Abstract
Purpose
The aim of this study was to describe changes in contrast agent kinetics in HCC following incomplete trans-arterial chemoembolization (TACE) on contrast-enhanced ultrasound (CEUS) and MRI/CT.
Methods
Patients with residual HCC proven by biopsy, retreatment angiography, or 4–8 month MRI demonstrating tumor progression were identified. Pre-treatment and 4–6-week follow-up CE-MRI/CT and CEUS exams were collected for blinded reads by two experienced readers for each modality to evaluate arterial phase hyper-enhancement (APHE) and washout within the residual HCC. A third reader provided tie-breaking decisions for any disagreements.
Results
Contrast-enhanced imaging data were collected from 29 patients with residual HCC post-TACE. On CEUS, 84.2% of patients with baseline APHE demonstrated APHE post-TACE (p = 0.25). On CE-MRI/CT, 57.1% of patients with baseline APHE later demonstrated APHE (p = 0.004). As for washout, on CEUS 33.3% of patients with baseline washout retained washout post-TACE (p = 0.01), while on CE-MRI/CT only 18.8% of patients with baseline washout later demonstrated washout (p < 0.001). Among CEUS readers, reader agreement was 100% for baseline APHE, 66.7% for baseline washout (K = 0.35), 84.2% for post-TACE APHE (K = 0.35), and 57.9% for post-TACE washout (K = − 0.09). On CE-MRI/CT, reader agreement was 65.5% for baseline APHE (K = 0.19), 55.2% for baseline washout (K = 0.12), 48.3% for post-TACE APHE (K = − 0.07), and 58.6% for post-TACE washout (K = 0.04).
Conclusion
Common diagnostic features of treatment-naïve HCC like APHE and washout can be substantially altered by TACE and should be considered when diagnosing residual disease on contrast-enhanced imaging.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Hepatocellular carcinoma (HCC) accounts for approximately 75% of all primary liver cancers, the prognosis of which remains relatively poor worldwide [1]. The incidence of HCC continues to rise given its association with risk factors like hepatitis B, hepatitis C, fatty liver disease, and cirrhosis from any etiology [2]. While HCC can be definitively treated early with surgical resection and transplantation, the disease is often discovered in its intermediate or late stages rendering patients ineligible for surgical intervention3,4. According to the Barcelona Clinic Liver Cancer (BCLC) Guidelines, locoregional therapy is often initiated in cases of intermediate disease as a bridging therapy to downstage the disease, prevent progression, or in more severe cases for palliation [5, 6]. Moreover, locoregional therapies can be used for potentially curative treatment. [6].
Locoregional therapies are minimally invasive procedures that use imaging guidance to introduce therapy directly to the tumor to induce necrosis within the malignant tissue [7]. There are various subtypes of locoregional therapies that utilize different techniques. They can use heat (thermal ablation), ischemia (embolization), radiation (radioembolization), or chemotherapy to induce tumor necrosis [4]. These locoregional therapies have the common goal of tumoral destruction.
Trans-arterial chemoembolization (TACE) is a commonly used locoregional therapy that improves HCC survival [8]. According to BCLC Guidelines, TACE is a preferred treatment for those with intermediate stage B disease and is also used as a bridging therapy for those awaiting transplantation [9, 10]. It is also used at many institutions for treatment of BCLC stage C disease [10]. In a conventional TACE procedure (c-TACE), a combination of lipiodol and chemotherapy is introduced via catheter intraarterially, followed by the embolization with gelfoam particles [11]. An alternative approach uses drug-eluting beads (DEB-TACE) in which poly-vinyl alcohol beads are loaded with doxorubicin to achieve localized chemotherapeutic effect. The goal of all TACE procedures is to create an ischemic environment while simultaneously trapping chemotherapy in the tumor.
The current reference standard for monitoring treatment response to TACE therapy is contrast-enhanced magnetic resonance imaging (CE-MRI) or contrast-enhanced computed tomography (CT) [12]. The Society of Interventional Radiology recommends follow-up CE-MRI or CT imaging approximately 4 weeks after TACE to evaluate tumor response to the procedure and every 3–6 months thereafter [4, 13]. Although, even after 4–6 weeks, it may still be difficult to discriminate viable tumor from simple inflammation. This limitation is particularly problematic when ring enhancement is present because of the low molecular weight and water solubility of commonly used CE-MRI/CT contrast agents [14].
Alternatively, contrast-enhanced ultrasound (CEUS) is another useful technique to evaluate treatment response and assess for viable HCC [15,16,17]. CEUS utilizes microbubble contrast agent to assess vascularity in real time via ultrasonography with high temporal resolution16. These microbubbles used in CEUS also have the advantage of allowing the use of contrast-based imaging without the risks of ionizing radiation and nephrotoxicity [17].
The American College of Radiology Liver Imaging Reporting and Data System (LI-RADS) represents standardized criteria to assess specific features of liver lesions on radiologic assessment. According to LI-RADS, a combination of arterial hyper-enhancement (APHE) and washout is hallmark appearance of HCC [18]. LI-RADS scoring ranges from LR-1 (“probably benign”) to LR-5 (“definitely HCC”). A LR-5 lesion would show diffuse or partial APHE in a 10 mm + nodule with late and/or mild washout (> 60 s) [19]. Both CE-MRI/CT and CEUS utilize very similar LI-RADS criteria evaluating APHE and washout in focal liver lesions [18, 20]. LI-RADS criteria for evaluating response to locoregional treatment are also in development. Based on current criteria, residual tumor following therapy is generally represented by the persistence of APHE in the treated lesion on CE-MRI/CT [21, 22]. While washout is included in these LI-RADS criteria, it is not independently necessary for the identification of viable tumor. However, because locoregional therapy at its core alters tumor vascularity, there are less well-defined criteria in the radiologic characterization of washout in residual HCC on CEUS.
Various studies show that CEUS and CE-MRI/CT agree strongly in the identification of residual HCC following locoregional therapy [23, 24]. However, there is little systematic review addressing how the parameters of blood flow kinetics might change despite the presence of viable tumor after TACE. While both CE-MRI/CT and CEUS appear to be reliable in identifying viable cancer, there is little work directly addressing the expected radiographic appearance of residual HCC on these modalities. Consequently, in this work we provide a blinded, multi-reader comparative study of CEUS and CE-MRI/CT in patients with proven residual HCC following TACE.
Methods
Data source
This retrospective analysis examined imaging results of 29 HCC patients receiving TACE as part of a larger clinical trial. As part of the IRB-approved trial (NCT#02,764,801), patients provided informed consent to receive both CEUS and CE-MRI or CT before TACE and 4–6 weeks post-treatment. In this study, we only examined baseline imaging and 4–6-week follow-up. Patients who received TACE therapy at Thomas Jefferson University in an ongoing clinical trial were identified. The sub-population of those with residual HCC proven on biopsy or clinically by 4–8-month MRI, retreatment angiography demonstrating disease progression (Table 1) were enrolled and their CE-MRI/CT and CEUS images were accessed for this study. De-identified imaging results from both the pre-treatment and at 4–6 weeks following TACE were collected for blinded reads.
CEUS imaging
Patients were scanned with a Logiq E9 ultrasound scanner (GE Healthcare, Waukesha, WI) using a C1–6 curved array transducer. The transducer was operated in a coded harmonic imaging mode with a low mechanical index (MI < 0.2). On baseline imaging, patients received 0.2 – 0.3 mL of Definity contrast agent (Lantheus Medical Imaging, North Billerica, MA) followed by a 10 mL saline flush. Before an injection of contrast agent, the sonographer obtained grayscale still images with and without measurements demarcating the tumor. The sonographer also used color and pulsed-wave Doppler to help visualize major vascularity surrounding the tumor area. Imaging data were collected during the arterial phase at midline of the tumor, followed by sweeping through the lesion for the first 60–90-s post-injection. Following arterial imaging, intermittent imaging (1 sweep approximately every 30 s) was recorded to evaluate vascularity while still minimizing microbubble destruction.
CE-MRI imaging
CE-MRI images were obtained with one of two scanners as part of the participant’s clinical standard of care. One scanner was a 1.5 T SIGNA scanner (GE Healthcare, Chicago, Illinois) using the following pulse sequences: 3-plane single-shot fast spin echo (SSFSE), coronal SSFSE, axial SSFSE, axial liver acquisition with volume acceleration flex, fast recovery fast spin echo array coil special sensitivity encoding, diffusion-weighted imaging, and fast imaging employing steady state. The other scanner was a 1.5 T Philips Achieve scanner (Philips Healthcare, Best, the Netherlands) using the following pulse sequences: axial SSFSE, balanced fast field echo, dual fast field echo, pre-contrast axial T1 high-resolution isotropic volume examination (THRIVE), arterial phase axial THRIVE, and venous phase axial THRIVE. Gadavist (Bayer, Leverkusen, Germany) gadolinium contrast agent was generally employed at a dose of 0.1 mmol/kg (approximately 7-10 mL), but occasionally titrated due to compromised renal function or BMI.
CE-CT imaging
CE-CT images were obtained using one of three scanners as part of the participant’s clinical care. The scanners were either a Philips Big Bore, Philips Brilliance 64, or Philips Brilliance iCT (Philips Healthcare, Best, the Netherlands). Slice thicknesses for each scan ranged from 2.0 to 5.0 mm. Omnipaque contrast agent (GE Healthcare, Chicago, Illinois) was used at a dose of 100 mL administered at a rate of 4 mL/s.
Analysis of imaging
Two readers for CEUS (6 and more than 20 years of experience in CEUS) and two for CE-MRI (10 and 17 years of experience in body imaging) were selected to analyze baseline and post-treatment scans for each patient. One additional reader with 14 years of experience was selected to provide consensus reads on cases of disagreement on both modalities. All scans were viewed using RadiAnt DICOM software (Medixant, Poznan, Poland).
For CE-MRI/CT, the readers were asked to use a binary yes or no if the image showed APHE and a binary yes or no if the image showed early tumoral washout relative to the surrounding liver in either the early or late phases (i.e., within the first 2–3 min). This process was repeated for both the baseline and follow-up exams. The readers in the MRI/CT group read both MRI and CT scans. For CEUS, readers first viewed a grayscale image of the tumor followed by CEUS from beginning of injection to 2–5 min following contrast injection. Similar to CE-MRI/CT, readers were asked to determine using a binary yes or no if the image showed APHE and a binary yes or no if the image showed any washout. This process was repeated for both the baseline and follow-up exams. Finally, the enhancement patterns for those cases that did show washout in viable tumor on CEUS were also identified and classified as either ‘no change,’ ‘partially treated,’ or ‘nodular peripheral enhancement’ to determine the influence of treatment on changes in tumor blood flow kinetics.
Statistical analyses
All statistics were performed in SPSS software (IBM, Armonk, NY) or MedCalc (MedCalc Software, Ostend, Belgium), with α < 0.05 used to determine statistical significance. All data were presented as mean ± standard deviation (SD). To assess any baseline differences between c-TACE and DEB-TACE, a chi-squared test was performed on retention of APHE and retention of washout. Cohen’s kappa was calculated to quantify reader reliability in each modality for the identification of APHE and washout on both baseline and one-month imaging. A McNemar’s test was conducted for APHE and washout for each reader and consensus data to evaluate differences in the radiologic characteristics of tumor vascularity in response to TACE. Importantly, a McNemar’s test was also conducted on a sub-population of patients who had APHE or washout on baseline to their 6–8-week counterparts. This more directly evaluates changes in vascular kinetics to the tumor following TACE. Additionally, to assess for inter-modality differences, we conducted an independent samples t test for baseline APHE and washout.
Results
Patient/tumor characteristics
In a subset of 72 HCC patients who received TACE therapy, 42 were identified as having proven residual HCC following therapy (58.3%). Reference standards to definitively diagnose residual HCC varied for each patient but included tumor growth or viability on 4–8-month CE-MRI/CT, confirmation of residual vascularity on angiography during retreatment, pathology, or tumor progression on imaging (Table 1). After excluding cases that were previously treated prior to initial TACE therapy in this study, we identified 29 cases to include in analysis. All cases were visible on CE-MRI or CT and 21 of the 29 cases were visible and analyzed on CEUS (72.4%). No significant differences were found between c-TACE and DEB-TACE for retention of APHE (p = 0.54). Similarly, there was also no significant difference between c-TACE and DEB-TACE in retention of washout (p = 0.82). Tumor characteristics are summarized in Table 1. Patient characteristics are summarized in Table 2.
Baseline imaging results
Summary statistics from reads across both time points and modalities are provided in Table 3. On baseline imaging, both CEUS readers diagnosed APHE within the mass in 100% of cases (21/21). This is consistent with the presence of an untreated HCC lesion in each patient. Inter-rater reliability could not be calculated for this condition because both readers ruled APHE on 100% of cases. On CE-MRI/CT, both readers diagnosed APHE in 72.4% of patients (21/29), although this was frequently diagnosed in different patients. Inter-rater reliability for diagnosis of APHE among CE-MRI/CT readers was low (Kappa = 0.19, p = 0.30).
Diagnosis of washout prior to treatment was more variable on both modalities. CEUS reader 1 diagnosed washout in 71.4% of cases (15/21) and CEUS reader 2 diagnosed washout in 47.6% of cases (10/21). Inter-rater reliability for diagnosis of washout among CEUS readers was moderate (Kappa = 0.35, p = 0.07). On CE-MRI/CT, reader 1 diagnosed washout in 48.3% of cases (14/29) and CE-MRI/CT reader 2 diagnosed washout on 79.3% of cases (23/29). Inter-rater reliability for diagnosis of washout among CE-MRI/CT readers was low (Kappa = 0.12, p = 0.41). Following consensus reads for cases of disagreement on CEUS, APHE was found in 100% of cases (21/21) and washout was found in 66.7% of cases (14/21). Following consensus reads of MRI/CT, APHE was found in 72.4% of cases (21/29) and washout was found in 55.2% of cases (16/29).
Post-treatment imaging results
One month following TACE, CEUS reader 1 diagnosed APHE in 79.0% of patients (15/19), representing no significant difference from baseline diagnosis of APHE (p = 0.13). CEUS reader 2 diagnosed APHE in 94.7% of patients (18/19) on one-month imaging also representing no significant difference from baseline diagnosis of APHE (p = 1.0). Inter-rater reliability for APHE between the CEUS readers was found to remain relatively high (Kappa = 0.35, p = 0.04). CE-MRI/CT reader 1 diagnosed APHE in 55.2% of patients (16/29), representing no significant difference from baseline (p = 0.58). CE-MRI/CT reader 2 diagnosed APHE in 44.8% of patients (13/29) which was significantly lower than baseline (p = 0.04). Inter-rater reliability for APHE among CE-MRI/CT readers was low (Kappa = − 0.07, p = 0.60).
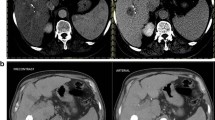
One month following TACE, CEUS reader 1 diagnosed washout in 26.3% of viable tumors (5/19) which was significantly lower than baseline (p = 0.01). CEUS reader 2 also diagnosed washout in 26.3% of patients (5/19), representing no significant difference from baseline (p = 0.45). Inter-rater reliability for diagnosis of washout among CEUS readers was low (Kappa = − 0.09, p = 0.71). CE-MRI/CT reader 1 diagnosed washout in 34.5% of cases (10/29), representing no significant difference from baseline (p = 0.48). CE-MRI/CT reader 2 diagnosed washout in 27.6% of cases (8/29) which was significantly less than baseline (p < 0.001). Inter-rater reliability for diagnosis of washout among CE-MRI/CT readers was low (Kappa = 0.04, p = 0.83). Following consensus reads of CEUS cases, APHE was found in 84.2% of cases (16/19, p = 0.25) and washout was found in 26.3% of cases (5/19, p = 0.04). In consensus CE-MRI/CT data, APHE was found in 48.3% of cases (14/29, p = 0.07) and washout was found in 20.7% of cases (6/29, p = 0.02). A general summary of these results is seen in Table 3. Figures 1 and 2 provide examples of how washout did or did not change from baseline on both modalities.
An example of an 8.4 cm segment 8 lesion that showed washout on baseline but not after TACE. Yellow arrows identify the tumor in each panel. All images are from the same patient. A Baseline CEUS imaging showing washout at time 2:59 following contrast injection. B Follow-up CEUS imaging showing no washout in residual tumor at time 1:11 following contrast injection. C Baseline delayed phase CE-MRI imaging showing washout. D Follow-up delayed phase CE-MRI imaging showing lack of washout of the residual tumor
An example of a 3.9 cm segment 4B lesion that showed washout both on baseline and after TACE. Yellow arrows identify the tumor in each panel. All images are from the same patient. A Baseline CEUS imaging showing washout at time 2:49 following contrast injection. B Follow-up CEUS imaging showing washout in residual tumor at time 3:06 following contrast injection. C Baseline delayed phase CE-MRI imaging showing washout. D Follow-up delayed phase CE-MRI imaging showing washout of the residual tumor
Tumor-specific changes to APHE/washout following treatment
A sub-analysis was also conducted on patients who had APHE or washout on baseline to their one-month counterparts. This more directly evaluates tumor-specific changes in parameters of vascular kinetics following TACE. On CEUS, 78.9% (15/19)—94.7% (18/19) of patients who had APHE at baseline demonstrated APHE post-TACE (p > 0.13). Following CEUS consensus reads, 84.2% of patients (16/19) who had APHE on baseline demonstrated APHE post-TACE (p = 0.25). On CE-MRI/CT, 52.4% (11/21)—57.1% (12/21) of patients who had APHE at baseline later demonstrated APHE within the residual tumor post-treatment (p < 0.02). CE-MRI/CT consensus data concluded that 57.1% of patients (11/21) who with baseline APHE later continued to show APHE following TACE (p = 0.004). Correspondingly, while evaluating changes in washout, on CEUS only 28.6% (4/14)—37.5% (3/8) of patients who showed washout pre-treatment retained this radiographic finding post-TACE (p = 0.002 & p = 0.06 for readers 1 and 2, respectively). CEUS consensus reads determined that 33.3% of patients (4/12) who showed washout on baseline retained washout on post-treatment imaging (p = 0.008). Figure 3 provides an example of CEUS and CE-MRI/CT disagreement on the persistence of washout after TACE. Similarly, on CE-MRI/CT only 21.4% (3/14)—30.4% (7/23) of patients who demonstrated washout at baseline later demonstrated washout within the viable tumor post-treatment (p < 0.001). According to consensus CE-MRI/CT reads, only 18.8% of cases (3/16) with baseline washout also showed washout on post-treatment imaging (p < 0.001).
An example of an 8.5 cm segment 4B lesion that elicited disagreement between CEUS and CE-MRI. CEUS diagnosed washout on baseline but not after TACE while CE-MRI diagnosed washout at both points. Yellow arrows identify the tumor in each panel. A Baseline CEUS imaging showing washout at time 2:35 following contrast injection. B Follow-up CEUS imaging showing lack of washout in residual tumor at time 2:50 following contrast injection. C Baseline delayed phase CE-MRI imaging showing washout. D Follow-up delayed phase CE-MRI imaging showing washout of the residual tumor
CEUS 2D enhancement patterns after treatment
CEUS readers identified 5 cases of residual tumor that retained the appearance of tumoral washout after treatment. Of these 5 cases, 3 (60%) were described as having an enhancement pattern representing “no change” from baseline on the diagnostic CEUS evaluation at the time of imaging. Interestingly, because most of the cases where washout was observed after treatment were described as not different as baseline, this could indicate that treatment did not affect tumor vascularity in these cases. If no changes are observed in the tumor, it makes sense that parameters of blood flow kinetics are not changing in these cases. Although, it is important to note that this pattern was only observed in 3 cases so a larger sample of similar cases would be needed to more confidently make these conclusions.
Assessing for inter-modality differences
When evaluating APHE across modalities, CEUS demonstrated a higher propensity for calling this criterion at baseline (100% ± 0% vs. 72.4% ± 0%). An example of CEUS calling persistent APHE while CE-MRI/CT did not can be seen in Fig. 4. There were no significant differences in the observation of tumoral washout between modalities at baseline (59.5% ± 16.8% vs. 63.8% ± 21.9%, p = 0.85).
An example of a 6.1 cm segment 2 lesion that showed persistence of APHE on CEUS but lack of persistence of APHE on CE-MRI. A Baseline CEUS imaging showing APHE at time 0:29 following contrast injection. B Follow-up CEUS showing APHE at time 0:24 following contrast injection. C Baseline arterial phase CE-MRI showing APHE. D Follow-up arterial phase CE-MRI showing lack of APHE in the residual tumor
Discussion/Conclusion
Specific radiographic findings of residual HCC following TACE remain relatively undefined. This study evaluated changes to these parameters (specifically APHE and early washout) 1-month post-treatment as well as comparing findings across modalities. Generally, APHE seemed to be much more pronounced in CEUS compared to CE-MRI/CT diagnoses on both baseline and post-treatment. This is likely reflective of the very high degree of temporal resolution that is achieved with real-time CEUS imaging [16, 25]. Additionally, this is also representative of the intravascular properties of CEUS contrast agent compared to the interstitial properties of the MRI/CT contrast agents, allowing vascularity to be highlighted. When diagnosing washout, all readers observed tumor washout either significantly or nearly significantly less often after treatment. Despite the presence of viable cancer tissue that had originally washed out, tumor vascular kinetics appeared to be affected by treatment. These variable findings of washout are interesting because although washout is a key criteria for the diagnosis of LR-4 or LR-5 lesions [20, 22], washout was only seen in a minority of cases according to one reader in each modality.
Moreover, of those that did show washout on both baseline and after treatment, CEUS-based descriptions of the residual enhancing component suggest that treatment may have had limited change to the overall tumor vascularity in the majority of these cases.
This study is important for the further refinement of criteria to evaluate residual HCC following TACE. The current LI-RADS CEUS guidelines recommend further evaluation of a treated lesion on CE-MRI/CT when it is encountered on CEUS because guidelines are not yet available [20]. However, according to the American College of Radiology LI-RADS criteria, either APHE or washout serves as criteria to favor the presence of residual cancer on CE-MRI/CT [22]. Our results show that both these findings can be lost over the course of TACE in tumors with confirmed residual enhancement. When comparing APHE vs. early washout, our data suggest that the presence of residual tumor may be more aptly identified with the presence of APHE and that this criterion is more frequently seen on CEUS because of its seemingly better ability to capture APHE both pre- and post-treatment with high temporal resolution. This would also indicate that the future CEUS guidelines should emphasize the presence of APHE over washout in the distinction of residual tumor following TACE.
While encouraging, this study has limitations involving sample population as well as readers. Specifically, we only used patients originating from a single institution. A multi-center study with additional locoregional therapies capturing a more diverse patient population would improve the generalizability of these results. Additionally, our sample size was rather small, consisting of 29 cases visible on CE-MRI/CT and 21 visible on CEUS. This sample size was further lessened in the sub-analyses considering only the cases that retained washout post-treatment. Also, only three readers were used for each modality in the present study, making their data more difficult to confidently assess. A future study with a larger sample of patients as well as more total readers would help make these findings more robust. Reader agreement in this specific study for both modalities was also relatively low, which may be related to the small sample size of the population. Finally, patients with locoregional therapy performed prior to TACE were omitted for data heterogeneity, but this should also be explored moving forward.
In conclusion, this study demonstrates that imaging patterns of tumor enhancement may be altered in cases with confirmed residual disease. Consequently, pre-treatment imaging hallmarks of HCC may be less relevant following TACE, which should be considered when evaluating for the presence of residual disease post-treatment with CE-MRI/CT and CEUS.
Data availability
Not applicable.
Code availability
Not applicable.
Abbreviations
- HCC:
-
Hepatocellular carcinoma
- BCLC:
-
Barcelona Clinic Liver Cancer
- TACE:
-
Trans-arterial chemoembolization
- c-TACE:
-
Conventional TACE
- DEB-TACE:
-
Drug-eluting bead TACE
- CE-MRI:
-
Contrast-enhanced magnetic resonance imaging
- CT:
-
Computed tomography
- CEUS:
-
Contrast-enhanced ultrasound
- APHE:
-
Arterial phase hyper-enhancement
- LI-RADS:
-
Liver Imaging Reporting and Data System
- IRB:
-
Institutional review board
- MI:
-
Mechanical index
- SSFSE:
-
Single-shot fast spin echo
- THRIVE:
-
T1 high-resolution isotropic volume examination
- SD:
-
Standard deviation
- IQR:
-
Inter-quartile range
References
McGlynn KA, Petrick JL, El‐Serag HB. Epidemiology of Hepatocellular Carcinoma. Hepatology. 2021;73(S1):4-13. doi:https://doi.org/https://doi.org/10.1002/hep.31288
Rawla P, Sunkara T, Muralidharan P, Raj JP. Update in global trends and aetiology of hepatocellular carcinoma. Contemp Oncol. 2018;22(3):141-150. doi:https://doi.org/10.5114/wo.2018.78941
El-Serag HB, Marrero JA, Rudolph L, Reddy KR. Diagnosis and Treatment of Hepatocellular Carcinoma. Gastroenterology. 2008;134(6):1752-1763. doi:https://doi.org/10.1053/j.gastro.2008.02.090
Makary MS, Khandpur U, Cloyd JM, Mumtaz K, Dowell JD. Locoregional Therapy Approaches for Hepatocellular Carcinoma: Recent Advances and Management Strategies. Cancers. 2020;12(7). doi:https://doi.org/10.3390/cancers12071914
Heckman JT, deVera MB, Marsh JW, et al. Bridging Locoregional Therapy for Hepatocellular Carcinoma Prior to Liver Transplantation. Ann Surg Oncol. 2008;15(11):3169-3177. doi:https://doi.org/10.1245/s10434-008-0071-3
Forner A, Reig ME, Lope CR de, Bruix J. Current Strategy for Staging and Treatment: The BCLC Update and Future Prospects. Semin Liver Dis. 2010;30(1):61-74. doi:https://doi.org/10.1055/s-0030-1247133
Huo T-I. Locoregional Therapy. In: Schwab M, ed. Encyclopedia of Cancer. Springer, Berlin, Heidelberg; 2011:2072-2073. doi:https://doi.org/10.1007/978-3-642-16483-5_3407
Varela M, Real MI, Burrel M, et al. Chemoembolization of hepatocellular carcinoma with drug eluting beads: Efficacy and doxorubicin pharmacokinetics. J Hepatol. 2007;46(3):474-481. doi:https://doi.org/10.1016/j.jhep.2006.10.020
Sciarra A, Ronot M, Tommaso LD, et al. TRIP: a pathological score for transarterial chemoembolization resistance individualized prediction in hepatocellular carcinoma. Liver Int. 2015;35(11):2466-2473. doi:https://doi.org/https://doi.org/10.1111/liv.12844
Tsurusaki M, Murakami T. Surgical and Locoregional Therapy of HCC: TACE. Liver Cancer. 2015;4(3):165-175. doi:https://doi.org/10.1159/000367739
Burrel M, Reig M, Forner A, et al. Survival of patients with hepatocellular carcinoma treated by transarterial chemoembolisation (TACE) using Drug Eluting Beads. Implications for clinical practice and trial design. J Hepatol. 2012;56(6):1330-1335. doi:https://doi.org/10.1016/j.jhep.2012.01.008
Nam K, Stanczak M, Lyshchik A, et al. Evaluation of Hepatocellular Carcinoma Transarterial Chemoembolization using Quantitative Analysis of 2D and 3D Real-time Contrast Enhanced Ultrasound. Biomed Phys Eng Express. 2018;4(3):035039. doi:https://doi.org/10.1088/2057-1976/aabb14
Gaba RC, Lokken P, Hickey R, et al. Quality Improvement Guidelines for Transarterial Chemoembolization and Embolization of Hepatic Malignancy. 2017;28(9):17.
Kono Y, Lucidarme O, Choi S-H, et al. Contrast-enhanced Ultrasound as a Predictor of Treatment Efficacy within 2 Weeks after Transarterial Chemoembolization of Hepatocellular Carcinoma. J Vasc Interv Radiol. 2007;18(1):57-65. doi:https://doi.org/10.1016/j.jvir.2006.10.016
Wessner CE, Shaw CM, Stanczak M, et al. Contrast-enhanced Ultrasound Identifies Patent Feeding Vessels in Transarterial Chemoembolization Patients With Residual Tumor Vascularity. Ultrasound Q. 2020;36(3):218-223. doi:https://doi.org/10.1097/RUQ.0000000000000513
Claudon M, Cosgrove D, Albrecht T, et al. Guidelines and Good Clinical Practice Recommendations for Contrast Enhanced Ultrasound (CEUS) - Update 2008. Ultraschall Med - Eur J Ultrasound. 2008;29(01):28-44. doi:https://doi.org/10.1055/s-2007-963785
Wilson SR, Greenbaum LD, Goldberg BB. Contrast-Enhanced Ultrasound: What Is the Evidence and What Are the Obstacles? Am J Roentgenol. 2009;193(1):55-60. doi:https://doi.org/10.2214/AJR.09.2553
Tang A, Bashir MR, Corwin MT, et al. Evidence Supporting LI-RADS Major Features for CT- and MR Imaging–based Diagnosis of Hepatocellular Carcinoma: A Systematic Review. Radiology. 2017;286(1):29-48. doi:https://doi.org/10.1148/radiol.2017170554
Yang HK, Burns PN, Jang H-J, et al. Contrast-enhanced ultrasound approach to the diagnosis of focal liver lesions: the importance of washout. Ultrasonography. 2019;38(4):289-301. doi:https://doi.org/10.14366/usg.19006
American College of Radiology Committee on LI-RADS. CEUS LI-RADS v2017. Accessed April 14, 2021. https://www.acr.org/Clinical-Resources/Reporting-and-Data-Systems/LI-RADS/CEUS-LI-RADS-v2017.
Goldberg SN, Grassi CJ, Cardella JF, et al. Image-guided Tumor Ablation: Standardization of Terminology and Reporting Criteria. J Vasc Interv Radiol. 2009;20(7, Supplement):S377-S390. doi:https://doi.org/10.1016/j.jvir.2009.04.011
American College of Radiology Committee on LI-RADS. MRI/CT LI-RADS v2017. Accessed April 14, 2021. https://www.acr.org/Clinical-Resources/Reporting-and-Data-Systems/LI-RADS/CT-MRI-LI-RADS-v2018.
Shaw CM, Eisenbrey JR, Lyshchik A, et al. Contrast-Enhanced Ultrasound Evaluation of Residual Blood Flow to Hepatocellular Carcinoma After Treatment With Transarterial Chemoembolization Using Drug-Eluting Beads. J Ultrasound Med. 2015;34(5):859-867. doi:https://doi.org/https://doi.org/10.7863/ultra.34.5.859
Lekht I, Gulati M, Nayyar M, et al. Role of contrast-enhanced ultrasound (CEUS) in evaluation of thermal ablation zone. Abdom Radiol. 2016;41(8):1511-1521. doi:https://doi.org/10.1007/s00261-016-0700-4
Jang H-J, Kim TK, Burns PN, Wilson SR. CEUS: An essential component in a multimodality approach to small nodules in patients at high-risk for hepatocellular carcinoma. Eur J Radiol. 2015;84(9):1623-1635. doi:https://doi.org/10.1016/j.ejrad.2015.05.020
Acknowledgements
Contrast-enhanced ultrasound data were obtained from an ongoing clinical trial (NCT#02764801) which received ultrasound contrast agent from Lantheus Medical Imaging (N. Billerica, MA) and equipment support from GE Healthcare (Milwaukee, WI).
Funding
Research funding was provided by NIH R01 CA194307 and NIH R01 CA215520.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
CEW declares that he is a consultant in Bracco Imaging. AL declares that he is a research supporter in GE Healthcare, Bracco Diagnostics, Siemens Healthineers, and Canon Medical Systems, is a member of advisory board in Bracco Diagnostics, is a consultant in GE Healthcare, Bioclinica, and WorldCare Clinical, and is one of the members of speaker panel in GE Healthcare, and has book royalties in Elsevier. JRE received grant and equipment support from GE Healthcare, equipment support from Siemens, and drug support and speaker honorarium from Lantheus Medical Imaging. All other authors have no conflict of interest to declare.
Ethical approval
This study was approved by the university’s Institutional Review Board.
Consent to participate
All patients in this trial provided informed consent to participate in the initial contrast-enhanced ultrasound study.
Consent for publication
Consent to publish de-identified images and data were included in the informed consent process.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Polikoff, A., Wessner, C.E., Balasubramanya, R. et al. Imaging appearance of residual HCC following incomplete trans-arterial chemoembolization on contrast-enhanced imaging. Abdom Radiol 47, 152–160 (2022). https://doi.org/10.1007/s00261-021-03298-z
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00261-021-03298-z