The remaining four LR-M nodules (10.0%) were benign inflammatory lesions, one in cirrhosis and three in chronic hepatitis. This finding confirmed that the LR-M category was highly correlated to malignancy but the pathological classification was different between cirrhosis and chronic hepatitis B. In our study, most LR-M nodules (73.3%) in patients with cirrhosis were moderately-poorly differentiated HCC, nevertheless, most LR-M nodules in patients with chronic hepatitis B were non-HCC malignancies.
The present data validated the high risk of HCC in lesions showing LR-4 pattern, 80% of nodules displaying LR-4 pattern in cirrhosis was proved to be HCC. This was approaching to the ratio reported by Terzi (with a range of 85–90% in the various centers) [18]. Therefore, it is reasonable to put the diagnosis of HCC into consideration when a nodule displaying LR-4 pattern in cirrhosis to avoid miss the malignant tumor. Conversely, only one moderately differentiated HCC (0.83%) was classified as LR-4 in patients with chronic hepatitis. The most common LR-4 category nodules in patients with chronic hepatitis were FNH (50%) and HAML (25%), indicating that when a nodule manifesting LR-4 pattern in chronic hepatitis, it is more likely to be a benign lesion rather than a HCC. On CEUS, FNH typically shows obvious and marked hyperenhancement in the arterial phase, with a rapid spoke-like enhancenment from the center outwards or an eccentric vascular supply [27]. During the portal and venous phase, FNH may remain slightly hyper-enhancement or become iso-enhancement and a centrally located scar may be seen [28]. However, few FNH nodules were lack of this typical enhancement pattern and manifested HCC like enhancement pattern. High flow or thrombosed hemangiomas and myomatous type or angiomatous type of HAMLs are lack of typical features on CEUS [29, 30], which are liable to be considered as possible HCC nodules in patients with high risk factors. These suspicious HCC nodules required biopsy, or alternative imaging (CT or MRI).
There are some limitations in the present study. We only enrolled the patients who were diagnosed pathologically in this retrospective study. Selection bias could not be avoided in our study because focal hepatic lesions displaying typical enhancement pattern of benign nodule are less likely undergo resection or biopsy. Some lesions of LR-1, LR-2 category were not included in our study. There were few LR-3 nodules in this retrospective study. In our daily work, LR-3 nodules usually being suggested follow-up and lack of indication of resection or biopsy. Therefore, the risk of HCC in the LR-3 nodules could not be quantified. A long time and large sample size prospective study will address these issues. Furthermore, the patient population with chronic hepatitis is relatively small, only 23 patients with chronic hepatitis are diagnosed as HCC. The possible reason is that the HCC has lower incidence in patients with chronic hepatitis. In addition, nodules with large size (> 50 mm) were excluded in our study, which may have typical LR-5 pattern and higher possibility of HCC in high risk patients. And these situations are not uncommon in clinical practice, especially in patients without routine surveillance. A possible higher sensitivity of CEUS may occur if CEUS is applied to patients with nodules larger than 50 mm at high risk of HCC.
Conclusions
The present data shows that CEUS LR-5 pattern provides an excellent PPV and indicate that CEUS LR-5 category is a reliable non-invasive diagnostic modality for HCC in cirrhotic patients. Nevertheless, CEUS LR-5 category still requires alternative imaging (CT or MRI) or histological histological confirmation in patients with chronic hepatitis B for its unsatisfactory PPV for HCC. Most nodules of CEUS LR-M are proved to be malignancy and strengthen the role of CEUS in the differential diagnosis of benign and malignant lesions with cirrhosis or chronic hepatitis B.
References
Torre LA, Bray F, Siegel RL,et al. (2015) Global cancer statistics, 2012. CA Cancer J Clin 65(2):87-108.
Lafaro KJ, Demirjian AN, Pawlik TM. (2015) Epidemiology of hepatocellular carcinoma. Surg Oncol Clin N Am 24(1):1-17.
Fehér J, Lengyel G. (2010) Hepatocellular carcinoma: occurence, risk factors, biomarkers. Orvosi Hetilap 151(23):933-940.
Llovet JM, Di Bisceglie AM, Bruix J, et al. (2008) Design and endpoints of clinical trials in hepatocellular carcinoma. J Natl Cancer Inst 100(10):698-711.
Strobel D, Seitz K, Blank W, et al. (2008) Contrast-enhanced ultrasound for the characterization of focal liver lesions--diagnostic accuracy in clinical practice (DEGUM multicenter trial). Ultraschall Med 29(5):499-505.
Claudon M, Dietrich CF, Choi BI, et al. (2013) Guidelines and good clinical practice recommendations for contrast enhanced ultrasound (CEUS) in the liver--update 2012: a WFUMB-EFSUMB initiative in cooperation with representatives of AFSUMB, AIUM, ASUM, FLAUS and ICUS. Ultraschall Med 34(1):11-29.
Italian Association for the Study of the Liver (AISF), AISF Expert Panel, AISF Coordinating Committee, et al. (2013) Position paper of the Italian Association for the Study of the Liver (AISF): the multidisciplinary clinical approach to hepatocellular carcinoma. Dig Liver Dis 45(9):712–723.
Omata M, Lesmana LA, Tateishi R, et al. (2010) Asian Pacific Association for the Study of the Liver consensus recommendations on hepatocellular carcinoma.Hepatol Int 4(2):439–474.
Kudo M, Matsui O, Izumi N, et al. (2014) Liver Cancer Study Group of Japan. JSH Consensus-based clinical practice guidelines for the management of hepatocellular carcinoma: 2014 update by the liver cancer study group of Japan. Liver Cancer 3(3–4):458–468.
Sherman M, Burak K, Maroun J, et al. (2011) Multidisciplinary Canadian consensus recommendations for the management and treatment of hepatocellular carcinoma.Curr Oncol 18(5):228–240.
Bruix J, Sherman M, American Association for the Study of Liver Diseases. (2011) Management of hepatocellular carcinoma: an update. Hepatology 53 (3):1020-1022.
Forner A, Vilana R, Ayuso C, et al. (2008) Diagnosis of hepatic nodules 20 mm or smaller in cirrhosis: Prospective validation of the noninvasive diagnostic criteria for hepatocellular carcinoma.Hepatology 47(1):97–104.
Sangiovanni A, Manini MA, Iavarone M, et al. (2010) The diagnostic and economic impact of contrast imaging techniques in the diagnosis of small hepatocellular carcinoma in cirrhosis.Gut 59(5):638–644.
Yuan MX, Li R, Zhang XH, et al. (2016) Factors affecting the enhancement patterns of intrahepatic cholangiocarcinoma (ICC) on contrast-enhanced ultrasound (CEUS) and their pathological correlations in patients with a single lesion. Ultraschall Med 37(6):609-618.
Wildner D, Bernatik T, Greis C, et al. (2015) CEUS in hepatocellular carcinoma and intrahepatic cholangiocellular carcinoma in 320 patients - early or late washout matters: a subanalysis of the DEGUM multicenter trial.Ultraschall Med 36(6):132–139.
American College of Radiology (2016). Liver imaging reporting and data system 2016.https://www.acr.org/-/media/ACR/Files/RADS/LI-RADS/CEUS-LIRADS_V3.pdf?la=en, 2016–06–24.
American College of Radiology (2017). Liver imaging reporting and data system 2017 Core.https://www.acr.org/Clinical-Resources/Reporting-and-Data-Systems/LI-RADS/CEUS-LI-RADS-v2017.
Terzi E, Iavarone M, Pompili M, et al. (2018) Contrast ultrasound LI-RADS LR-5 identifies hepatocellular carcinoma in cirrhosis in a multicenter restropective study of 1,006 nodules. J Hepatol 68(3):485-492.
Terrault NA, Lok ASF, McMahon BJ, et al. (2018) Update on prevention, diagnosis, and treatment of chronic hepatitis B: AASLD 2018 hepatitis Bguidance. Hepatology 67(4):1560-1599.
Bolondi L, Correas JM, Lencioni R, et al. (2007) New perspectives for the use of contrast-enhanced liver ultrasound in clinical practice. Dig Liver Dis 39 (2):187-195.
Forner A, Vilana R, Bianchi L, et al. (2015) Lack of arterial hypervascularity at contrast-enhanced ultrasound should not define the priority for diagnostic work-up of nodules <2 cm. J Hepatol 62(1):150-155.
Jang HJ, Kim TK, Burns PN, et al. (2007) Enhancement patterns of hepatocellular carcinoma at contrast-Enhanced US: comparison with histologic differentiation. Radiology 244(3):898-906.
Boozari B, Soudah B, Rifai K, et al. (2011) Grading of hypervascular hepatocellular carcinoma using late phase of contrast enhanced sonography - a prospective study. Dig Liver Dis 43(6):484-490.
Loria, F, Loria G, Basile S, et al. (2012) Contrast-enhanced ultrasound of hepatocellular carcinoma: correlation between enhancement pattern and cellular differentiation on histopathlogy. Updates Surg 64(4):247-255.
von Herbay A, Vogt C, Westendorff J, et al. (2009) Correlation Between SonoVue Enhancement in CEUS, HCC Differentiation and HCC Diameter: Analysis of 130 Patients with Hepatocellular Carcinoma (HCC). Ultraschall Med 30(6):544-550.
Fan ZH, Chen MH, Dai Y, et al. (2006) Evaluation of primary malignancies of the liver using contrast-enhanced sonography: correlation with pathology. AJR Am J Roentgenol 186(6):1512-1519.
Dietrich CF, Schuessler G, Trojan J, et al. (2005) Differentiation of focal nodular hyperplasia and hepatocellular adenoma by contrast-enhanced ultrasound. Br J Radiol 78(932):704-707.
Piscaglia F, Gianstefani A, Ravaioli M, Golfieri R, Cappelli A, Giampalma E, Sagrini E, Imbriaco G, Pinna AD, Bolondi L; Bologna Liver Transplant Group. Criteria for diagnosing benign portal vein thrombosis in the assessment of patients with cirrhosis and hepatocellular carcinoma for liver transplantation. Liver Transpl. 2010;16(5):658–667.
Dietrich CF, Mertens JC, Braden B, et al. (2007) Contrast-enhanced ultrasound of histologically proven liver hemangiomas. Hepatology 45(5):1139-1145.
Li R, Tang CL, Cai P, et al. (2016) Comparison of CT and contrast-enhanced ultrasound findings in hepatic angiomyolipoma with pathological correlations. Abdom Radiol (NY) 41(2):248-256.
Funding
No funding was received for conducting this study.
Author information
Authors and Affiliations
Contributions
RL: designed the study and revise the manuscript. Interpreted the results of CEUS, drafted the manuscript and revised it. DY: interpreted CEUS images and collected clinical data. Performed the statistical analysis and drafted the manuscript. HH: designed the study and interpreted the results of CEUS. CLT: interpreted CEUS images and collected cases. KSM: interpreted clinical data, performed liver surgery and biopsy. DYG: interpreted the pathology of HCC.All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Yang, D., Hu, H., Li, R. et al. The diagnostic value of contrast-enhanced ultrasound LI-RADS for hepatocellular carcinoma in patients with cirrhosis and chronic hepatitis B. Abdom Radiol 47, 630–639 (2022). https://doi.org/10.1007/s00261-021-03345-9
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00261-021-03345-9