Abstract
Refractory bile leaks represent a damaging sequela of hepatobiliary surgery and direct trauma. Management of bile leaks represents a challenging clinical problem. Despite advances in endoscopic techniques, interventional radiology continues to play a vital role in the diagnosis and management of refractory bile leaks. This article reviews strategies for optimizing the diagnosis and management of bile leaks and provides an overview of management strategies, including the management of complicated biliary leaks.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Bile leaks are an uncommon but feared complication of hepatobiliary surgery and trauma [1,2,3]. The incidence of post-operative bile leaks has increased over the past 30 years, due in part to the development of laparoscopic surgery, but also due to an increase in the number of patients who now qualify for hepatobiliary surgery [4]. When associated with hepatobiliary surgery, bile leaks can result in diminished survival and long-term biliary stricture as well as increased morbidity and mortality [1, 4]. In addition to the morbidity and mortality associated with post-operative bile leaks, the cost of repair of an operative bile duct injury may be substantial due to extended hospital stay, repeat surgery and multiple imaging studies to diagnose and follow-up treatment. For these reasons, non-surgical interventions are often preferred over surgical re-interventions. And despite advances in endoscopic techniques, interventional radiologists continue to play an important role in the management of patients with biliary leaks [5] and are often called upon to assist in the diagnosis and management of challenging cases. The purpose of this article is to provide a comprehensive review of the etiology, imaging presentation and role of interventional radiology in the management of bile leaks.
Etiology
Bile leaks most commonly develop as a result of complications of hepatobiliary surgery or abdominal trauma [6,7,8,9].
Hepatobiliary Surgery
Hepatic resection
Bile leakage from the hepatic resection margin is one of the main causes of morbidity after major liver resections (Fig. 1). In a prospective analysis of 467 consecutive patients who underwent liver resections, post-operative bile leaks after hepatectomy were associated with a 5.4% morbidity rate [10]. Factors that may contribute to an increased risk of postoperative bile leakage include resection of at least three segments, operative times longer than 3 h, non-anatomic resections, and the presence of total vascular occlusion [10, 11].
Cholecystectomy
Laparoscopic cholecystectomy, which was introduced 30 years ago as a less invasive alternative to open surgery, is now one of the most commonly performed surgical procedures worldwide and is considered the gold standard for the management of cholelithiasis [12, 13]. However, along with the introduction of laparoscopic cholecystectomy was an increase in the rate of bile leaks; the rate increased from 0.1 to 0.2% with open cholecystectomy to approximately 3% with the laparoscopic procedure [14,15,16,17]. One factor that may account for this increased risk is difficulty in defining surgical planes as a result of bleeding, inflammation, and scarring. Injury of the common bile duct during the procedure is the most common cause of bile leak after cholecystectomy; the second most common cause is injury to the ducts of Luschka. The ducts of Luschka are small accessory bile ducts that form tributaries of the right hepatic ductal system and enter the gallbladder bed [18]. When injured, these ducts can leak bile directly into the gallbladder fossa [18].
Liver transplant
Bile leak after liver transplant occurs in up to 27% of cases and is more commonly associated with related living-donor transplants [19]. These leaks most commonly occur as anastomotic leaks between the donor and recipient bile ducts, and leaks from the hepaticojejunostomy when a Roux-en-Y anastomosis is created [20, 21]. Anastomoses involving three or more bile ducts tend to be associated with a higher risk of leak [22]. With related living-donor transplants, bile leak can also occur along the cut liver surface.
Abdominal trauma
The incidence of major bile leak after traumatic biliary injury is low [23]. When these leaks do occur, they can arise from penetrating injuries to the liver or blunt abdominal trauma. In contrast to iatrogenic injuries, blunt trauma injuries often go undetected until they present as a delayed complication of bile peritonitis [24]. Management of blunt abdominal trauma has evolved over the past 20 years from exploratory laparotomy to conservative management in up to 90% of cases [25], which helps to explain why bile leak often presents as a delayed complication in these patients [23, 26].
Imaging presentation
Imaging studies are critical in the workup of a suspected bile duct injury, as these studies allow determination of the site and extent of injury and to initiate treatment planning. Imaging studies available to evaluate the biliary tree include ultrasonography (US), computed tomography (CT), hepatobiliary scintigraphy, magnetic resonance imaging/cholangiopancreatography (MRI/MRCP), endoscopic retrograde pancreatography (ERCP), and percutaneous transhepatic cholangiography (PTHC).
Ultrasound
Hepatic US can be helpful for the initial assessment of a suspected bile duct leak. Ultrasound, which is readily available in most institutions, can be used as a screening modality to easily assess the bile ducts and abdominal fluid collections. However, when compared with other cross-sectional imaging modalities such as CT or MRI, US has lower sensitivity, which can lead to a delay in diagnosis [27]. Sonographic findings that may suggest the presence of bile duct injury or leak include perihepatic and intrahepatic fluid and complex ascites.
Computed tomography
In the setting of trauma, (CT) findings of bile duct leak may be subtle and nonspecific. The presence of perihepatic fluid and ascites should raise a high level of suspicion for bile duct injury. If serial imaging demonstrates enlarging perihepatic or abdominal fluid collections, a biloma from a bile leak should be strongly suspected, and additional studies with nuclear scintigraphy or MRI should be performed. Similarly, low-attenuation or complex fluid collections that develop in the perihepatic space or abdomen after cholecystectomy, liver transplant, or hepatic resection should be suspected as a possible bile leak.
Hepatobiliary scintigraphy
Hepatobiliary scintigraphy allows for physiologic evaluation of the biliary tree with the potential to reveal biliary extravasation into the peritoneal or intrahepatic tissues. A characteristic finding of bile leak on scintigraphy is a gradual accumulation of radiotracer within the abdomen that does not correspond to the expected appearance of the gastrointestinal tract [28]. Single-photon emission CT may provide enhanced anatomic resolution of the site of bile leak with fewer false-positive diagnoses when compared to planar scintigraphy alone [29].
MRCP
An alternative to hepatobiliary scintigraphy is MRCP. When performed with a hepatobiliary contrast agent, MRCP is capable of detecting with high certainty the specific site of a bile leak [30]. In particular, gadolinium-based hepatobiliary contrast agents provide the ability to evaluate both the hepatic parenchyma and the bile ducts, as these agents are excreted through hepatic and renal tissues [31]. When compared to T2-weighted MRCP, MRCP with hepatobiliary contrast agents provides superior evaluation of the biliary tree and thus also provides superior evaluation of the bile leak site [31].
Percutaneous transhepatic cholangiography
PTC is commonly used for the visualization of biliary anatomy for patients who are not candidates for ERCP or as a preliminary step for percutaneous drainage [32,33,34]. Ultrasound or fluoroscopic guidance is used to access the bile ducts with a 20- or 22-g needle. Once the biliary system is accessed, injection of contrast into the bile ducts allows for direct, visualization of bile duct leaks, obstructions, and strictures. However, some research has demonstrated an increased incidence of complications in patients undergoing PTC who lack dilation of the intrahepatic ducts [32].
Management
Initial management
Management of bile leaks requires a multidisciplinary approach involving hepatobiliary surgeons, gastroenterologists, and interventional radiologists. Medical stabilization with fluids and electrolytes is the initial priority. The presence of cholangitis and infected bilomas should prompt the use of antibiotics and early drainage for source control. Interventional management is dictated by the location and character of the leak, the surgical candidacy of the patient, and the interval since biliary injury (early or delayed).
Iatrogenic common bile duct leaks after cholecystectomy and hepatobiliary surgery are discovered immediately in up to 33% and 80% of cases, respectively [35, 36], either by direct observation or by the presence of postoperative bilious output from surgical drains. Diagnosis within 72 h should prompt consideration of early surgical repair for more rapid resolution, despite a reoperation mortality rate that is higher than the mortality rate of the initial operation [37]. Successful reoperation relies on rapid diagnosis, surgical candidacy, and appropriate surgical expertise (e.g., a hepatobiliary surgeon rather than the surgeon who caused the injury, to reduce subsequent morbidity, mortality, and cost [38, 39].
For early surgical repair planning [40, 41], CT can provide cursory identification of bilomas and duct obstruction, whereas MRCP can more accurately characterize the leak [42]. ERCP and percutaneous transhepatic cholangiography (PTHC) are more invasive options that can be used for preoperative drainage and leak characterization. The surgeon may prefer to use preoperative drainage for a number of reasons. A PTBD exiting a transected duct and terminating in a biloma guides intraoperative visualization of obscure leaks and serves as a scaffold for the creation of small or complex anastomoses (Fig. 2) [39]. The surgeon can modify the catheter length and side-hole position intraoperatively and leave the drain in place postoperatively. Some surgeons prefer to use preoperative, external-only PTBD for intraoperative injection to locate small, cut-edge leaks that can be oversewn.
Fluoroscopic images from a 67-year-old man with bile leak after cadaveric left lateral segment liver transplant. a A percutaneous transhepatic biliary drain was placed from the segment 3 duct across an intact hepaticojejunostomy (white arrowhead). However, injection of a biloma drain showed spillage into the abdomen from a missed segment 2 duct (white arrow). b A percutaneous transhepatic cholangiogram from segment 2 confirmed the bile leak (white arrowhead). c A percutaneous transhepatic biliary drain terminating in the biloma was placed (black arrowhead) for use as a guide for surgical creation of a separate hepaticojejunostomy for segment 2. d Follow-up cholangiography through existing biliary drains demonstrated intact, separate anastomoses for segments 2 and 3
In some cases, particularly after hepatic resection, discovery of biliary leakage is delayed for weeks. These leaks are more persistent [43], abdominal fibrosis is more advanced, and immediate surgical drainage is typically not considered. Long-term interventional management of these leaks involves drainage of the biliary system and bilomas which, in the absence of infection, can typically be performed any time within 3 days of diagnosis without adverse effects on the outcome [6]. This strategy serves to temporize the clinical status, attempt definitive treatment via minimally invasive means, and minimize further abdominal fibrosis in the event that interval hepaticojejunostomy becomes necessary.
Percutaneous transhepatic cholangiography
Percutaneous transhepatic cholangiography accurately characterizes the extent and location of bile leaks in 85% of cases [44] and provides rapid access when drainage is necessary. PTBD allows the option of using external drainage in cases involving uncrossable transections and obstructions (Fig. 3). Success rates can be as high as 92% with this technique, even in nondilated and pediatric cases [45, 46].
65-year-old man with a bile leak after hepatic resection for hepatocellular carcinoma. Two separate biliary-enteric anastomoses were required: a posterior anastomosis for segments 5–7 and an anterior anastomosis for segment 8. a Coronal T2-weighted MR cholangiopancreatogram image shows a bile leak along the hepatic resection margin (white arrows). The segment 8 duct is seen coursing along the cut edge (white arrowhead). b Contrast-enhanced axial T1-weighted MR image shows both the anterior and posterior anastomoses adjacent to the surgical drain (black arrows). c Percutaneous transhepatic cholangiogram from segment 7 shows obstruction (white arrow) and a bile leak (white arrowhead). d Fluoroscopic image from a percutaneous transhepatic cholangiogram from segment 8 shows isolation of this duct and obstruction of its separate anastomosis (white arrow). e Internal–external percutaneous biliary drainage was achieved for segment 8 (black arrow), and external-only percutaneous biliary drainage was performed for segments 5–7 (black arrowhead) because the anastomosis could not be crossed with a wire. f One week later, puncture across the occluded anastomosis was achieved from segment 5, resulting in 2 internal–external percutaneous biliary drainages and eventual resolution of the bile leak
Percutaneous transhepatic cholangiography is accomplished by passing a 21-gauge needle into the hepatic parenchyma, preferably under US guidance [47], and withdrawing the needle during continuous contrast injection under fluoroscopic observation until a bile duct is opacified. To identify the location of the leak, contrast is injected under digital subtraction angiographic guidance (Fig. 4) before opacification of the related biloma obscures fluoroscopic visualization. If free contrast obscures the leak, identification may still be possible via subsequent cholangiography performed using fluoroscopic subtraction techniques such as roadmap imaging. Preliminary MRCP performed before PTHC can help to guide selective drainage, and intraprocedural cone-beam CT or combined fluoroscopy/CT can facilitate access into specific ducts.
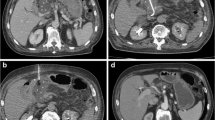
58-year-old woman with large bile leak after laparoscopic cholecystectomy. The patient had sepsis, and the case was deemed unsuitable for reoperation because of the patient’s deteriorating clinical status. a Contrast-enhanced axial CT image shows a large biloma with a surgical drain in place (white arrow). b Percutaneous transhepatic cholangiography image shows a large leak from the common duct at the former location of the cystic duct (black arrow). c A covered stent was placed across the leak with plans for later retrieval
Classification of biliary injury
Bismuth classification
The Bismuth classification of injury to the common bile duct is based on the location of the injury in the biliary tree [36, 48]. This classification describes five distinct types of injuries relative to their distance from the hilum. Type I injuries involve the low common hepatic duct and common bile duct. Type II injuries are injuries to the common hepatic duct less than 2 cm from the confluence. Type III injuries result when there is no residual common hepatic duct left intact. Type IV injuries involve isolation of the left and right hepatic ducts. Type V injuries involve injury or separation of an aberrant right hepatic duct in isolation or in combination with injury to the common hepatic duct. A limitation of the Bismuth classification is that it relates injuries to the common bile duct only and excludes classification of bile leaks with traumatic or post-liver transplant etiologies.
Strasberg classification
A modification of the Bismuth classification, the Strasberg classification of bile duct injuries differentiates between small leaks (i.e., leaks from the cystic duct or aberrant sectoral branches) and major leaks of larger bile ducts [31, 49]. In this system, five types of injury are described, classified as Types A through E. Types A, C, and D involve biliary leaks. A Type A leak is a bile leak from the cystic duct remnant or from the duct of Luschka in the gallbladder fossa. A Type C leak originates from a divided posterior sector duct, and a Type D leak originates from injury of the common bile duct at the hepatic hilum.
McMahon classification
The McMahon classification uses a scheme similar to the Bismuth and Strasberg classification systems and describes bile injury as either minor or major depending on the extent of laceration to the common bile duct after laparoscopic cholecystectomy [50]. Minor injuries are lacerations that involve less than 25% of the common bile duct. Transections or lacerations that involve more than 25% of the common bile duct are categorized as major injuries.
Hanover classification
The Hanover system describes five types of bile duct injury (A-E) with several subtypes within each category, for a total of 21 injury patterns that may be seen after laparoscopic cholecystectomy [11, 51]. Of these, Types A1 and A2 are similar to the Type A leaks described in the Strasberg classification in that both types describe leaks from the cystic duct remnant or duct of Luschka.
Percutaneous transhepatic biliary drainage (PTBD)
Percutaneous approach to the biliary system is the preferred method for leak characterization, biliary decompression, and biliary diversion in all cases. This is especially relevant for cases involving altered anatomy, total transections, or total obstructions, or failed transpapillary access. PTBD is typically performed in the setting of leaking hepaticojejunostomy (Fig. 5).
67-year-old man with bile leak after related living-donor liver transplant. a Contrast-enhanced axial image shows a biloma near the hepaticojejunostomy (white arrow). Patient has a percutaneous gastrostomy (black asterisk). b Image from a segment 3 percutaneous transhepatic cholangiogram shows partial obstruction (black arrow) of the anastomosis and a bile leak (black arrowhead). c One month after percutaneous transhepatic biliary drainage and biloma drain placement, cholangiography demonstrated resolution of bile leak. The catheters were removed
Percutaneous transhepatic biliary drainage is accomplished via initial biliary access with a 0.018-inch wire, transitional dilation to allow passage of a 0.035-inch wire, and serial dilatation to place a biliary drain (typically 8 to 12 French). When transections are uncrossable, external PTBD may be necessary. Leaks associated with total obstruction may be crossable using percutaneous techniques. Alternatively, a wire placed within a biloma from one side of the transection can be snared from the other side using rendezvous techniques that combine ERCP and PTHC, and a standard internal EBD or internal–external PTBD can be placed. Similarly, a rendezvous technique within a biloma can facilitate placement of a “U tube,” a single tube that enters the biloma and exits the skin via the bile ducts to facilitate common and secure drainage. In addition, rendezvous techniques facilitate access across an obstructed, leaking anastomosis. Finally, bilateral PTBDs may be indicated to decompress bilateral or hilar leaks and to assist the surgeon with the typically challenging repair and reconstruction associated with hilar leaks (Fig. 6).
64-year-old woman with bile leak after laparoscopic cholecystectomy. An endoscopic retrograde pancreatography was performed, but the leak could not be crossed with a wire. a Percutaneous transhepatic cholangiography image shows massive hilar leakage into the peritoneal cavity (white arrow), consistent with high transection. b A percutaneous biliary drain was placed from the left ducts (white arrow) terminating in the biloma to guide surgical reconstruction. In some cases, transection occurs above the hilar confluence, and the placement of bilateral percutaneous transhepatic biliary drains can assist surgeons in the creation of a complex surgical anastomosis
Reports of placing covered self-expanding metal stents (CSEMS) across large benign biliary injuries are limited [52], but for poor surgical candidates with leakage refractory to other methods of repair, CSEMS may be an option. Resistant to gastrointestinal, pancreatic, and biliary secretions, these stents are composed of a metallic skeleton that is covered by a biocompatible synthetic material [53]. Deliverable via percutaneous approaches, CSEMS offer more effective tamponade of leakage when compared with plastic catheters and stents. However, CSEMS have migration rates as high as 37% [54] and retrieval rates as low as 75% [55]. Surgical removal and reconstruction may become necessary in some cases after resolution of leakage. To avoid the need for stent retrieval, biodegradable biliary stents have been deployed across bile leaks with success rates equivalent to those of EBD with plastic stents [56].
Biloma drainage
Imaging-guided percutaneous catheter drainage of all bilomas is necessary if immediate surgical repair is not an option. Undrained bilomas can lead to substantial morbidity, including pain, abscess, peritonitis, sepsis, and abdominal fibrosis, all of which can interfere with future surgical reconstruction. Biloma drainage is accomplished via serial dilatation under US, fluoroscopic, or CT guidance followed by placement of as many percutaneous catheter drains as necessary. Percutaneous catheter drainage of extrahepatic bilomas through the liver parenchyma is safe for gallbladder fossa collections resulting from leaking ducts of Luschka [57].
Follow-up and outcomes
Success rates for biliary drainage of traumatic leaks, small leaks, cystic duct leaks, and duct of Luschka leaks exceed 90% without the need for subsequent surgery [58,59,60], and protracted drainage periods of 2 to 3 months are common. Larger complex leaks are more likely to require surgical reconstruction. Follow-up appointments every 4 to 6 weeks in hepatology, interventional radiology, and surgery clinics can direct the need for biliary reconstruction in the event of persistent leakage or obstruction. At each visit, patients should undergo clinical assessment for complications, routine catheter exchange, and catheter removal when there is cessation of output from the biloma drains and no demonstrable leakage or obstruction. Signs of infection should prompt consideration of catheter manipulation, CT to rule out abscess formation, and antibiotics to treat cholangitis. Complications of long-term external drainage include decreased serum bicarbonate levels caused by excessive loss of bicarbonate-rich bile, prerenal insufficiency, hyponatremia, and malnutrition. Complications of catheter and duct obstruction include peri catheter leakage, pruritus, and cholangitis.
Advanced management techniques
When bile leaks are complicated by anatomic idiosyncrasies, fistula formation, previous surgical disruption of the biliary tree, or liver function abnormalities, other management techniques may be more suitable, such as the use of proximal occlusion balloons, absolute alcohol injection, N-butyl-cyanoacrylate adhesion, ethylene–vinyl alcohol copolymer occlusion, and direct portal vein occlusion. It should be noted that data regarding the use of these techniques is limited and that their used is rare and performed in select cases.
Use of a proximal occlusion balloon
Proximal occlusion balloons are commonly used by interventional radiologists to reversibly occlude flow in the setting of selective angiography, vascular pre-operative vascular occlusion, and acute hemorrhage. For complicated cases of biliary leakage in which the biliary anatomy is not amenable to other approaches such as a Billroth II gastrectomy or Roux-en-Y bilio-enteric reconstruction and for cases of entero-biliary fistula percutaneous drainage remains the preferred option. With the use of a proximal balloon occlusion technique, two guide wires are placed percutaneous into the affected bile duct. A balloon-occlusion catheter is advanced over one of the guide wires and positions just proximal to the site of bile leakage. A biliary drainage catheter is then advanced over the second guide wire and positioned proximal to the occlusion balloon within the bile duct. This arrangement facilitates drainage of bile away from the site of leakage, thus facilitating healing [61].
Absolute alcohol is a widely available, efficacious, inexpensive drug that induces permanent necrosis of the secretory epithelium by causing membrane lysis and protein denaturation [62]. Treatment with ethanol leads to atrophy of the treated segment or lobe and hypertrophy of the untreated segments or lobe. In the biliary system, necrotizing obliteration of the biliary ducts leads to decreased bile production from the involved hepatic segment and thus decreases leakage from the biliary fistula. However, for the same reason, ethanol is typically not used when there is an internal communication with the biliary system [63]. Recent case reports described biliary fistulae in two patients that were successfully treated using percutaneous cannulation of the leaking bile ducts, occlusion balloons, and absolute alcohol [66]. Absolute alcohol injection has few drawbacks; however, this treatment does usually require multiple injections and can therefore be time consuming [64]. In addition, systemic absorption can lead to pain, hypotension, fever, flushing, and leukocytosis [65].
N-butyl-cyanoacrylate (NBCA) is a tissue adhesive that causes obliteration of the ducts by inducing inflammation. The initial exudative phase is followed by proliferation of the granulation tissue, leading to scar formation at the site of NBCA instillation [66]. Vu et al. [67] suggested that the most effective way to manage a communicating biliary cutaneous fistula involves placing a coil at the neck of the fistula and then instilling NBCA using an external approach; the goal of this treatment is to obliterate the bile ducts, leading to complete cessation of bile production by these ducts. In their study assessing this technique, the researchers glued the biliary duct to the level of the skin surface [67]. In this scenario, the best duct to access for NBCA obliteration is one that will allow the glue to enter the duct without the risk of glue spilling into the peritoneal cavity as the tract is glued to the skin surface.
Ethylene–vinyl alcohol copolymer (Onyx®; Medtronic, Minneapolis, MN, USA) that is dissolved in dimethyl sulfoxide, is non-adhesive and radio-opaque. Injection of this agent into the bile ducts results in precipitation of the chemical agents into a solid, spongy embolic material which acts a cast within the bile ducts, thus limiting leakage. In one case report, this agent was used to control refractory bile leak after left hepatic trisegmentectomy, with less viscous Onyx 18 (6% ethylene vinyl alcohol) leading to better biliary cast formation than Onyx® 34 (8% ethylene vinyl alcohol) [68]. Onyx 34 casts can fracture, and fractured casts can migrate to central bile ducts. To avoid this complication, coils or plugs can be deployed in the nonisolated ducts [69, 70]. Biliary occlusion by Onyx may be less hepatotoxic than occlusion by NBCA, as Onyx causes a less abrupt decrease in biliary output and allows more time for compensatory hypertrophy of nonembolized liver [68]. In addition, NBCA carries a risk of adhering the catheter into the bile duct, as well as the risk of possible acute mortality after obliteration of the bile ducts due to insufficient functional residual liver volume.
The enterohepatic circulation maintains the return of bile salts to the liver. Embolization of a branch of the portal vein causes interruption of the enterohepatic circulation in that particular segment of the liver, leading to atrophy and fibrosis of the hepatocytes and cessation of bile production by the embolized segment. There is compensatory hypertrophy of the nonembolized segments due to diversion of the portal vein flow from the embolized segment to the nonembolized segment [64]. In one study, the technique of portal vein embolization via the ileal vein after small laparotomy was used [71, 75], but other research has demonstrated that the portal vein branches can be easily approached via the percutaneous transhepatic approach [68].
Conclusion
Biliary duct leaks may be precipitated and affected by a variety of factors, and current management strategies reflect this heterogeneity by providing an array of methods to visualize and remediate nearly all leaks with a high degree of safety and efficacy. A thorough understanding of all methodologies will lead to enhanced patient outcomes and reduced cost through improved efficiency. In most cases, through a variety of techniques, interventional radiology plays an increasingly important role in managing refractory bile leaks.
References
Fong ZV, Pitt HA, Strasberg SM, et al. Diminished survival in patients with bile leak and ductal injury: management strategy and outcomes. J Am Coll Surg 2018;226(4):568–576
Vadvala H V, Arellano RS. Imaging and Intervention of Biliary Leaks and Bilomas. Dig Dis Interv 2017;1(1):14–21
Bouras G, Burns EM, Howell A-M, et al. Systematic review of the impact of surgical harm on quality of life after general and gastrointestinal surgery. Ann Surg 2014;260(6):975–83
Zimmerman MA, Baker T, Goodrich NP, et al. Development, management, and resolution of biliary complications after living and deceased donor liver transplantation: a report from the adult-to-adult living donor liver transplantation cohort study consortium. Liver Transplant 2013;19(3):259–67
Gupta P, Maralakunte M, Rathee S, et al. Percutaneous transhepatic biliary drainage in patients at higher risk for adverse events: experience from a tertiary care referral center. Abdom Radiol (NY): 2020;45(8):2457–2553.
Adler DG, Papachristou GI, Taylor LJ, et al. Clinical outcomes in patients with bile leaks treated via ERCP with regard to the timing of ERCP: a large multicenter study. Gastrointest Endosc 2017;85(4):766–72.
Arend J, Schütte K, Weigt J, et al. Gallenleckage nach Leberresektion. Der Chir 2015; 86:(2)132–8
Bala M, Gazalla SA, Faroja M, et al. Complications of high grade liver injuries: management and outcomewith focus on bile leaks. Scand J Trauma Resusc Emerg Med 2012;20:20
Kirimlioglu V, Tatli F, Ince V, et al. Biliary complications in 106 consecutive duct-to-duct biliary reconstruction in right-lobe living donor liver transplantation performed in 1 year in a single center: a new surgical technique. Transplant Proc 2011;43(3):917–20
Dell AJ, Krige JEJ, Jonas E, et al. Incidence and management of postoperative bile leaks: A prospective cohort analysis of 467 liver resections. S Afr J Surg 2016;54(3):18–22
Panaro F, Hacina L, Bouyabrine H, Al-Hashmi A-W, Herrero A, Navarro F. Risk factors for postoperative bile leakage: a retrospective single-center analysis of 411 hepatectomies. Hepatobiliary Pancreat Dis Int 2016;15(1):81–6
Pucher PH, Brunt LM, Davies N, et al. Outcome trends and safety measures after 30 years of laparoscopic cholecystectomy: a systematic review and pooled data analysis. Surg Endosc 2018;32(5):2175–2183
Stinton LM, Shaffer EA. Epidemiology of gallbladder disease: cholelithiasis and cancer. Gut Liver 2012;6(2):172–87
Pesce A, Portale TR, Minutolo V, Scilletta R, Destri GL, Puleo S. Bile duct injury during laparoscopic cholecystectomy without intraoperative cholangiography: a retrospective study on 1,100 selected patients. Dig Surg 2012;29(4):310–4
Navez B, Ungureanu F, Michiels M, et al. Surgical management of acute cholecystitis: results of a 2-year prospective multicenter survey in Belgium. Surg Endosc 2012;26(9):2436–45
Törnqvist B, Strömberg C, Akre O, Enochsson L, Nilsson M. Selective intraoperative cholangiography and risk of bile duct injury during cholecystectomy. Br J Surg 2015;102(8):952–958
Club SS. A prospective analysis of 1518 laparoscopic cholecystectomies. N Engl J Med 1991; 324:1075–8
Nigro C Lo, Geraci G, Sciuto A, Volsi FL, Sciumè C, Modica G. Bile leaks after videolaparoscopic cholecystectomy: duct of Luschka. Ann Ital Chir 2012;83(4):303–12
Testa G, Broelsch CE. Biliary anastomosis in living related liver transplantation using the right liver lobe: techniques and complications. Liver Transplant 2000;6(6):710–4
Gastaca M, Matarranz A, Muñoz F, et al. Biliary complications in orthotopic liver transplantation using choledochocholedochostomy with a t-tube. Transplant Proc 2012;44(6):1554–6
Gunawansa N, McCall JL, Holden A, Plank L, Munn SR. Biliary complications following orthotopic liver transplantation: a 10-year audit. HPB (Oxford) 2011;13(6):391–9
Freise CE, Gillespie BW, Koffron AJ, et al. Recipient morbidity after living and deceased donor liver transplantation: findings from the A2ALL Retrospective Cohort Study. Am J Transplant 2008;8(12):2569–79
Anand RJ, Ferrada PA, Darwin PE, Bochicchio G V, Scalea TM. Endoscopic retrograde cholangiopancreatography is an effective treatment for bile leak after severe liver trauma. J Trauma 2011; 71(2):480–5
Yuan K-C, Wong Y-C, Fu C-Y, Chang C-J, Kang S-C, Hsu Y-P. Screening and management of major bile leak after blunt liver trauma: a retrospective single center study. Scand J Trauma Resusc Emerg Med 2014;22:26
Bouras A-F, Truant S, Pruvot F-R. Management of blunt hepatic trauma. J Visc Surg 2010; 147(6):e351–8
Hommes M, Nicol AJ, Navsaria PH, Folmer ER, Edu S, Krige JEJ. Management of biliary complications in 412 patients with liver injuries. J Trauma Acute Care Surg 2014;77(3):448–51
Chiu WC, Wong-You-Cheong JJ, Rodriguez A, Shanmuganathan K, Mirvis SE, Scalea TM. Ultrasonography for interval assessment in the nonoperative management of hepatic trauma. Am Surg 2005;71(10):841–6
Gupta V. Bile leak detection by radionuclide scintigraphy. Kathmandu Univ Med J (KUMJ) 2006;4(1):82–5
Arun S, Santhosh S, Sood A, Bhattacharya A, Mittal BR. Added value of SPECT/CT over planar Tc-99m mebrofenin hepatobiliary scintigraphy in the evaluation of bile leaks. Nucl Med Commun 2013; 34(5):459–66
Khalid TR, Casillas VJ, Montalvo BM, Centeno R, Levi JU. Using MR Cholangiopancreatography to Evaluate Iatrogenic Bile Duct Injury. AJR Am J Roentgenol 2001; 177(6):1347–52
Kantarci M, Pirimoglu B, Karabulut N, et al. Non-invasive detection of biliary leaks using Gd-EOB-DTPA-enhanced MR cholangiography: Comparison with T2-weighted MR cholangiography. Eur Radiol 2013;23(10):2713–22
Teplick SK, Flick P, Brandon JC. Transhepatic cholangiography in patients with suspected biliary disease and nondilated intrahepatic bile ducts. Gastrointest Radiol 1991;16(3):193–7
Popat B, Thakkar D, Deshmukh H, Rathod K. Percutaneous Transhepatic Biliary Drainage in the Management of Post-surgical Biliary Leaks. Indian J Surg 2017;79(1):24–28
Cozzi G, Severini A, Civelli E, et al. Percutaneous transhepatic biliary drainage in the management of postsurgical biliary leaks in patients with nondilated intrahepatic bile ducts. Cardiovasc Intervent Radiol 2006;29(3):380–8
Maddah G, Mashhadi MTR, Mashhadi MP, Nooghabi MJ, Hassanpour M, Abdollahi A. Iatrogenic injuries of the extrahepatic biliary system. J Surg Res 2017;213:215–221
Törnqvist B, Strömberg C, Persson G, Nilsson M. Effect of intended intraoperative cholangiography and early detection of bile duct injury on survival after cholecystectomy: population based cohort study. BMJ 2012;345:e6457Chun K. Recent classifications of the common bile duct injury. Korean J Hepatobiliary Pancreat Surg 2014;18(3):69–72
Lessing Y, Pencovich N, Nevo N, et al. Early reoperation following pancreaticoduodenectomy: impact on morbidity, mortality, and long-term survival. World J Surg Oncol 2019;17(1):26
Fischer CP, Fahy BN, Aloia TA, Bass BL, Gaber AO, Ghobrial RM. Timing of referral impacts surgical outcomes in patients undergoing repair of bile duct injuries. HPB (Oxford) 2009;11(1):32–7
Dageforde LA, Landman MP, Feurer ID, Poulose B, Pinson CW, Moore DE. A cost-effectiveness analysis of early vs late reconstruction of iatrogenic bile duct injuries. J Am Coll Surg 2012;214(6):919–27
Lau WY, Lai ECH, Lau SHY. Management of bile duct injury after laparoscopic cholecystectomy: a review. ANZ J Surg 2010;80(1–2):75–81
Stewart L, Way LW. Bile duct injuries during laparoscopic cholecystectomy: factors that influence the results of treatment. Arch Surg 1995;130(10):1123–8
Petrillo M, Ierardi AM, Tofanelli L, et al. Gd-EOB-DTP-enhanced MRC in the preoperative percutaneous management of intra and extrahepatic biliary leakages: does it matter? Gland Surg 2019;8(4):174–183
Kaibori M, Shimizu J, Hayashi M, et al. Late-onset bile leakage after hepatic resection. Surgery 2015;157(1):37–44
Fidelman N, Kerlan Jr RK, LaBerge JM, Gordon RL. Accuracy of percutaneous transhepatic cholangiography in predicting the location and nature of major bile duct injuries. J Vasc Interv Radiol 2011;(6)22:884–92
Funaki B, Zaleski GX, Straus CA, et al. Percutaneous biliary drainage in patients with nondilated intrahepatic bile ducts. AJR Am J Roentgenol 1999;173(6):1541–4
Lorenz JM, Funaki B, Leef JA, Rosenblum JD, Van Ha T. Percutaneous transhepatic cholangiography and biliary drainage in pediatric liver transplant patients. AJR Am J Roentgenol 2001;176(3):761–5
Wagner A, Mayr C, Kiesslich T, Berr F, Friesenbichler P, Wolkersdörfer GW. Reduced complication rates of percutaneous transhepatic biliary drainage with ultrasound guidance. J Clin Ultrasound 2017;45(7):400–407
Chun K. Recent classifications of the common bile duct injury. Korean J Hepatobiliary Pancreat Surg 2014;18(3):69–72
Strasberg SM, Hertl M, Soper NJ. An analysis of the problem of biliary injury during laparoscopic cholecystectomy. J Am Coll Surg 1995; 180(1):101–25
McMahon AJ, Fullarton G, Baxter JN, O’dwyer PJ. Bile duct injury and bile leakage in laparoscopic cholecystectomy. Br J Surg 1995;82(3):307–13
Bektas H, Schrem H, Winny M, Klempnauer J. Surgical treatment and outcome of iatrogenic bile duct lesions after cholecystectomy and the impact of different clinical classification systems. Br J Surg 2007; 94(9):1119–27
Mangiavillano B, Luigiano C, Tarantino I, et al. Fully covered, self-expandable metal stents for first-step endoscopic treatment of biliary leaks secondary to hepato-biliary surgery: a retrospective study. Dig Liver Dis 2013;45(5):430–2
Shao H, Arellano R. Percutaneous Management of Benign and Postoperative Biliary Strictures. Dig Dis Interv 2017;1(1):28–35
Ali SE, Frandah WM, Su L, Fielding C, Mardini H. Should a fully covered self-expandable biliary metal stent be anchored with a double-pigtail plastic stent? A retrospective study. World J Gastrointest Endosc 2019;11(5):365–372
Devière J, Reddy DN, Püspök A, et al. Successful management of benign biliary strictures with fully covered self-expanding metal stents. Gastroenterology 2014;147(2):385–95
Siiki A, Vaalavuo Y, Antila A, et al. Biodegradable biliary stents preferable to plastic stent therapy in post-cholecystectomy bile leak and avoid second endoscopy. Scand J Gastroenterol 2018;53(10–11):1376–1380
Ciftci TT, Akinci D, Akhan O. Percutaneous transhepatic drainage of inaccessible postoperative abdominal abscesses. AJR Am J Roentgenol 2012;198(2):477–81
Kulaylat AN, Stokes AL, Engbrecht BW, McIntyre JS, Rzucidlo SE, Cilley RE. Traumatic bile leaks from blunt liver injury in children: a multidisciplinary and minimally invasive approach to management. J Pediatr Surg 2014;49(3):424–7
Tewani SK, Turner BG, Chuttani R, Pleskow DK, Sawhney MS. Location of bile leak predicts the success of ERCP performed for postoperative bile leaks. Gastrointest Endosc 2013;77(4):601–8
Rainio M, Lindström O, Udd M, Haapamäki C, Nordin A, Kylänpää L. Endoscopic therapy of biliary injury after cholecystectomy. Dig Dis Sci 2018;63(2):474–480
Pedicini V, Poretti D, Mauri G, et al. Management of post-surgical biliary leakage with percutaneous transhepatic biliary drainage (PTBD) and occlusion balloon (OB) in patients without dilatation of the biliary tree: preliminary results. Eur Radiol 2010;20(5):1061–8
Sasaki M, Hori T, Furuyama H, et al. Postoperative biliary leak treated with chemical bile duct ablation using absolute ethanol: a report of two cases. Am J Case Rep 2017;18:871–877
Kusano T, Kida H, Nishiwaki Y, et al. Percutaneous sclerotherapy for intractable xternal biliary fistula after hepatectomy. Int Surg 2003;88(2):72–5
Sadakari Y, Miyoshi A, Ohtsuka T, Kohya N, Takahashi T, Matsumoto K, et al. Percutaneous transhepatic portal embolization for persistent bile leakage after hepatic resection: report of a case. Surg Today 2008;38(7):668–71
Kyokane T, Nagino M, Sano T, Nimura Y. Ethanol ablation for segmental bile duct leakage after hepatobiliary resection. Surgery 2002;131(1):111–3
Matsumoto T, Nemhauser GM, Soloway HB, Heisterkamp C, Aaby G. Cyanoacrylate tissue adhesives: an experimental and clinical evaluation. Mil Med 1969;134(4):247–52
Vu DN, Strub WM, Nguyen PM. Biliary duct ablation with N-butyl cyanoacrylate. J Vasc Interv Radiol 2006;17(1):63–9
Wible BC, Gooden C, Saucier N, Borsa JJ, Cummings LS, Cho KH. Ethylene-vinyl alcohol copolymer endobiliary obliteration of hepatic segments in a patient with isolated bile leaks. J Vasc Interv Radiol 2014;25(11):1821–5
Uller W, Müller-Wille R, Loss M, et al. Percutaneous management of postoperative bile leaks with an ethylene vinyl alcohol copolymer (Onyx). Rofo 2013;185(12):1182–7
Wilson KA, Haskal ZJ. Durable plug and Onyx occlusion of a refractory bile leak. J Vasc Interv Radiol 2013;7(7):1067–9
Yamakado K, Nakatsuka A, Iwata M, et al. Refractory biliary leak from intrahepatic biliary–enteric anastomosis treated by selective portal vein embolization. J Vasc Interv Radiol 2002;13(12):1279–81
Funding
No internal or external funding was used in the preparation of this manuscript.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
All authors report no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Arellano, R.S., Reid, N.J., Kapoor, B. et al. The role of interventional radiology in the management of refractory bile leaks. Abdom Radiol 47, 1881–1890 (2022). https://doi.org/10.1007/s00261-021-03016-9
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00261-021-03016-9