Abstract
Objective
The purpose of this article is to describe clinical and imaging characteristics of confirmed cases of cryptogenic multifocal ulcerous stenosing enteritis (CMUSE).
Methods
Retrospective review of electronic medical records identified patients considered for a diagnosis CMUSE over 20-years in a single large tertiary center. Clinical data were abstracted and diagnosis was confirmed based on published criteria. Two GI radiologists reviewed CT and MR enterography (CTE/MRE) exams in consensus of confirmed patients to characterize the imaging features of CMUSE.
Results
Eight patients with confirmed CMUSE diagnosis were included for image review, and 9 CTEs and 1 MRE were analyzed. Most patients were males (75%) with a median age at diagnosis of 59.5 years (25–71) presenting with iron deficiency anemia (75%). Patients were commonly refractory (87.5%) to their first therapy, including steroids, with half being refractory to surgical intervention. Major imaging features included multiple (≥ 5; 88%; 7/8), short (< 2 cm; 100%; 8/8), circumferential (100%; 8/8) strictures with moderate wall thickening (6–9 cm), and stratified hyper enhancement (100%; 8/8) located in the ileum (100%; 8/8). Median proximal small bowel dilation was 2.95 cm (2.5–4.1 cm). No CMUSE cases demonstrated penetrating disease (e.g., abscess, fistula).
Conclusion
CT and MR enterography are invaluable tools in the multidisciplinary diagnostic evaluation of CMUSE, a rare cause of small bowel strictures with overlapping clinical and imaging features of Crohn’s disease and NSAID enteropathy.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Cryptogenic multifocal ulcerous stenosing enteritis (CMUSE) is a rare illness characterized by chronic or relapsing obstructive symptoms resulting from multiple fibrous strictures and shallow ulcers of the small bowel [1]. It was first described in the early 1960s, with about 60 cases reported in the literature [1,2,3,4,5,6,7,8,9,10]. The most notable pathophysiologic characteristic of this condition is the excessive production of collagen [1, 11]. An overlap between CMUSE and intestinal vasculitis has been proposed; however, the underlying etiology remains unknown [1, 12, 13].
Diagnostic criteria have been proposed based on the presence of occult blood loss from the gastrointestinal (GI) tract in the setting of characteristic endoscopic small bowel lesions, which include: multiple stricturing ulcers that are < 4 cm apart; sharply demarcated borders; circumferential or oblique alignment; geographic or linear shape; and no involvement of the proper muscular layer [14]. Microscopically, these lesions are characterized by infiltration of plasma cells, lymphocytes and eosinophils [14]. However, these features are not specific, and the diagnosis also requires excluding other more common causes of small bowel ulcerations and stenosis, including non-steroidal anti-inflammatory drugs (NSAID) enteropathy and Crohn’s Disease (CD), which requires integration of clinical, endoscopy, radiology, and pathology data.
A limited number of case series demonstrate variability of clinical presentation, radiology features, and treatment outcomes of confirmed cases [12, 13, 15, 16]. Furthermore, the role of surgery and biologic therapies is also not well defined [17]. As such, we aim to retrospectively describe our experience in the diagnosis and management of CMUSE over the past two decades.
Methods
Patient selection
This is an IRB approved retrospective, descriptive study of patients who had a diagnosis of CMUSE considered from January of 1999 to January 2019 at a tertiary medical center. Electronic medical records were queried for the presence of one of the following terms at least once in a patients chart(obtained based on literature review): CMUSE; cryptogenic multifocal ulcerating stenosing enteritis; cryptogenic multifocal ulcerous stenosing enteritis; cryptogenic plurifocal ulcerative stenosing enteritis; cryptogenic multifocal stenosing ulceration of the small intestine; multifocal idiopathic stenosing enteritis; and chronic nonspecific multiple ulcers of the small intestine.
CMUSE diagnosis was determined independently by an experienced gastroenterologist with over 30 years of practice and charts were reviewed to confirm cases that met described diagnostic criteria [13, 14]. Reference standard criteria are as follows: (1) persistent and occult blood loss from the GI tract except during bowel rest or postoperative period; (2) confirmation of characteristic small intestinal lesions by macroscopy, radiography, or enteroscopy; and (3) exclusion of more common small bowel conditions which could have clinical/radiologic/enteroscopic overlap (e.g., NSAID enteropathy, Crohn’s disease, celiac disease,, and small bowel malignancies). Characteristic features of lesions at enteroscopy/macroscopy include the following: (i) circular or oblique in alignment; (ii) sharply demarcated from surrounding normal mucosa; (iii) geographic or linear in shape; (iv) multiplicity in numbers with < 4 cm distance from each other; (v) ulcers not reaching proper muscular layer; and (vi) scarred ulcers presumed to be the healing stage of those characterized by i–v in cases treated by bowel rest [14]. Clinically, NSAID enteropathy was usually excluded if lesions and clinical symptoms persisted after discontinuation of suspect medications for at least 3 months. Celiac disease and complications were excluded based on adherence to gluten-free diet, serological markers, and biopsy results. Crohn’s disease was often excluded in the absence of a constellation of typical features including small intestinal transmural inflammatory process or ulceration, granulomas on biopsy, penetrating (fistula) complications, and extra-intestinal manifestations. Gastrointestinal malignancies were excluded following imaging and pathology studies.
Patients not meeting the reference standard criteria for the diagnosis of CMUSE, after clinical review, in addition to those without one-year follow-up and/or missing data were excluded from the study. Data were abstracted for demographics, clinical (e.g., presenting symptoms, medication record), endoscopic (e.g., report impressions and therapeutic interventions), radiographic (described below), laboratory (e.g., hemoglobin, c-reactive protein, auto-antibodies), and treatment features (e.g., type, duration, and response to medications/surgeries). JMP software (Version 14.1, SAS Institute Inc., Cary NC, 1989–2019) was used for data management and analysis.
Imaging protocol
CT enterography (CTE) and MR enterography (MRE) examinations were performed in accordance with Society of Abdominal Radiology guidelines and joint society practice parameters [18, 19]. Our institutions CTE and MRE protocols are summarized in Supplementary Table 1. Before the enterography examinations, patients drank approximately 1500 mL of a mannitol-based flavored-beverage (Breeza; Beekly Medical, Bristol, CT, USA) or 1350 mL of low-contrast barium solution (Volumen; Bracco Diagnostics, Princeton, NJ, USA), followed by 500 mL of water over 60 min before the scan. For CTE, iodinated contrast was given by intravenous injection using a weight-based protocol at 4 mL/s, followed by scanning during the enteric phase (50–60 s). CT images were reconstructed in all three planes with high spatial resolution (≤ 3 mm). MRE was performed with 1.5 T magnet using an 8-channel phased array coil with the patient prone. Glucagon (0.5 mg) was administered intravenously at the beginning of the scan to alleviate motion artifact from bowel peristalsis. Gadobutrol was given by intravenous injection using a weight-based protocol (0.1 mmol/kg IV) at 1.5 mL/s for the contrast-enhanced acquisitions.
Image analysis
Two fellowship-trained GI radiologists (31 and 18 years of experience) retrospectively analyzed prior CT and MR enterography (CTE/MRE) studies to characterize the radiologic findings of the confirmed CMUSE patients. All available CTE/MRE examinations were sequentially reviewed, and the morphologic parameters were recorded based on consensus. When there were multiple exams showing disease, we chose the exam that showed the most severe disease to be representative. This included extent of small bowel involvement number of strictures and severity of inflammation. If a patient had imaging studies with significant interval morphologic change, then each study was analyzed, and all reported observations were summarized based on reader consensus of the dominant features shared among the reviewed examinations.
Strictures were defined as small bowel segments demonstrating > 50% unequivocal luminal narrowing with or without proximal bowel dilation and were characterized using the following morphologic parameters: presence or absence bowel wall thickening (> 3 mm), maximum wall thickness in any lesion, stricture location (jejunum vs. ileum), the total number of strictures (1, 2–5, 6–10, > 10), stricture length (< 0.5 cm (web), 0.5–2 cm, 2–5 cm, > 5 cm), morphology (e.g., circumferential, asymmetric, or both), degree of contrast enhancement [mild (> nearby, non-contracted normal small bowel segments and less than renal cortex), moderate (similar to renal cortex), severe (greater than renal cortex and similar to intravascular enhancement in nearby vascular structures))], pattern of contrast enhancement, and diffusion-weighted imaging (DWI) signal (increased or isointense to adjacent normal small bowel). Additional features catalogued included the percentage of small bowel involvement (distance between the most proximal to most distal stricture relative to the total length of small bowel), maximum upstream luminal diameter of small bowel related to any stricture (mild 3–4 cm, moderate to severe > 4 cm), and any change in small bowel fold pattern (normal, decreased, or thickened) [20, 21].
Extra-enteric parameters included the following: increased mesenteric vascularity defined as engorgement of the vasa recta relative to the vascularity of normal adjacent small bowel and/or a dilated superior mesenteric vein greater or equal to the aorta at the same level, mesenteric edema or ascites, number and size of the mesenteric lymph nodes, penetrating complications (e.g., fistula, abscess), and the presence or absence of colon abnormality.
Results
CMUSE: clinical characteristics
Our initial search of the electronic medical record using the previously described search criteria identified 33 patients who had CMUSE considered as a diagnosis during initial clinical evaluation. A different diagnosis was confirmed in 21 patients and 4 patients did not meet CMUSE diagnostic criteria and were excluded. Crohn’s disease (n = 7), drug-induced enteropathy (n = 7), celiac disease (n = 2), scleroderma (n = 1), cytomegalovirus (n = 1), angioedema, ischemia (n = 1), and nonfunctioning mutation in prostaglandin transporter gene SLCO2A1(n = 1) were the other confirmed diagnoses excluded from the cohort. Confirmed diagnosis of CMUSE was established 8 patients who were included in the final cohort.
Table 1 summarizes demographic, clinical, and treatment characteristics of the 8 clinically confirmed CMUSE cases. Median duration of symptoms prior to diagnosis was 63.5 months (interquartile range [IQR] 21.5–106 months). Most patients presented with iron deficiency anemia (75%) at initial evaluation, with a median hemoglobin of 11.4 g/dL (IQR 9.5–12.7). It has been previously described that CMUSE could be associated with other systemic vasculitis [13]. However, in our cohort, autoimmune and vasculitis antibodies were not consistently obtained in all patients with three patients displaying positive antibodies at presentation: two anti-nuclear antibodies (ANA) and one anti-Saccharomyces cerevisiae antibodies (ASCA). All strictures were initially identified by CT/MR enterography with subsequent enteroscopy confirmation. All cases had multiple ulcers at enteroscopy. Endoscopically, these ulcers were well demarcated in 87.5% and circumferential in 75% of patients. Lymphocyte-predominant infiltration was reported on three out of four biopsies; in some cases, pathologists did not comment on the predominant cells.
CMUSE: imaging features
Eight patients were included for image review, and 9 CTEs and 1 MRE were analyzed. The morphologic features for all cases are summarized in Table 2 and illustrated in Figs. 1, 2, 3, 4, 5, 6, and 7. One patient had three separate sequential CTEs evaluated due to a change in appearance and disease morphology was noted by the readers between the individual examinations (Fig. 2.)
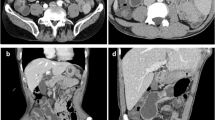
Most common imaging features observed in CMUSE. a Coronal CTE image demonstrates multiple multifocal circumferential strictures the ileum (solid white arrows) with proximal small bowel dilatation near 3 cm (dashed bracket). The longer stricture noted in the ileum (dot-dashed white arrow) demonstrates characteristic stratified hyperenhancement. b, c Axial and coronal CTE images demonstrate a characteristic ileal stricture with stratified mural hyperenhancement in long axis (white arrow) and en face orientation (dotted white arrow). Identifying lesions en face helps with characterization and lesion detection
59-year-old male with partial small bowel obstruction secondary to CMUSE. a Coronal CTE image before surgery demonstrates multiple (> 15) multifocal short strictures (white arrows) involving both the ileum and jejunum. Note that one of the lesions has slight asymmetry mimicking the appearance of CD (dashed white arrow). b Coronal CTE image from the same study demonstrating moderate proximal small bowel dilatation and the small bowel feces sign (white circle). c Follow-up coronal CTE images 4 months after surgery demonstrate a circumferentially thickened long segment of proximal jejunum with mucosal undulations and stratified mural hyperenhancement compatible with active inflammation and ulcerations (white arrow) with associated mesenteric adenopathy (dotted white arrow). d Coronal CTE image 4 years later demonstrates resolution of the findings within the jejunum and new strictures sequelae of prior inflammation and ulceration (white circle). e Anterograde double balloon enteroscopy with a cap of a different patient demonstrating a crater-like ulcer in within the jejunum illustrating ulcerations in CMUSE (white arrow)
Differential diagnosis of multifocal short small bowel strictures should always include CD, NSAID enteropathy, and CMUSE. a. Multiplanar CTE images from a CMUSE demonstrate a circumferential short (< 2 cm) stricture in the ileum nearly identical to diaphragm disease from excessive NSAID use (black box). These two etiologies cannot confidently be differentiated based on imaging features alone. b Anterograde double balloon enteroscopy with a cap demonstrating the classic endoscopic appearance of a small bowel diaphragm (white arrow)
Differential diagnosis of multifocal short small bowel strictures should always include CD, NSAID enteropathy, and CMUSE. a, b Coronal and axial CTE images of different CMUSE patient demonstrating multifocal short circumferential strictures with fat attenuation within the bowel wall (white arrow). This is a frequent imaging feature observed in long standing small bowel CD from chronic inflammation. CD can be confidently diagnosed if penetrating disease and extra-intestinal sequels are present. c Anterograde double balloon enteroscopy with a cap demonstrating ulcerating stricture in the jejunum with a similar appearance to CD (white arrow)
Imaging features of treatment response in CMUSE. a, b Baseline coronal and axial CTE images of a 68-year-old male with CMUSE demonstrating a short segment circumferential stricture with stratified hyperenhancement in the ileum (white arrow). c, d Two-year follow-up CTE with interval budesonide therapy shows subtle improvement in the wall thickening and enhancement of the previously seen stricture (dotted white arrow). Most patients in our cohort were observed to have partial or no response to medical therapy
CMUSE mimicking multifocal small bowel carcinoid tumors. a, b Initial CTE of a 67-year-old male referred for evaluation of anemia and possible CD. Multiplanar CTE images demonstrate a multiple enhancing mass-like lesions within the ileum (white arrow). Subtle areas of asymmetric plaque-like wall thickening and luminal narrowing were noted and deemed difficult to distinguish from normal small peristalsis (dashed white arrow). Initial interpretation of the CTE concluded that findings were most compatible with small bowel carcinoid and a DOTATE PET study was recommended. c Representative axial fused image of the patient’s subsequent whole body 68Ga-DOTANOC PET/MRI examination showed no radiotracer uptake previously noted small bowel mass-like lesion or areas of mural thickening, excluding carcinoid tumors. Six-month follow-up CTE was recommended after completion of a budesonide taper. d, e Multiplanar CTE images at 6 months follow-up demonstrate multifocal short segment strictures involving the proximal and mid ileum (dotted white arrows). The previously noted small bowel nodule (black arrow) was unchanged and thought to be a small bowel polyp or pseudopolyp rather than a mass. The interpreting radiologist’s impression included CMUSE and NSAID enteropathy on the differential and the diagnosis of CMUSE was confirmed after unrevealing clinical work-up
28-year-old male with CMUSE detected on MRE. a, b. Coronal SSFE without and with fat saturation demonstrate moderate circumferential thickening of the jejunum with increase T2 signal within bowel wall compatible with edema and associated peri-enteric edema (white arrows). c. Axial T1 post-contrast demonstrates stratified hyperenhancement of the previously noted segment of jejunum (white arrows). d, e. Coronal SSFE demonstrates multifocal short segment strictures in the distal ileum (white rectangle/circle) with moderate generalized proximal bowel dilation. f Coronal SPGR post-contrast image shows homogeneous hyperenhancement of the ileal stricture (white square). Smaller lesions (< 5 mm) may be difficult to detect on MRE compared to CTE because of lower spatial resolution; however, having multiple sequences aids in differentiating physiologic narrowing/peristalsis from true strictures
The most common major morphologic feature observed in every patient was short, multifocal small bowel strictures measuring < 2 cm in length with circumferential luminal narrowing and stratified hyperenhancement (Fig. 1). Multiple strictures were commonly seen with ≥ 5 strictures involving < 25–50% of the small bowel observed in seven patients, and four of those patients had > 10 strictures. The ileum was the most common site of involvement and found in every patient. Isolated disease within the jejunum did not occur, but three patients had concomitant disease within the ileum and jejunum (Figs. 2, 3, 4, 6 and 7). The maximum wall thickness of the lesions for each patient was in the range of moderate severity (6–9 mm). The upstream small bowel luminal diameter ranged from 2.5 to 4.1 cm, indicative of partial obstructing physiology in cases with measurements > 3 cm in three patients which included two patients with mild upstream dilation (3.5 cm) and one patient with moderate upstream dilation (4.1 cm) (Fig. 2).
Coexisting asymmetric stricture morphology occurred in four patients (Figs. 2 and 6). However, no case demonstrated penetrating disease. The presence of numerous mesenteric lymph nodes was found in all patients, but lymph node enlargement (> 1 cm short axis) was only present in four patients (Fig. 2). Other extra-enteric findings were infrequent and included three patients with ascites (Fig. 7), two patients with increased mesenteric vascularity, and one patient with mesenteric edema and colonic inflammation.
Treatment and outcomes
Budesonide and prednisone were the most common therapies (87.5%) (Table 1; Fig. 5). Three biologic medications were successfully utilized in three individual cases: one patient was treated with vedolizumab after partial clinical response to methotrexate; a second patient was also treated with vedolizumab after failing both budesonide and mesalamine therapies; and one patient initially treated with infliximab with good clinical response and later switched to adalimumab/azathioprine combination therapy following skin adverse event. Most patients recurred after first treatment (87.5%) after a median time of 4 months (IQR, 2–19.5 months) (Supplementary Table 2). Surgery for refractory intestinal obstruction was required in half of cases with 50% recurrence of ulcerating disease post-operatively (Fig. 2). The three patients treated with endoscopic balloon dilation had recurrence of obstructive symptoms during study follow-up. Even though one of these patients had a symptom-free window of 2 years after the first dilation, the other two patients and subsequent dilations on the first patient had recurrence of symptoms within 2 to 3 months. History of hospitalizations for obstruction during the observed follow-up period was common (75%) and two patients died of non-GI-related causes.
Discussion
This is the largest single tertiary care center experience of adults with CMUSE in North America. Our study augments the limited and needed evidence describing the clinical and imaging characteristics of CMUSE in a US population. Clinically, the majority of patients were males, in the 5th and 6th decades of life, presenting with iron deficiency anemia (75%) with median duration of symptoms of 63.5 months. Most patients were refractory (87.5%) to their first therapy, including steroids, with half being refractory to surgical intervention. The most common imaging features included multiple short circumferential strictures (< 2 cm in length; < 1 cm thick) with layered hyperenhancement pattern (> 90%) most commonly involving the ileum (90%).
The findings in our study are similar to the radiologic findings described by Hwang et al. in the largest available study of 20 patients with confirmed CMUSE in South Korea [22]. However, there were subtle differences [22]. For instance, the mean number of strictures per patient was higher in our cohort, with the majority of patients having 5 or more strictures (88%; 7/8) with half (50%; 4/8) having more than 10 strictures versus a mean of 2.6 strictures (range 1–6) per patient in their study [22]. Moreover, a unique feature of long segmental small bowel wall thickening with inflammation that later evolved into multiple short strictures on subsequent follow-up imaging was observed the one patient in our cohort (Fig. 2). Interestingly, this phenomenon was also observed in 7 patients (35%) in the previously mentioned study [22]. The authors postulated that the long segmental small bowel wall thickening and inflammatory change likely represented multiple superficial mucosal ulcerations of the involved small bowel segment, but this was never confirmed with endoscopy or pathology [22]. This observation suggests CMUSE may have an active ulcerating phase and chronic inflammatory/fibrotic phase that manifest along a temporal spectrum at various frequencies of recurring episodes. Early phases likely have varying degrees superficial small bowel (mucosa and submucosa) ulceration with associated surrounding inflammation (e.g., long segmental involvement). In the later phases, localized chronic inflammation and submucosal fibrosis likely develop in the small bowel segments with ulceration leading to the characteristic strictures. The two phases are likely not mutually exclusive since strictures with superficial ulcerations have also been observed pathologically [20].
A separate cohort of 12 patients, identified from a consortium of French centers over a period of 40 years, has described CMUSE as a gastrointestinal manifestation of systemic vasculitis [13]. In our cohort, two patients demonstrated elevated serum autoantibody levels (+ ANA); however, biopsy specimens did not demonstrate findings suggestive of vasculitis. Currently, there is no recommendation regarding accepted surgical or medical therapy for patients with CMUSE. Half of our cohort underwent surgery (e.g., small bowel resection), at a slightly lower frequency of the other published cohorts (65% and 92%) [13, 16]. While surgery is effective, disease recurrence remained common (50%). Glucocorticoids (87.5%) were usually tried as first line and had limited benefit given most patients had symptom/disease recurrence and became refractory to therapy (86%). Biologic therapy was used in three patients who all demonstrated response to therapy. An isolated case report has shown similar effect with biologics; however, the demonstrated benefit is anecdotal with further research and understanding of CMUSE needed [17].
A potential clinical and radiologic diagnostic challenge is differentiating Crohn’s disease, drug-induced enteropathy, and CMUSE as a final diagnosis because of their overlapping characteristics (Figs. 3 and 4). However, characteristic imaging features do exist and knowledge of them is crucial for accurate interpretation and diagnosis. Enterography studies demonstrating multiple long segments of small bowel involvement that are patchy and asymmetric favor CD. CMUSE lesions may demonstrate subtle asymmetry, as noted in two patients in our cohort, but higher proportion circumferential lesions were present. Identifying penetrating disease such as a fistula, abscess, or perianal disease will exclude CMUSE and NSAID enteropathy. Disease location (e.g., colon, duodenum, stomach, and esophagus) and extra-enteric complications (e.g., primary sclerosing cholangitis, sacroiliitis, etc.) can also be used as differentiating signs for CD. Additionally, sequela of such as mesenteric vein thrombosis, fibrofatty proliferation, and pseudosacculations will favor CD. Overlapping imaging features that are less specific include the degree of bowel wall thickening (6–9 mm), increased stratified enhancement pattern, degree of proximal bowel dilation, number and size of the mesenteric lymph nodes, increased mesenteric vascularity, and the presence of mesenteric fluid. The imaging features of drug-induced enteropathy are more similar to CMUSE than CD. In a previously reported case series of 12 patients by Flicek et al., 90% of NSAID enteropathy strictures were circumferential, ring-like, 5–10 mm (mean length 2.6 cm; range 5 mm to 10 cm) in length, and symmetric with respect to the bowel lumen [23]. Mild-to-moderate segmental bowel wall thickening (5–8 mm) was observed in two-thirds of their patients and half demonstrated mild-to-moderate mural hyperenhancement and mild proximal bowel dilation (3.8 cm) [23]. The terminal ileum was never involved which might be useful feature for differentiating from CD [23, 24]. All the aforementioned features overlap with the imaging features of CMUSE in our cohort and previous studies. Consequently, differentiating the two entities on imaging features alone is uncertain, thus clinical (NSAID use), pathology, and radiology data need to be integrated for accurate diagnosis.
Our study has limitations. For instance, the small sample size, retrospective nature, and incomplete data availability for all patients limit our ability to make generalizable conclusions. However, these limitations are difficult to address and avoid because the diagnosis of CMUSE is extremely rare and not completely understood. The goal of this report is to increase awareness and identification of patients with this condition which will hopefully lead to needed research to improve patient care given the current lack of knowledge and available evidence. CT and MR enterography are invaluable tools for diagnostic evaluation of CMUSE, but have limitations requiring a knowledgeable multidisciplinary team and integration of all clinical and pathologic data for accurate diagnosis.
Data availability
Data available on request due to privacy/ethical restrictions.
References
Kohoutova D, Bartova J, Tacheci I, Rejchrt S, Repak R, Kopacova M, Bures J (2013) Cryptogenic multifocal ulcerous stenosing enteritis: a review of the literature. Gastroenterol Res Pract 2013:918031. https://doi.org/10.1155/2013/918031
Graham DY, Bynum TE (1974) Primary nonspecific small bowel ulceration as a source of chronic bleeding. Report of a case and review of the approach to localization of the site of small bowel hemorrhage. Am J Gastroenterol 62 (4):350–355
Hopkins JE, Deaver JM (1966) Stenosing small-bowel ulceration. Pa Med 69 (8):35–39
Doutre LP, Paccalin J, Perissat J, Traissac FJ (1966) [Plurifocal ulcerous stenosing enteritis. A further case]. Arch Fr Mal App Dig 55 (6):537–540
Morgenstern L, Freilich M, Panish JF (1965) The Circumferential Small-Bowel Ulcer: Clinical Aspects in 17 Patients. JAMA 191:637–640. https://doi.org/10.1001/jama.1965.03080080027006
Lindholmer B, Nyman E, Raef L (1964) Nonspecific Stenosing Ulceration of the Small Bowel: A Preliminary Report. Acta Chir Scand 128:310–311
Debray C, Besancon F, Hardouin JP, Martin E, Marche C, Khoury K (1964) [Cryptogenetic Plurifocal Ulcerative Stenosing Enteritis]. Arch Mal Appar Dig Mal Nutr 53:193–206
Watson MR (1963) Primary Nonspecific Ulceration of the Small Bowel. Arch Surg 87:600–603. https://doi.org/10.1001/archsurg.1963.01310160062010
Rocha A, Artigas V (1959) [Stenosing ulcerous disease of the jejuno-ileum]. Arch Mal Appar Dig Mal Nutr 48:1230–1236
Cattan R, Frumusan P, Pineau P, Nivet P, Habib R (1957) [Stenosing ulcer of the ileum]. Arch Mal Appar Dig Mal Nutr 46 (10):984–990
Boydstun JS, Jr., Gaffey TA, Bartholomew LG (1981) Clinicopathologic study of nonspecific ulcers of the small intestine. Dig Dis Sci 26 (10):911–916. https://doi.org/10.1007/bf01309496
Freeman HJ (2009) Multifocal stenosing ulceration of the small intestine. World J Gastroenterol 15 (39):4883–4885. https://doi.org/10.3748/wjg.15.4883
Perlemuter G, Guillevin L, Legman P, Weiss L, Couturier D, Chaussade S (2001) Cryptogenetic multifocal ulcerous stenosing enteritis: an atypical type of vasculitis or a disease mimicking vasculitis. Gut 48 (3):333–338. https://doi.org/10.1136/gut.48.3.333
Matsumoto T, Iida M, Matsui T, Yao T (2007) Chronic nonspecific multiple ulcers of the small intestine: a proposal of the entity from Japanese gastroenterologists to Western enteroscopists. Gastrointest Endosc 66 (3 Suppl):S99–107. https://doi.org/10.1016/j.gie.2007.01.004
Matsumoto T, Iida M, Matsui T, Yao T, Watanabe H, Yao T, Okabe H (2004) Non-specific multiple ulcers of the small intestine unrelated to non-steroidal anti-inflammatory drugs. J Clin Pathol 57 (11):1145–1150.https://doi.org/10.1136/jcp.2003.015735
Chung SH, Park SU, Cheon JH, Kim ER, Byeon JS, Ye BD, Keum B, Shim KN, Jung SA, Kim JO, Jeon SR, Song HJ, Moon JS, Chang DK (2015) Clinical Characteristics and Treatment Outcomes of Cryptogenic Multifocal Ulcerous Stenosing Enteritis in Korea. Dig Dis Sci 60 (9):2740–2745. https://doi.org/10.1007/s10620-015-3595-y
De Schepper H, Macken E, Van Marck V, Spinhoven M, Pelckmans P, Moreels T (2013) Infliximab induces remission in cryptogenic multifocal ulcerous stenosing enteritis: first case. World J Gastroenterol 19 (10):1661–1664. https://doi.org/10.3748/wjg.v19.i10.1661
Baker ME, Hara AK, Platt JF, Maglinte DD, Fletcher JG (2015) CT enterography for Crohn’s disease: optimal technique and imaging issues. Abdom Imaging 40 (5):938–952. https://doi.org/10.1007/s00261-015-0357-4
Grand DJ, Guglielmo FF, Al-Hawary MM (2015) MR enterography in Crohn’s disease: current consensus on optimal imaging technique and future advances from the SAR Crohn’s disease-focused panel. Abdom Imaging 40 (5):953–964. https://doi.org/10.1007/s00261-015-0361-8
Deepak P, Fletcher JG, Fidler JL, Barlow JM, Sheedy SP, Kolbe AB, Harmsen WS, Loftus EV, Hansel SL, Becker BD, Bruining DH (2016) Radiological Response Is Associated With Better Long-Term Outcomes and Is a Potential Treatment Target in Patients With Small Bowel Crohn’s Disease. Am J Gastroenterol 111 (7):997–1006. https://doi.org/10.1038/ajg.2016.177
Guglielmo FF, Anupindi SA, Fletcher JG, Al-Hawary MM, Dillman JR, Grand DJ, Bruining DH, Chatterji M, Darge K, Fidler JL, Gandhi NS, Gee MS, Grajo JR, Huang C, Jaffe TA, Park SH, Rimola J, Soto JA, Taouli B, Taylor SA, Baker ME (2020) Small Bowel Crohn Disease at CT and MR Enterography: Imaging Atlas and Glossary of Terms. Radiographics 40 (2):354–375. https://doi.org/10.1148/rg.2020190091
Hwang J, Kim JS, Kim AY, Lim JS, Kim SH, Kim MJ, Kim MS, Song KD, Woo JY (2017) Cryptogenic multifocal ulcerous stenosing enteritis: Radiologic features and clinical behavior. World J Gastroenterol 23 (25):4615–4623. https://doi.org/10.3748/wjg.v23.i25.4615
Flicek KT, Hara AK, De Petris G, Pasha SF, Yadav AD, Johnson CD (2014) Diaphragm disease of the small bowel: a retrospective review of CT findings. AJR Am J Roentgenol 202 (2):W140–145. https://doi.org/10.2214/AJR.13.10732
Frye JM, Hansel SL, Dolan SG, Fidler JL, Song LM, Barlow JM, Smyrk TC, Flicek KT, Hara AK, Bruining DH, Fletcher JG (2015) NSAID enteropathy: appearance at CT and MR enterography in the age of multi-modality imaging and treatment. Abdom Imaging 40 (5):1011–1025. https://doi.org/10.1007/s00261-015-0367-2
Funding
None.
Author information
Authors and Affiliations
Contributions
GPR involved in original concept, study design, data abstraction, manuscript draft, review, and editing. DJB performed study design, data abstraction, manuscript draft, figure, and legend editing. ACB did data abstraction and critical review of the manuscript. DHB did critical review of the manuscript. JLF contributed to expert review of radiology studies and critical review of the manuscript. SPS participated in study design, expert review of radiology studies, and critical review of the manuscript. JM involved in study design and critical review of the manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflicts of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Guilherme Piovezani Ramos and David J. Bartlett sharing co-first authorship.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Ramos, G.P., Bartlett, D.J., Bledsoe, A.C. et al. Cryptogenic multifocal ulcerous stenosing enteritis (CMUSE): a 20-year single-center clinical and radiologic experience. Abdom Radiol 46, 3798–3809 (2021). https://doi.org/10.1007/s00261-021-03005-y
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00261-021-03005-y