Abstract
Objectives
To evaluate the diagnostic performance of DCE MRI and DWI in the assessment of pathologic complete response (pCR) after neoadjuvant chemotherapy (NAC) in patients with muscle-invasive bladder cancer (MIBC).
Methods
This prospective study included 90 patients with MIBC who finished NAC. Two radiologists independently assessed MRI for the determination of semi-quantitative parameters (wash-in rate and wash-out rate) and apparent diffusion coefficient (ADC) value. The correlation between pCR and wash-in rate, wash-out rate, ADC value were analyzed. The area under the ROC curve (AUC) was used to evaluate the diagnostic performance for detecting pCR. Inter-reader agreement was assessed using the ICC statistics.
Results
On cystectomy specimens, pCR was confirmed in (43.3%, 39/90). pCR is negatively correlated with wash-out rate (r = − 0.701, p = 0.01) and ADC value (r = − 0.621, p = 0.01). ADC value is positively correlated with wash-out rate (r = 0.631, p = 0.001). The diagnostic accuracy of ADC value (cut-off value: 0.911 × 10−3mm2/s) and wash-out rate (cut-off value: 0.677 min−1) in the identification of pCR was (92% for reader 1, 91% for reader 2), and (90% for reader 1, 88% for reader 2), respectively. The sensitivity, specificity for predicting pCR using ADC value + washout rate cut off values were 95.4%, 97.7% for reader 1, and 96%, 97% for reader 2, respectively. AUC was 0.981 for reader 1, 0.971 for reader 2. The overall reproducibility of the mean ADC value and wash out rate was excellent (ICC = 0.83–0.90). The ICC values for the mean ADC value, washout rate was 0.89 (95% CI 0.84–0.89) and 0.87 (95% CI 0.86–0.91), respectively.
Conclusion
Semi-quantitative parameter (wash-out) derived from DCE-MRI and ADC has the potential to assess the tumor’s complete pathologic response. The two parameters using together can offer the best possibility to identify complete response to NAC in MIBC.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
For patients with MIBC, radical cystectomy (RC) is the reference standard of care. However, postsurgical morbidity and urinary diversion performed concurrently with radical cystectomy potentially compromise patients’ quality of life. So, bladder-sparing approaches have been investigated to overcome these issues. The trimodality protocol (transurethral resection, chemotherapy, and radiotherapy) has yielded the most favorable clinical outcomes, the 5-year overall survival rate was 50% to 60% and it was comparable to the contemporary immediate RC series [1, 2].
After NAC, patients who achieve pCR can be subjected to bladder preservation, whereas those who have residual disease are recommended to undergo RC [3]. Also, there is a strong impact of response to NAC on the survival even though patients undergo RC with curative intent [4, 5]. Thus, accurate identification of pCR will optimize patient selection for bladder preservation.
Several previous studies defined clinical complete response to NAC by negative cytology, cystoscopy after transurethral resection of a bladder tumor (TURB), and imaging examinations [6,7,8]. The accuracy of biopsy or resection methods for the prediction of pCR was low. Half of the negative cases have demonstrated pathologic residual disease after RC [9]. Thus, the addition of imaging findings to cystoscopy findings can improve the performance of restaging after NAC.
However, to date, RECIST 1.1 criteria are limited to designating pCR in bladder cancer and there are no reliable imaging criteria to evaluate CR [10], especially in the presence of bladder wall thickening without measurable lesions. So, the diagnosis of a pCR could be less reproducible and highly subjective [11].
After NAC, inflammation, or fibrosis related to transurethral resection or chemotherapy made the radiological assessment of treatment response to be difficult [12, 13]. The diagnostic performance of CT [14], PET [15] to assess tumor response have been previously reported. FDG-PET/CT demonstrated good sensitivity (75%) and specificity (89.7%) for identifying CR [15]. Recently, the urothelial phase CT demonstrated high diagnostic performance in the assessment of pCR after NAC [14].
In the local staging of bladder cancer, MRI is superior to CT [16, 17]. So, the role of MRI in restaging is promising. To the best of our knowledge, only a small cohort study (n = 12) has addressed the diagnostic performance of DCE MRI in this issue [18] and showed encouraging results in the evaluation of tumor response to NAC [18].
The utility of DW-MRI in monitoring response to CRT has been previously reported in 20 patients with MIBC who were treated using CRT-based-selective bladder-sparing strategy [19]. The specificity and accuracy of DWI in the diagnosis of pCR (92%, 80%, respectively) were higher than T2WI (45% and 44%, respectively), and DCE-MRI (18% and 33%, respectively).
Also, DW-MRI is a potential biomarker for predicting CRT sensitivity in multimodal organ preservation strategies for MIBC [20]. We conducted this study in our setting to evaluate the diagnostic performance of DCE MRI and DWI in the assessment of tumor response to NAC in MIBC.
Methods
Study population
Between May 2017 and Feb 2019, this prospective study was approved by our institutional review board. Written informed consent was obtained from all patients who participated in the study.
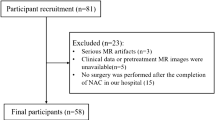
Inclusion criteria were (a) patients with pure urothelial muscle-invasive bladder cancer (proved by transurethral resection of the tumor or cystoscopic biopsy) with clinical stage T2-4aN0M0 disease scheduled for NAC and subsequent cystectomy. (b) Pre and post-NAC MRI imaging, and subsequent radical or partial cystectomy and pelvic lymphadenectomy within 4 weeks after the completion of NAC. Patients were excluded from the study as detailed in (Fig. 1).
MRI technique
Image acquisition
All MRI examinations were acquired on a 1.5 Tesla MRI scanner (Ingenia; Philips Healthcare, Best, The Netherlands) with a phased array body coil. All examinations were made through bladder catheterization to allow for consistent bladder wall distension.
The imaging protocol consisted of triplanar, high spatial resolution T2-weighted fast spin-echo sequences, diffusion-weighted imaging (DWI) in transverse planes at different b-values, dynamic contrast enhancement (DCE) after the injection of contrast agent (0.1 ml/kg Gadovist, Bayer HealthCare, Osaka, Japan), and delayed axial turbo spin-echo T1-weighted images with fat suppression. Images were acquired in multiple phases of contrast enhancement in the sagittal and oblique axial (OA) planes. DCE-MRI was performed using axial fast spin gradient echo sequences with a temporal resolution of 5 s, which was repeated for 5–6 min. The contrast agent was administered intravenously at an injection rate of 2 ml/s using a power injector.
Imaging analysis
Two radiologists (with 10 and 13 years’ experience in genitourinary imaging) independently evaluated MRI sequences. At T2WI, Bladder abnormalities were analyzed regarding the number, location, and morphology (nodules/masses or wall thickening without measurable mass formation). For those lesions classified as nodules or masses, the tumor diameter was measured.
On DCE-MRI images, a region of interest (ROI) was manually drawn on one dynamic image at the slice that showed maximal tumor diameter (to maximally cover the tumor and excluding the surrounding urine and the stalk). For those cases with no suspicious lesions, we put the ROIs on the corresponding area of the primary tumor.
From a series of images in DCE MRI, the signal–time curve was reconstructed and semi-quantitative parameters were evaluated: time to peak (TTP), wash-in rate (speed of contrast uptake), and wash-out rate (clearance rate of contrast agent). Magnetic resonance imaging parameters are summarized in (table 1).
Similar to the previous study [21], normalized ADC was used to reduce the variability of technical and biological factors. ADC of the gluteus muscles rather than the normal bladder wall was used as the reference because ADC of gluteus muscles was relatively constant and easier to measure.
On the axial ADC map, the ADC of lesion and gluteus muscles were automatically calculated by placing a region of interest (ROI) on the largest solid port of the lesion and muscles (with minimum areas of 1 cm2). All ADC values were manually measured three times and the normalized average ADC of lesions was calculated as a ratio (ADClesion/ADCgluteus).
Histopathology reference standard
All patients underwent surgery (a radical or partial cystectomy/cystoprostatectomy and a pelvic lymphadenectomy). The cystectomy specimens were evaluated by an expert pathologist regarding the location and size of any residual tumor, pathologic T-stage (ypT0–T4), N-stage, the status of the surgical margin, and the presence of urothelial CIS. Pathologic absence of tumor (ypT0) was considered a complete response.
Statistical analysis
Quantitative data are presented as means ± standard deviation or median (range) and categorical data are presented as a percentage.
The normalized ADC of complete response (ADC complete response/ADC gluteus) and non-complete response (ADCnon-complete response/ADCgluteus) were compared using an independent- samples t-test.
Analysis of the ADC value, wash-in rate, and wash-out rate was performed using a one-way analysis of variance (ANOVA). The correlation between different variables was assessed by the Pearson correlation test.
Receiver operating characteristic (ROC) curve analysis was performed to evaluate their accuracy in the differentiation between pCR and non-complete response based on the areas under the ROC curves (AUC). The optimal cutoff value was calculated from the ROC analysis. The sensitivity, specificity, and AUC of parameters for the identification of complete responses were calculated by the optimal cutoff value. Inter-reader agreement was evaluated using interclass correlation (ICC) statistics.
Statistical analyses were performed using SPSS software version 20 (SPSS Inc., Chicago, IL). p-value < 0.05 was considered statistically significant.
Results
Table 2 shows the patient and tumor-related characteristics. Seventy-nine patients (87.8%) had complete TURB before NAC. On pathologic specimen, 39 (43.3%) had pCR and 51 (56.7%) had residual disease. Concurrent CIS was detected in 39 patients (43.3%). Multifocal lesions were detected in 19 (21.1%).
Of 39 cystectomy specimens with confirmed pCR, (51.3%) were diagnosed accurately on T2WI with no gross lesions that could be detected. The remaining 49.7% were falsely diagnosed as having residual tumor due to focal bladder wall thickening (mean 10 mm ± 3 mm, range 5–14 mm) at the site of the primary tumor.
The wash-out rate and mean ADC values were both significantly higher in patients having a pCR than those having residual lesions (0.87 min−1 vs. 0.53 min−1) and (1.19 ± 0.16 × 10−3 mm2/s vs 0.84 ± 0.11 × 10−3 mm2/s, respectively (Figs. 2 and 3). There was no statistically significant difference in the TPP and the wash-in rate between both groups (p > 0.1) (Table 3).
A 59-year-old man with MIBC stage T3bNOMO. Pre-NAC MRI images (a, b, c). a Axial T2WI showed polypoid soft tissue mass at the anterior and left anterolateral wall of the UB with interruption of the muscle layer and haziness of the perivesical adipose tissue (arrow). b Axial DWI image showed hyperintense signal, c ADC image showed hypointense signal with ADC of 0.825 × 10−3 mm2/s. Post-NAC MRI images (d–g). d Axial T2WI showing disappearance of the soft tissue mass with noted thickening of the anterior bladder wall. e On DCE-MRI, there is focal enhancement of the anterior bladder wall (arrow); wash out rate was 0.87 min−1. f On DWI at b = 1000, the anterior wall showed focal hyperintense signal. g ADC image showed iso to hypo intense signal with mean ADC value of 1.19 × 10−3 mm2/s. Pathology confirmed chronic inflammation with no residual tumor
A 62-year-old woman with MIBC. Pre-NAC MRI images (a, b, c). a Axial T2WI showing huge polypoid solid mass with lobulated contour involving all UB walls sparing the anterior wall with adipose tissue infiltration. b Axial DWI image showed hyperintense signal, c ADC image showed hypointense signal with ADC of 0.731 × 10−3 mm2/s. Post-NAC MRI images (d–g). d Axial T2WI showed marked reduction of the tumor size with residual soft tissue mass at the left lateral wall with preserved muscle layer. e On DCE-MRI, the mass shows homogenous enhancement, wash out rate was 0.53 min−1. f On DWI at b = 1000, the mass showed hyperintense signal. g ADC image showed mean ADC value of 0.748 × 10−3 mm2/s. Pathology confirmed residual tumor
pCR had negative correlation with the wash-out rate (r = − 0.701, p = 0.01), ADC value (r = − 0.621, p = 0.01) and had no significant correlation with wash-in rate (r = 0.187, p = 0.14), TPP value (r = − 0.211, p = 0.113). Also, the correlation between the tumor ADC value and TPP, wash-in rate, wash-out rate was evaluated. The mean ADC value had positive correlation with wash-out rate (r = 0.631, p = 0.001) and had no significant correlation with TPP, wash-in rate. There was negative correlation between wash-out and wash-in rate (r = − 0.311, p = 0.01) and no significant correlation was found between tumor TPP and wash-in, wash-out rate.
Using the mean ADC cut-off value of 0.911 × 10−3 mm2/s, the sensitivity and specificity for predicting pCR were 91.2%, 89.3% for reader 1, and 90%, 91% for reader 2, respectively. AUC was 0.921 for reader 1, 0.911 for reader 2 (figure 4).
Illustrated the association of ADC values (a) and wash-out rate (b) with the response to NAC. Boxplots demonstrate the distribution of ADC values and wash-out rate. Significant differences in mean ADC and wash-out rate were observed between complete response and non-complete response groups (p < 0.01)
Using cut-off wash-out rate of 0.677 min−1, the sensitivity and specificity for predicting pCR were 89.5%, 75% for reader 1, and 88.4%, 77% for reader 2, respectively. AUC was 0.89.5 for reader 1, 0.88 for reader 2.
The sensitivity, specificity for predicting pCR using ADC value + washout rate cut off values were 95.4%, 97.7% for reader 1, and 96%, 97% for reader 2, respectively. AUC was 0.981 for reader 1, 0.971 for reader 2. (Table 4) (Fig. 5).
The overall reproducibility of the mean ADC value and wash out rate was excellent (ICC = 0.83–0.90). The ICC values for the mean ADC value, washout rate was 0.89 (95% CI 0.84–0.89) and 0.87 (95% CI 0.86–0.91), respectively.
Discussion
The morphologic changes after NAC demonstrated variable manifestations including complete normal appearance, nodular enhancement, or enhancing soft tissue in conjunction with transurethral resection or chemotherapy-induced fibrosis/inflammation [14]. Particularly, pCR can be presented as a completely normal appearance or diffuse bladder wall thickening. Our findings indicate that the diagnostic performance of DCE-MRI and DWI in the assessment of tumor response to NAC in MIBC improved for the combination of wash-out rate and ADC value (AUC 0.971–0.981). Interestingly, we demonstrated high specificity (97–97.7%) and excellent reproducibility for the cut-off values of combined wash-out rate and ADC value in the identification of pCR.
Relevant capabilities of DCE MRI and DWI in the assessment of tumor response to NAC in bladder cancer have been previously described. However, morphological MRI imaging alone has suboptimal capability in the differentiation between viable tumor tissue and postoperative changes, it may also underestimate or overestimate the residual tumor size [21]. These false-positive results can misrecognize a proportion of complete responses and lead to suboptimal clinical management [19].
DWI has been used to differentiate scar tissue from tumor recurrence after bladder cancer resection and it is considered to be an imaging biomarker for cancer [22, 23]. In our study, DWI had 90–91% accuracy in the detection of pCR, which is higher than previously reported (80%) by Yoshida et al. [19]. Their lower accuracy was attributed to the qualitative assessment of diffusion coefficient by comparing the images at 2 different b-values (500 and 1000 s/mm2), instead of quantitative analysis of ADC value. They overcome this problem in their next study evaluating the role of DWI in predicting chemo-radiotherapy (CRT) sensitivity [20]. They found an inverse correlation between ADC values of the tumor and CRT sensitivity in 23 MIBC patients who underwent cystectomy following induction CRT [20]. The CRT-sensitive tumors that showed pCR (median 0.63 × 103 mm2/s; range 0.43–0.77 mm2/s) had significantly lower ADC values compared with CRT-resistant tumors (non-pathological CR: median 0.84 × 103 mm2/s; range, 0.69–1.09 mm2/s; p = 0.0003). The cut-off ADC value of 0.74 × 103 mm2/s had 92% sensitivity, 90% specificity, and 91% accuracy in the prediction of CRT sensitivity [20]. That study concluded that DWI could provide a useful tool for the identification of good responders to NAC in MIBC, and may provide a useful guide for optimization of patient selection for bladder-sparing approaches which are consistent with our findings. Pearson et al. [24] found statistically significant rises in mean ADC values after NAC or CRT and considered it as early indicators of pathological response in bladder tumors [24]. We think DWI is a promising imaging tool and can be added to the imaging protocols to evaluate the response to NAC because it has many advantages such as non-invasive technique, short acquisition time with no ionizing radiation, and can be performed without breath-holding.
DCE-MRI provides perfusion characteristics of the tumors [18]. It depends on the permeability of blood vessels and contrast extravasation into the surrounding tissue. Malignant vessels with leaky endothelia had higher permeability than normal vessels.
Subjective assessment of DCE-MRI in the evaluation of therapeutic response to NAC has been previously reported [19] and yielded low specificity (18%) and accuracy (33%). Dobson et al. [25] reported that DCE-MRI is reliable in the exclusion of residual or recurrence at 4 and 12 months post-radiotherapy with negative predictive values of 100% and 93%, respectively. The sensitivity, specificity, and positive predictive values were 80% and 76%, 50% at 12 months and 100% and 48%, 48% at 4 months, respectively.
The data of DCE-MRI can be analyzed in a semi-quantitative or quantitative manner. Semi-quantitative parameters represented contrast-related signal intensity changes and were derived from a signal intensity–time curve. The commonly used semi-quantitative parameters were the wash-in rate and wash-out rate [26]. Schrier et al. [27] first investigated fast DCE-MRI technique to predict NAC response for locally advanced bladder cancer; they reported 92% accuracy, 91% sensitivity, and 93% specificity. On a series of 21 patients, Donaldson et al. [28] reported that pre-and post-NAC DCE-MRI can be used to differentiate residual tumor from treatment effect. They described that residual tumors had significantly higher perfusion and rSI80s than those with treatment effect. Recently, Chakiba et al. [18] investigated the performance of DCE-MRI parameters including relative enhancement at the arterial (rSI35s) and venous phases (rSI80s) to assess the therapeutic response of NAC on bladder cancer. pCR was demonstrated in (50%, 6/12) patients. They described a statistically significant difference in rSI80s between pCR and non-pCR patients. With a cut-off value of 40%, sensitivity, specificity, and accuracy to identify pCR were 83.33%. That study concluded that DCE-MRI is a useful biomarker for patients with localized bladder cancer and can improve selection before surgery, which is consistent with our findings. Nguyen et al. [29] investigated the role of DCE‐MRI pharmacokinetic parameters (k‐means clustering) in the early prediction of the bladder tumor’s chemotherapeutic response. When they compared the MRI studies (pre-NAC and mid-NAC), they found a significant difference between responders and non-responders. In their next study, DWI with ADC maps was performed before NAC to evaluate tumor heterogeneity and micro cellularity. pCR was identified in (75%, 15/20). Non-responders had significant heterogeneous ADC values which indicated higher cellular dis-homogeneity. Moreover, initial survival data described the worst survival outcomes for those patients who had the most heterogeneous tumors [30].
The correlation between ADC value and wash-out rate in this study could be explained by the relation of malignant tissues to the permeability of blood vessels and abnormal microstructure including dense cellularity and large cellular size. Therefore, the accuracy of identifying pCR using the ADC value and wash-out rate together was improved.
In our study, the wash-out rate can effectively identify MIBC patients with pCR after NAC. We used only a semi-quantitative analysis because we could not adequately estimate the perfusion parameters. Even promising, perfusion MRI on the bladder remains challenging particularly on 1.5 T systems. Recently, Ciolina et al. [31] reported that DCE-MRI perfusion parameters provide a promising role in predicting response to CRT in locally advanced rectal cancer. They described that MRI-derived parameter Ktrans is a reliable indicator of complete response to CRT. MRI perfusion studies on MIBC are needed to evaluate its role in the assessment of response to NAC.
MRI volumetry is increasingly integrated into treatment response evaluation in rectal cancer [32] and cervical cancer [33]. It is accepted as an effective tool for the identification of good responders to neoadjuvant therapy in locally advanced rectal cancer [32]. MRI volumetry studies are warranted for the evaluation of treatment response in MIBC.
Finally, our study showed that combined DWI and DCE-MRI is helpful to identify pCR. It is of great clinical value to provide a useful guide for the optimization of patient selection for CRT-based bladder-sparing approaches. In our study, 8 patients who had pCR after NAC with contraindication to surgery received bladder preservation therapy. Five of them showed no residual lesions on follow-up cystoscopy for 15 months.
Limitations of our study include, the normalized ADC value could be a reasonable index of diffusion environment in MIBC, however the lack of standardization of ADC measurement technique represents an inherent limitation of DWI, and it depends on the coil systems, vendors, and imagers. Thus, the standardization of diffusion assessment is required. However DCE-MRI semi-quantitative analysis provides promising results, it is known to be hampered by individual patient-based factors and results may vary between MRI scanners and radiofrequency coils. Further multicenter studies are needed.
Conclusion
Semi-quantitative parameter (wash-out) derived from DCE-MRI and ADC have the potential to assess the tumor’s complete pathologic response. The two parameters using together can offer the best possibility to identify complete response to NAC in MIBC.
Abbreviations
- MIBC:
-
Muscle-invasive bladder cancer
- NAC:
-
Neoadjuvant chemotherapy
- DCE-MRI:
-
Dynamic contrast-enhanced magnetic resonance imaging
- DWI:
-
Diffusion-weighted image
- pCR:
-
Pathologic complete response
- RC:
-
Radical cystectomy
References
Krause FS, Walter B, Ott OJ, et al. 15-year survival rates after transurethral resection and radiochemotherapy or radiation in bladder cancer treatment. Anticancer Res 2011;31:985e990.
Rodel C, Weiss C, Sauer R. Trimodality treatment and selective organ preservation for bladder cancer. J Clin Oncol 2006;24:5536e5544.
Shipley WU, Kaufman DS, Tester WJ, et al. Overview of bladder cancer trials in the Radiation Therapy Oncology Group. Cancer 2003; 97:2115e2119.
Sandler HM, Mirhadi AJ. Current status of radiation therapy for bladder cancer. Expert Rev Anticancer Ther 2010;10:895e901.
Koga F, Yoshida S, Kawakami S, et al. Low-dose chemoradiotherapy followed by partial or radical cystectomy against muscle-invasive bladder cancer: An intent-to-treat survival analysis. Urology 2008; 72:384e388.
A. Meyer, R. Ghandour, A. Bergman, C. Castaneda, M. Wosnitzer, G. Hruby, M. Benson, J. McKiernan, The natural history of clinically complete responders to neoadjuvant chemotherapy for urothelial carcinoma of the bladder, J. Urol. 192 (2014) 696–701.
D. Robins, J. Matulay, M. Lipsky, A. Meyer, R. Ghandour, G. DeCastro, C. Anderson, C. Drake, M. Benson, J.M. McKiernan, Outcomes following clinical complete response to neoadjuvant chemotherapy for muscle-invasive urothelial carcinoma of the bladder in patients refusing radical cystectomy, Urology 111 (2018) 116–121.
P. Mazza, G.W. Moran, G. Li, D.J. Robins, J.T. Matulay, H.W. Herr, G.J. Decastro, J.M. McKiernan, C.B. Anderson, Conservative management following complete clinical response to neoadjuvant chemotherapy of muscle invasive bladder cancer: contemporary outcomes of a multi-institutional cohort study, J. Urol. 200 (2018) 1005–1013.
A.B. Alexa Meyer, P. Nichols, M. Kates, N. Hahn, Mark Schoenberg, and Trinity Bivalacqua SUO 2017: Inaccuracy of Clinical Staging after Neoadjuvant Chemotherapy for Muscle Invasive Bladder Cancer, (2017).
J.E. Husband, L.H. Schwartz, J. Spencer, L. Ollivier, D.M. King, R. Johnson, R. Reznek, S. International Cancer Imaging, Evaluation of the response to treatment of solid tumours – a consensus statement of the International Cancer Imaging Society, Br. J. Cancer 90 (2004) 2256–2260.
E.A. Eisenhauer, P. Therasse, J. Bogaerts, L.H. Schwartz, D. Sargent, R. Ford, J. Dancey, S. Arbuck, S. Gwyther, M. Mooney, L. Rubinstein, L. Shankar, L. Dodd, R. Kaplan, D. Lacombe, J. Verweij, New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1), Eur. J. Cancer 45 (2009) 228–247.
V. Kundra, P.M. Silverman, Imaging in the diagnosis, staging, and follow-up of cancer of the urinary bladder, Am. J. Roentgenol. 180 (2003) 1045–1054.
P. Ramchandani, H.M. Pollack, Radiology of drug-related genitourinary disease, Semin. Roentgenol. 30 (1995) 77–87.
Choi SJ, Park KJ, Lee G, Kim MH, Kim JK. Urothelial phase CT for assessment of pathologic complete response after neoadjuvant chemotherapy in muscle-invasive bladder cancer. Eur J Radiol. 2020;126:108902.
A. Soubra, M. Gencturk, J. Froelich, P. Balaji, S. Gupta, G. Jha, B.R. Konety, FDGPET/ CT for assessing the response to neoadjuvant chemotherapy in bladder cancer patients, Clin. Genitourin. Cancer 16 (2018) 360–364.
R.J. de Haas, M.J. Steyvers, J.J. Fütterer, Multiparametric MRI of the bladder: ready for clinical routine? Am. J. Roentgenol. 202 (2014) 1187–1195.
S. Verma, A. Rajesh, S.R. Prasad, K. Gaitonde, C.G. Lall, V. Mouraviev, G. Aeron, R.B. Bracken, K. Sandrasegaran, Urinary bladder cancer: role of MR imaging, RadioGraphics 32 (2012) 371–387.
C. Chakiba, F. Cornelis, E. Descat, M. Gross-Goupil, P. Sargos, G. Roubaud, N. Houede, Dynamic contrast enhanced MRI-derived parameters are potential biomarkers of therapeutic response in bladder carcinoma, Eur. J. Radiol. 84 (2015) 1023–1028.
Yoshida S, Koga F, Kawakami S et al. Initial experience of diffusion-weighted magnetic resonance imaging to assess therapeutic response to induction chemoradiotherapy against muscle-invasive bladder cancer. Urology 2010; 75: 387–91.
Yoshida S, Koga F, Kobayashi S et al. Role of diffusion-weighted magnetic resonance imaging in predicting sensitivity to chemoradiotherapy in muscle-invasive bladder cancer. Int. J. Radiat. Oncol. Biol. Phys. 2012; 83: e21–7.
- Wang HJ, Pui MH, Guo Y, Yang D, Pan BT, Zhou XH. Diffusion-weighted MRI in bladder carcinoma: the differentiation between tumor recurrence and benign changes after resection. Abdomen Imaging. 2014; 39:135–141.
Padhani AR, Liu G, Koh DM, et al. (2009) Diffusion-weighted magnetic resonance imaging as a cancer biomarker: consensus and recommendations. Neoplasia 11:102–125
-El-Assmy A, Abou-El-Ghar ME, Refaie HF, Mosbah A, El-Diasty T (2012) Diffusion-weighted magnetic resonance imaging in followup of superficial urinary bladder carcinoma after transurethral resection: initial experience. BJU Int 110:E622–E627
Pearson RA, Thelwall PE, Snell J, et al. Evaluation of early response to neoadjuvant chemotherapy in muscle-invasive bladder cancer using dynamic contrast-enhanced MRI and diffusion-weighted MRI: MARBLE study. J Clin Oncol 2016;34:403.
Dobson MJ, Carrington BM, Collins CD, et. al.: The assessment of irradiated bladder carcinoma using dynamic contrast-enhanced MR imaging. Clin Radiol 2001; 56: pp. 94-98.
Zhou G, Chen X, Zhang J, Zhu J, Zong G, Wang Z. Contrast-enhanced dynamic and diffusion-weighted MR imaging at 3.0T to assess aggressiveness of bladder cancer. Eur J Radiol. 2014;83(11):2013-2018.
Schrier BP, Peters M, Barentsz JO, Witjes JA. Evaluation of chemotherapy withmagnetic resonance imaging in patients with regionally metastatic or unre-sectable bladder cancer. Eur Urol 2006;49(4):698–703.
Donaldson SB, Bonington SC, Kershaw LE, Cowan R, Lyons J, Elliott T, et al.Dynamic contrast-enhanced MRI in patients with muscle-invasive transitionalcell carcinoma of the bladder can distinguish between residual tumour andpost-chemotherapy effect. Eur J Radiol 2013;82(12):2161–8.
Nguyen HT, Jia G, Shah ZK, et al. Prediction of chemotherapeutic response in bladder cancer using K-means clustering of dynamic contrast-enhanced (DCE)-MRI pharmacokinetic parameters. J Magn Reson Imaging. 2015 May;41(5):1374-82.
Nguyen HT, Mortazavi A, Pohar KS, Zynger DL, Wei L, Shah ZK, Jia G, Knopp MV. Quantitative Assessment of Heterogeneity in Bladder Tumor MRI Diffusivity: Can Response be Predicted Prior to Neoadjuvant Chemotherapy? Bladder Cancer. 2017 Oct 27;3(4):237-244.
Ciolina M, Caruso D, De Santis D, et al. (2019) Dynamic contrast-enhanced magnetic resonance imaging in locally advanced rectal cancer: role of perfusion parameters in the assessment of response to treatment. Radiol med 124:331–338
Seierstad T, Hole KH, Grøholt KK, et al. MRI volumetry for prediction of tumour response to neoadjuvant chemotherapy followed by chemoradiotherapy in locally advanced rectal cancer. Br J Radiol. 2015;88(1051):20150097.
Wang JZ, Mayr NA, Zhang D, Li K, Grecula JC, Montebello JF, et al. . Sequential magnetic resonance imaging of cervical cancer: the predictive value of absolute tumour volume and regression ratio measured before, during, and after radiation therapy. Cancer 2010; 116: 5093–101.
Disclosure
The scientific guarantor of this publication is DR: Shimaa Abdalla Ahmed. The authors of this manuscript declare no relationships with any companies, whose products or services may be related to the subject matter of the article.
Funding
No disclosure of funding received for this work from any organization.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
Institutional Review Board approval was obtained.
Informed consent
Written informed consent was obtained from all patients participating in the study.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Ahmed, S.A., Taher, M.G.A., Ali, W.A. et al. Diagnostic performance of contrast-enhanced dynamic and diffusion-weighted MR imaging in the assessment of tumor response to neoadjuvant therapy in muscle-invasive bladder cancer. Abdom Radiol 46, 2712–2721 (2021). https://doi.org/10.1007/s00261-021-02963-7
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00261-021-02963-7