Abstract
Objective
To explore which computed tomography (CT) features can predict pathological complete response (pCR) (ypT0N0) after neoadjuvant chemotherapy (NAC) in patients with gastric adenocarcinoma (GC).
Materials and Methods
This study reviewed an institutional database of patients who underwent resection of GC after NAC and identified patients with pCR from January 2010 to December 2013. The correlations between pre-chemotherapy and post-chemotherapy CT features and pCR were analyzed.
Results
Eleven of 199 patients with GC who achieved ypT0N0 status after NAC were classified as the pCR group in this study. After matching pCR (n = 11) and non-pCR patients (n = 44) in the ratio of 1:4, a total of 55 cases were analyzed. The binary logistic regression analysis showed that the post-chemotherapy short diameter of the largest lymph node and tumor thickness ratio reduction were independent predictors of pCR, with an area under the curve (AUC) of 0.94 on the receiver operating characteristic (ROC) curve analysis.
Conclusion
Two CT features, including the short diameter of the largest lymph node post-chemotherapy and tumor thickness ratio reduction, are good predictors of pCR after NAC in patients with GC.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Gastric cancer (GC) or adenocarcinoma of the esophagogastric junction (AEG) is an aggressive disease, with most patients having locally advanced disease upon presentation. In such cases, the National Comprehensive Cancer Network (NCCN) recommends neoadjuvant chemotherapy (NAC) to downsize the tumor followed by radical surgery [1]. Previous clinical trials have reported significant improvements in the overall survival and disease-free survival rates of patients with locally advanced GC or AEG after undergoing neoadjuvant chemotherapy [2, 3]. A meta-analysis found that patients with resectable GC or AEG who achieved complete pathological response (pCR) after NAC had significantly better survival outcomes than patients without pCR [4]. The pCR rate in GC and AEG is approximately 8-20%, yet the patients who have pCR show better long-term survival outcomes in the clinic [5]. In patients with rectal cancer, a clinical complete response can be treated by the watch-and-wait approach to avoid radical surgery, while those patients who develop regrowth can undergo salvage surgery [6]. Currently, the accurate prediction of pCR preoperatively in GC after neoadjuvant therapy is an unsolved problem.
Multi-detector computed tomography (CT) is currently the routine technique of choice for preoperative staging of GC [7,8,9,10,11,12]. However, restaging by CT after NAC is still inaccurate in patients with locally advanced GC [13]. The radiological T-stage after NAC should not be considered for decision-making [14]. To the best of our knowledge, there are no known CT features for the prediction of pCR after NAC in patients with GC. In this study, we examined several CT features to determine the best predictors of pCR after NAC in patients with GC.
Materials and methods
Patients
This retrospective study was approved by the institutional review board, and the requirement for informed consent was waived. We collected the clinicopathological data of 206 patients with pathologically confirmed GC who underwent gastrectomy after NAC from January 2010 to December 2013. These patients received 2–3 cycles of NAC. All patients underwent twice contrast-enhanced CT scans of the abdomen and pelvis at the base line and within 2 weeks before the sugery. Patients with poor quality CT images causing by poor breathless (3 cases) were excluded. Patients who were found to have metastatic disease on post-chemotherapy CT scan and did nonradical surgery (4 cases of palliative surgery) were excluded from the study.
Out of 199 patients with GC who underwent radical gastrectomy after NAC, 11 patients had pCR (ypT0N0 stage) status on postoperative pathology. These 11 cases were classified as the pCR group for this study. Next, we randomly matched the 11 pCR cases with 44 non-pCR cases in the ratio of 1:4. The total enrollment consisted of 55 cases (45 male and 10 female, median age: 57 years).
CT protocol
A CT scanner with 64 rows of detectors was used (LightSpeed 64; GE Healthcare, Milwaukee, WI, USA). After > 8 h of fasting, the patients were given 8 g of oral gas-producing crystals for achieving gastric distension, along with an intramuscular injection of 10 mg anisodamine for reducing gastric motility (raceanisodamine hydrochloride, Hangzhou Minsheng Pharmaceutical Co. Ltd., China). At 10-15 min post-injection, a non-contrast CT scan from the dome of diaphragm to 2 cm below the lower margin of the air-distended gastric body was acquired (collimation: 0.625 mm, peak tube voltage: 120 kVp, tube current–time product: automatic). Next, 100 mL of a non-ionic contrast medium (Ultravist, 370 mg/mL; Bayer, Munich, Germany) was administered intravenously at 3.5 mL/s using an automatic injector. The arterial phase images were captured using bolus tracking. The arterial phase scanning was initiated when the radiodensity in the abdominal aorta at the level of the celiac artery was 100 HU. The mean delay after the trigger threshold to the onset of arterial phase scanning was 22 s (range: 20-25 s). Portal phase scanning began 35 s after arterial phase scanning.
Image analysis
Image analysis was performed jointly by two radiologists with more than 10 years of combined experience using the workstation (Carestream Health, Shanghai, China). Any discrepancies were resolved by consulting another radiologist with 20 years of experience to achieve a consensus. They reviewed the axial, sagittal, and coronal CT images of the stomach to detect tumors. The correlation between CT images and results of endoscopy was made by radiologists to confirm the location of the tumor for every case. The CT features of GC observed and measured in this study were as follows:
Gastric tumor thickness
The thickest diameter of the gastric tumor on the axial CT image measured before and after NAC. With the application of oral gas contrast and IV anisodamine medicine of our CT protocol, the adequate gastric distension and reduced gastric motility ensured the accuracy and consistency of measurement of tumor thickness. For some tumor difficult to identify like signet-ring cell cancers and ulcer-like cancers, we checked the location in endoscopy and compared the changes of gastric wall before and after chemotherapy to confirm the measurement site of the lesion.
CT attenuation of gastric tumor
CT attenuation values of the gastric tumor with an oval region-of-interest (ROI) in the same portion of the tumor on the axial portal phase CT image measured before and after NAC.
Only linear mucosal enhancement on post-chemotherapy CT
Presence of only linear mucosal enhancement on the portal phase axial, coronal, and sagittal images, based the MPR images at the tumor site, observed after NAC.
Resolution of the tumor ulcer
Disappearance of the tumor ulcer on post-chemotherapy CT images.
Short diameter of the largest lymph node
The shortest diameter of the largest regional lymph node was measured on pre-chemotherapy and post-chemotherapy CT images.
All lymph nodes less than 5 mm
The short diameter of regional lymph nodes was less than 5 mm on post-chemotherapy CT image.
Reduction in tumor thickness, CT attenuation and diameter of lymph node
Reduced tumor thickness, CT attenuation, and lymph node diameter values on CT after chemotherapy. We subtracted these measured values directly.
Reduced ratio in tumor thickness, CT attenuation and diameter of lymph node
Reduced ratio of tumor thickness, CT attenuation, and diameter of lymph node ratios on CT after chemotherapy.
Statistical analysis
The continuous and categorical data were present as mean ± standard deviation (SD) and frequency (%), respectively. Data processing and analysis were performed using SPSS/PC + version 22.0 (SPSS Inc., IL, Chicago, USA). The CT features of the pCR and non-pCR groups were compared using the independent-samples t test and Mann–Whitney U test. Univariate logistic regression was conducted, from which the statistically significant variables tested were substituted into multivariate logistic regression to determine the independent CT features that predicted pCR. Receiver operating characteristic (ROC) curve analysis was performed to determine the diagnostic accuracy of the CT features in predicting pCR. P-values less than 0.05 were considered to be statistically significant.
Results
Patient and tumor characteristics
There were 55 patients included in this study (Fig. 1). The patient and tumor characteristics are summarized in Table 1. The majority of the patients had moderately and poorly differentiated (30.9%) adenocarcinomas, while signet-ring cell carcinoma was present in eight patients (14.5%). The most common sites of the primary tumor were the gastroesophageal junction (38.2%) and the distal stomach (34.5%). In this study, 11 (20%), 9 (16.4%), 7 (12.7%), 8 (14.5%), and 20 (36.4%) patients had ypT0, ypT1, ypT2, ypT3, and ypT4 disease, respectively. Twenty-eight patients (50.9%) had ypN0 disease. Eleven patients achieved pathological complete response (ypT0N0) after NAC. The NAC regimens included SOX (37 cases, S-1 plus oxaliplatin), XELOX (11 cases, oxaliplatin plus capecitabine), mFOLFOX7 (five cases, modified regimen of leucovorin, fluorouracil, and oxaliplatin), XP (one case, cisplatin plus capecitabine), and XELOX plus Herceptin (one case).
Comparison of CT features between pCR and non-pCR groups
Since all patients in this group were of advanced gastric cancer, the CT manifestations of these primary cancers were relatively obvious. All tumors found on endoscopy were detected by CT successfully.
Univariate analysis showed that several CT features were significantly different between the pCR and non-pCR groups, including tumor thickness, tumor attenuation, only linear mucosal enhancement on post-chemotherapy CT, the incidence of tumor ulcer disappeared on post-chemotherapy CT image, the post-chemotherapy short diameter of the largest lymph node, all lymph nodes less than 5 mm, reduction in tumor thickness, reduction in tumor attenuation, reduced ratio of tumor thickness, reduced ratio of tumor attenuation, and reduced ratio of the diameter of the lymph node (Table 2).
Univariate logistic regression results are listed in Table 3. Statistically significant variables tested by univariate logistic regression were substituted into multivariate logistic regression. On the multivariate analysis with forward selection, two CT features, namely, the short diameter of the largest lymph node and reduction in the ratio of tumor thickness, were the independent predictors for pCR, and ORs were 2.86 (95% CI, 1.38–5.91) and 0.75(95% CI, 0.60–0.94), respectively. A multivariate logistic regression model was constructed as (Y = 1.05 × Post-chemotherapy short diameter of the largest lymph node-0.289 × Reduction in tumor thickness). The ROC curves showed that the multivariate logistic regression model achieved the largest ROC curve (0.94) for predicting pCR (Table 4; Fig. 2).
Discussion
Previous studies have demonstrated that tumor responses, as visualized by imaging studies, such as IV contract-enhanced CT and endoscopic ultrasonography, are often associated with histopathological response and survival of patients with GC treated with NAC [15]. However, the early assessment of NAC response through imaging studies has shown limited benefit [15]. The sensitivity and specificity of imaging modalities in response assessment are not very encouraging in clinical practice. Some studies found that CT perfusion imaging, diffusion-weighted magnetic resonance imaging (MRI), and positron emission tomography (PET) can distinguish between responders with non-responders in patients with GC after NAC [16,17,18]. Nevertheless, the accuracy of restaging remains low for patients with GC after NAC. Previously, Park et al. reported accuracies of 47% and 57% for EUS and CT, respectively, for T-restaging and 39% and 37% for N-restaging, respectively [13]. The ability of CT or other imaging modalities to diagnose pCR has not been evident.
In this study, we analyzed the accuracy of various CT imaging features in predicting pCR after NAC in patients with GC. Our results showed that CT findings prior to NAC were not useful in predicting pCR. We speculate that pre-chemotherapy CT findings only reflect the initial stage of the disease and cannot predict tumor response. Other CT features of primary gastric tumor, including post-chemotherapy tumor thickness, tumor attenuation, reduction in tumor thickness, and reduction in tumor attenuation, were significantly different between pCR and non-pCR groups in this study. Although the response to NAC of primary tumors of the gastrointestinal tract cannot be assessed by the Response Evaluation Criteria in Solid Tumours (RECIST) version 1.1, the CT features of the stomach may help diagnose pCR in patients with GC. The reduction in the ratio of tumor thickness is an independent predictor for pCR in this study. In our institution, oral gas-producing crystals and injection of 10 mg anisodamine are routinely used during CT for every patient without contraindication. This helps in achieving consistency in the gastric cavity distension during the CT examination and reduces the impact of gastric wall motility on the gastric tumor thickness. We found that linear mucosal enhancement on post-chemotherapy CT appeared in 54.5% (6/11) patients with pCR. The CT feature of linear enhancement with or without mucosal thickening has been used to diagnose the T1a stage of GC in previous studies [11, 12]. When the enhancement of advanced GC changes from the full-thickness wall enhancement to only linear mucosal enhancement after NAC, it may indicate that the lesion has completely resolved (ypT0 stage) or limited residual disease in the mucosal layer (ypT1 stage)(Fig. 3). Complete resolution of the tumor ulcer on post-chemotherapy CT image appeared in 27.3% (3/11) patients with pCR in this study. Although, on the univariate analysis, the incidence of resolution of the tumor ulcer was different between pCR and non-pCR patients, this CT feature cannot be used in protruding tumors or those with diffuse infiltration. Moreover, CT is less accurate than endoscopy for the assessment of tumor ulcers. Hence, we believe it is not an accurate predictor for pCR in clinical practice, and endoscopy is a better alternative to assess tumor ulcer healing.
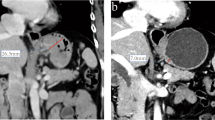
a–d Male, 68 years, gastroscopy revealed mass of gastroesophageal junction. The pathological result of biopsy was poorly differentiated adenocarcinoma and intestinal type of Lauren classification. The clinical stage was diagnosed as cT3N1 by the ultrasonic gastroscopy. This patient received 2 cycles of S-1 plus oxaliplatin (SOX) chemotherapy and total gastrectomy. Postoperative pathology showed chronic inflammatory cell infiltration with fibrous tissue hyperplasia in the original tumor bed, and no residual carcinoma was observed. No metastases were found in lymph nodes dissected in surgery (0/24). a, b, the axial and coronal baseline CT images revealed thickened wall of cardia with obviously contrasted enhancement in portal phase. 3C-3D, the axial and coronal post-chemotherapy CT images showed the thickness of tumor shrunk. The enhancement of tumor decreased and became only linear mucosal enhancement on CT
Stark et al. reported that patients of GC or GEC with ypN + disease have substantially reduced survival compared to those with ypT0 disease after preoperative therapy [5]. In the current study, we found that lymph node status is a useful predictor in diagnosing pCR (pT0N0). The mean post-chemotherapy short diameter of the largest lymph node (4.27 ± 1.01) of the pCR group was significantly smaller than that of the non-pCR group (8.11 ± 3.84) (p < 0.001). The post-chemotherapy short diameter of the largest lymph node was another independent predictor for predicting pCR in the logistic analysis. Also, the size of all visualized lymph nodes less than 5 mm could be used to predict pCR. In the study by Park et al., the accuracy of N-restaging by CT was low at 37%. They considered the lymph nodes to be suggestive of having metastasis if the longest diameter was > 1.0 cm or if the size was between 0.7 and 1.0 cm with hyper-enhancement, a round shape, central necrosis, or perinodal infiltration [13]. In other studies, regional lymph nodes were considered to have metastases if their long-axis diameter was > 8 mm, they were solitary, or showed marked enhancement (attenuation > 85 HU in the portal venous phase) [19]. Chen and colleagues reported the accuracy of CT for N-staging to be 78% [19]. Some studies have shown that lymph node size is not a reliable indicator for metastasis in GC [20]. For example, Noda et al. indicated that metastases can be present in small lymph nodes, and ignoring them could lead to staging errors in GC. If all nodes less than 5 mm in size were ignored, 37.8% of all metastatic nodes would be missed in the clinic [21]. Therefore, we believe that reducing the diagnostic threshold for lymph nodes from 10 mm or 8 mm to 5 mm for the short-axis diameter might be suitable for N-restaging and pCR prediction for patients with GC after NAC.
The multivariate logistic regression model of this study, which combined two CT features (post-chemotherapy short diameter of the largest lymph node and reduction in the ratio of tumor thickness), had the largest area of ROC curve (0.94) compared to other individual CT features for predicting pCR (Fig. 2).
In the current study, we did not include all 188 non-pCR patients for the analysis. The reason was that the number of pCR patients (n = 11) was relatively small compared to non-pCR patients (n = 188). This imbalance in sample size could lead to bias in the statistical analysis. Hence, we randomly selected non-pCR cases to obtain the ratio of 4:1.
This study has some limitations. First, it is a retrospective study that relies on the data gathered from databases. Secondly, the number of pCR patients (n = 11) was small and may have affected our ability to identify the best predictors of patient outcomes. Third, we did not include other features, such as endoscopy findings (before and after NAC), type of chemotherapy, or other imaging studies like PET/CT, in the multivariate analysis of the predictors of pCR. Our results need to be confirmed by larger prospective studies in the future. Although PET/CT was recommended in all patients with no evidence of metastatic disease from NCCN guideline version 2013, the high price of PET/CT examination leads to its low applied rate in China, and it was less commonly used for monitoring efficacy after neoadjuvant chemotherapy in gastric cancer patients in China.
In conclusion, we found that specific CT features of the tumor and lymph nodal status, including the post-chemotherapy short diameter of the largest lymph node and reduction in the ratio of tumor thickness, may be suitable for predicting pCR after NAC in patients with GC.
Abbreviations
- CT:
-
Computed tomography
- pCR:
-
Pathological complete response
- NAC:
-
Neoadjuvant chemotherapy
- ROC:
-
Receiver operating characteristic curve
References
Jaffer A Ajani, Thomas A D’Amico, Khaldoun Almhanna, David J Bentrem, Joseph Chao, Prajnan Das, et al. Gastric Cancer, Version 3.2016, NCCN Clinical Practice Guidelines in Oncology. J Natl Compr Canc Netw 2016;14:1286-1312.
David Cunningham, William H Allum, Sally P Stenning, Jeremy N Thompson, Cornelis J H Van de Velde, Marianne Nicolson, et al. Perioperative Chemotherapy Versus Surgery Alone for Resectable Gastroesophageal Cancer. N Engl J Med 2006;355:11-20.
Marc Ychou, Valérie Boige, Jean-Pierre Pignon, Thierry Conroy, Olivier Bouché, Gilles Lebreton, et al. Perioperative Chemotherapy Compared With Surgery Alone for Resectable Gastroesophageal Adenocarcinoma: An FNCLCC and FFCD Multicenter Phase III Trial. J Clin Oncol 2011;29:1715-1721
Ziyu Li, Fei Shan, Yinkui Wang, Yan Zhang, Lianhai Zhang, Shuangxi Li, et al. Correlation of Pathological Complete Response With Survival After Neoadjuvant Chemotherapy in Gastric or Gastroesophageal Junction Cancer Treated With Radical Surgery: A Meta-Analysis. PLoS One 2018;13:e0189294.
Alexander P Stark, Naruhiko Ikoma, Yi-Ju Chiang, Jeannelyn S Estrella, Prajnan Das, Bruce D Minsky, et al. Characteristics and Survival of Gastric Cancer Patients With Pathologic Complete Response to Preoperative Therapy. Ann Surg Oncol;26:3602-3610.
Fahima Dossa, Tyler R Chesney, Sergio A Acuna, Nancy N Baxter. A Watch-And-Wait Approach for Locally Advanced Rectal Cancer After a Clinical Complete Response Following Neoadjuvant Chemoradiation: A Systematic Review and Meta-Analysis. Lancet Gastroenterol Hepatol 2017;2:501-513.
A Y Kim, H J Kim, H K Ha. Gastric Cancer by Multidetector Row CT: Preoperative Staging. Abdom Imaging 2005;30:465-72.
Robert Michael Kwee, Thomas Christian Kwee. Imaging in Assessing Lymph Node Status in Gastric Cancer. Gastric Cancer 2009;12:6-22.
Seishi Kumano, Takamichi Murakami, Tonsok Kim, Masatoshi Hori, Riccardo Iannaccone, Saki Nakata, et al. T Staging of Gastric Cancer: Role of Multi-Detector Row CT. Radiology 2005;237:961-966.
Joon Seok Lim, Mi Jin Yun, Myeong-Jin Kim, Woo Jin Hyung, Mi-Suk Park, Jin-Young Choi, et al. CT and PET in Stomach Cancer: Preoperative Staging and Monitoring of Response to Therapy. Radiographics 2006;26:143-156.
Jin Woong Kim, Sang Soo Shin, Suk Hee Heo, Yoo Duk Choi, Hyo Soon Lim, Young Kyu Park. Diagnostic Performance of 64-section CT Using CT Gastrography in Preoperative T Staging of Gastric Cancer According to 7th Edition of AJCC Cancer Staging Manual. Eur Radiol 2012;22:654-662.
In Joon Lee, Jeong Min Lee, Seung Ho Kim, Cheong-Ii Shin, Jae Young Lee, Se Hyung Kim, et al. Diagnostic Performance of 64-channel Multidetector CT in the Evaluation of Gastric Cancer: Differentiation of Mucosal Cancer (T1a) From Submucosal Involvement (T1b and T2). Radiology 2010;255:805-814.
Sook Ryun Park, Jong Seok Lee, Chan Gyoo Kim, Hark Kyun Kim, Myeong-Cherl Kook, Young-Woo Kim, et al. Endoscopic Ultrasound and Computed Tomography in Restaging and Predicting Prognosis After Neoadjuvant Chemotherapy in Patients With Locally Advanced Gastric Cancer. Cancer 2008;112:2368-2376.
Takaki Yoshikawa, Kazuaki Tanabe, Kazuhiro Nishikawa, Yuichi Ito, Takanori Matsui, Yutaka Kimura, et al. Accuracy of CT Staging of Locally Advanced Gastric Cancer After Neoadjuvant Chemotherapy: Cohort Evaluation Within a Randomized Phase II Study. Ann Surg Oncol 2014;21 Suppl 3:S385-9.
Robert Michael Kwee, Thomas Christian Kwee. Role of Imaging in Predicting Response to Neoadjuvant Chemotherapy in Gastric Cancer. World J Gastroenterol 2014;20:1650-1656.
Francesco Giganti, Francesco De Cobelli, Carla Canevari, Elena Orsenigo, Francesca Gallivanone, Antonio Esposito, et al. Response to Chemotherapy in Gastric Adenocarcinoma With Diffusion-Weighted MRI and (18) F-FDG-PET/CT: Correlation of Apparent Diffusion Coefficient and Partial Volume Corrected Standardized Uptake Value With Histological Tumor Regression Grade. J Magn Reson Imaging 2014;40:1147-1157.
. Zongqiong Sun, Xiaofang Cheng, Yuxi Ge, Lin Shao, Yinghua Xuan, Gen Yan. An Application Study of Low-Dose Computed Tomography Perfusion Imaging for Evaluation of the Efficacy of Neoadjuvant Chemotherapy for Advanced Gastric Adenocarcinoma. Gastric Cancer 2018;21:413-420.
Katja Ott, Ulrich Fink, Karen Becker, Alexander Stahl, Hans-Joachim Dittler, Raymonde Busch, et al. Prediction of response to preoperative chemotherapy in gastric carcinoma by metabolic imaging: results of a prospective trial. J Clin Oncol 2003;21:4604-4610.
Chiao-Yun Chen, Jui-Sheng Hsu, Deng-Chyang Wu, Wan-Yi Kang, Jan-Sing Hsieh, Twei-Shiun Jaw, et al. Gastric Cancer: Preoperative Local Staging With 3D Multi-Detector Row CT--correlation With Surgical and Histopathologic Results. Radiology 2007;242:472-482.
S P Mönig , T K Zirbes, W Schröder, S E Baldus, D G Lindemann, H P Dienes, A H Hölscher. Staging of Gastric Cancer: Correlation of Lymph Node Size and Metastatic Infiltration. AJR Am J Roentgenol 1999;173:365-367.
N Noda, M Sasako, N Yamaguchi, Y Nakanishi. Ignoring Small Lymph Nodes Can Be a Major Cause of Staging Error in Gastric Cancer. Br J Surg 1998;85:831-834.
Acknowledgements
This work was supported by the Digestive Medical Coordinated Development Center of Beijing Municipal Administration of Hospitals (No. XXT20), Beijing Municipal Administration of Hospitals Clinical Medicine Development of Special Funding Support(No. ZYLX201803), and Beijing Hospitals Authority’ Ascent Plan (Code: 20191103).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflicts of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Wang, ZL., Li, YL., Li, XT. et al. Role of CT in the prediction of pathological complete response in gastric cancer after neoadjuvant chemotherapy. Abdom Radiol 46, 3011–3018 (2021). https://doi.org/10.1007/s00261-021-02967-3
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00261-021-02967-3