Abstract
Multiple myeloma represents a subset of plasma cell dyscrasias characterized by the proliferation of plasma cells typically in the bone marrow, representing approximately 1% of all cancers and 15% of hematologic malignancies. Often multiple myeloma is limited to the skeletal system; however, a small percentage (<5%) of patients will develop extraosseous manifestations. We review the current WHO classification of plasma cell dyscrasias and use multimodality imaging including US, CT, MRI, and PET-CT to illustrate the spectrum of extraosseous multiple myeloma in the abdomen and pelvis. Because extraosseous multiple myeloma is associated with a poorer prognosis and decreased survival, it is important for the radiologist to become familiar with a variety of extraosseous manifestations in the abdomen and pelvis, especially in a patient with a known diagnosis of multiple myeloma and the development of an abdominal or pelvic mass.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Multiple myeloma is a monoclonal gammopathy characterized by the proliferation of plasma cells typically in the bone marrow. It is a common malignancy in patients over the age of 40. Median age of patients at the time of diagnosis is approximately 65 years. It accounts for 1% of all malignancies and approximately 10% of all hematological disease. More than 20,000 new cases are diagnosed annually in the United States [1].
Patients most commonly present with bone pain, anemia, renal failure, hypercalcemia, and recurrent infections. Extraosseous manifestations of multiple myeloma point to the presence of myeloma outside the skeletal system. Classically this has been considered rare and thought to be found in <5% of patients. However, with longer patient survival and more sensitive imaging modalities, the incidence of extraosseous multiple myeloma may be increasing. Still, radiographic descriptions of extraosseous manifestations of multiple myeloma are limited. We review the spectrum of extraosseous myeloma in the abdomen and pelvis utilizing multimodality imaging.
Discussion
Pathologic classification
Plasma cell neoplasms are characterized by clonal proliferation of plasma cells in the bone marrow. The current World Health Organization (WHO) classification places plasma cell neoplasms under the broad category of mature B-cell neoplasms. Subcategories of mature B-cell neoplasms include monoclonal gammopathy of unknown significance (MGUS), Waldenstrom macroglobulinemia (WM), solitary plasmacytoma (SP), multiple myeloma (MM), extraosseous multiple myeloma (EOMM), and monoclonal immunoglobulin deposition diseases [2]. Diagnosis and classification predominantly rely on patients’ symptoms and laboratory values. Commonly utilized laboratory values include total serum protein, serum albumin, serum and urine protein electrophoresis, quantitative immunoglobulins, immunofixation in serum and urine, B2-microglobulin, and detection of immunoglobulin free light chains [3]. In addition, patients undergo bone marrow aspirate and biopsy to determine the percentage of plasma cells and for prognostic studies [3].
MGUS is the most common type of plasma cell dyscrasia and is seen in more than 3% of the general population aged 50 years or older [4]. The clinical significance of MGUS is that it is considered premalignant and a precursor to MM with an overall 1% per year risk of progression to MM. Imaging in patients with MGUS is not routinely used, although it may be considered for those patients with higher risk or if there is clinical concern [5].
WM is a distinct subtype of plasma cell dyscrasia characterized by lymphoplasmacytic lymphoma in the bone marrow with an associated IgM monoclonal gammopathy [3]. WM is rare with an incidence of approximately 3 per million people per year [6]. Clinical and radiologic features are similar to lymphoma with bulky lymphadenopathy and hepatosplenomegaly [3].
Asymptomatic smoldering myeloma (SMM) refers to a stage of myeloma, which is often a precursor to the development of multiple myeloma. There is an increased circulating M protein greater than 3g/dL and/or greater than 10% but less than 60% bone marrow clonal plasma cells [3]. It is an intermediate stage between MGUS and symptomatic MM. Most patients with SMM progress to symptomatic myeloma; however, these patients can remain stable over long periods of time. It is important for these patients to be imaged regularly to determine the presence of any bone lesions to look for disease progression [3].
Solitary plasmacytoma is defined as proliferation of clonal plasma cells without evidence of significant bone marrow plasma cell infiltration. Two separate entities have been described: solitary plasmacytoma of bone (SPB) and solitary extramedullary plasmacytoma. The distinction between solitary extramedullary plasmacytoma and extraosseous multiple myeloma is based on radiological and laboratory findings. In solitary plasmacytoma, no M protein is found in the serum and/or urine. The bone marrow biopsy is not consistent with MM. Myeloma-related organ or tissue impairment is not seen in solitary plasmacytoma. In solitary plasmacytoma, there are no abnormalities such as increased calcium levels, increased creatinine levels, or decreased hemoglobin [3]. SPB constitutes approximately 5% of cases of plasma cell myeloma [7]. A little more than half of patients with SPB progress to MM [8].
MM accounts for 1.3% of all malignancies and 15% of hematologic cancers with men more frequently affected than women and increased incidence in Africans and African Americans compared to whites [9]. It is almost always preceded by MGUS or SMM. The definition of MM is clonal bone marrow plasma cells greater than or equal to 10% or biopsy proven bony or extramedullary plasmacytoma. Myeloma bone disease is defined as osteolytic lesions or presence of osteoporosis with compression fractures attributable to clonal plasma cell disorder [10].
Extraosseous multiple myeloma
Extraosseous multiple myeloma (EOMM) is the presence of myeloma deposits outside the skeletal system. Although considered rare, multiple autopsy series have demonstrated the presence of extraosseous malignancies in approximately 63.5% of myeloma patients with lymph nodes, pleura, and the liver most commonly involved [11]. In a study of 1003 consecutive patients, there was a suggestion that clinical or radiological extraosseous disease is seen in almost 13% of myeloma patients [12]. The study concluded the use of sensitive imaging techniques such as MRI and CT may partially explain this finding. It also theorized the use of high dose therapy and novel therapies with better supportive care led to significant improvement of survival leading to increased detection of extraosseous disease [12]. It is reported that there is an increased incidence of extraosseous manifestations after autologous or allogenic stem cell transplantation [12]. It is theorized that extraosseous locations act as sanctuary sites that are not successfully treated by the stem cell transplantation and are the source of the higher rate of extraosseous recurrence in these patients [13].
The clinical significance of EOMM is the association with poorer survival rates and prognosis. It is associated with shorter overall and progression free survival [12]. The median survival after relapse of medullary myeloma with subsequent development of extraosseous disease in the course of MM in a single study was shown to be only 38 days [14]. Response to conventional treatment therapies are found to be poor in extraosseous multiple myeloma. Therefore, it has become important for involved physicians and radiologists to be familiar with extraosseous manifestations of multiple myeloma as it significantly affects patients’ outcomes and treatment [15].
With the advancements made in cross sectional imaging (CT/MRI) and increased availability of functional imaging such as FDG PET, imaging has become an integral component of staging multiple myeloma. Cross sectional and functional imaging has contributed to the increased detection of EOMM [15]. Ultrasound and CT fluoroscopy are useful in percutaneous tissue biopsy of suspected sites of extraosseous disease [15].
Extraosseous multiple myeloma pathology appearance
On hematoxylin and eosin stain (H&E stain), EOMM has a characteristic appearance. Tissues are diffusely infiltrated by plasma cells which appear as ovoid cells with abundant deep blue cytoplasm. These plasma cells have characteristic appearance on H&E stain which differentiates them from B-lymphocytes. Plasma cells have eccentric nuclei and exhibit perinuclear clearing which appears on H&E stain as a halo surrounding the nucleus or a clearing corresponding to the Golgi apparatus [16]. All plasma cells regardless of abnormalities are detectable by their expression of certain antigens on the cytoplasmic membrane. CD38 and CD138; also known as syndecan-1 are expressed by plasma cells and CD20 and CD19 antigens are not [17]. CD138 and CD38 are commonly used to detect plasma cells with these antigens on the cell membrane characteristically staining as a brown rim and are present in nearly all cases [17]. Abnormal plasma cells as seen in primary extraosseous plasmacytoma have abnormal weak expression of CD56, which is not seen in extraosseous myeloma [18].
Abdominal and pelvic extraosseous manifestations of multiple myeloma
Extraosseous multiple myeloma frequently affects the solid organs of the upper abdomen. Common affected sites include the liver, kidney, pancreas, and the lymph nodes with less common involvement of the bowel and gonads.
Liver
EOMM can affect any organ in the abdomen and pelvis; however, the liver is most frequently involved according to multiple autopsy series [11, 19]. The reported incidence of hepatic involvement is in the range of 28–30% in autopsy studies [11]. The most common pattern of involvement per multiple autopsy studies is diffuse plasma cell infiltration with nodular involvement being less common. Symptomatology varies from asymptomatic to hepatomegaly, jaundice, and ascites to fulminant liver failure.
By imaging, hepatic involvement may be diffuse, unifocal, or multifocal. Diffuse involvement can be due to innumerable lesions or occasionally be organ infiltration (Fig. 1). Multifocal pattern of involvement usually presents with hepatomegaly with innumerable small low attenuating lesions which is the appearance we have most commonly seen [15, 20]. Focal pattern of involvement on ultrasound has been noted to be hypoechoic or target/bullseye in character. On CT, focal lesions are generally described as low attenuating without calcification or significant contrast enhancement [21]. On MRI, focal lesions may be hyper- or hypointense on T1-weighted images and hyperintense on T2-weighted images with minimal gadolinium enhancement [22]. In our experience, when multiphasic post-contrast imaging is performed with either CT or MRI, myelomatous lesions in the liver are commonly hypervascular on late arterial phase imaging and low attenuating on venous phase images (Fig. 2). EOMM in the liver is commonly hypermetabolic on 18-FDG PET-CT (Fig. 3).
A 68-year-old female presented with weight loss and new onset jaundice. MRI was performed as part of the workup. Axial MR T2 fat-saturated (a), T1 fat-saturated (b), T1 fat-saturated late arterial phase post-contrast (c), and T1 fat-saturated venous phase post-contrast (d) of the liver demonstrate infiltrative perivascular soft tissue pattern (white arrows) with increased T2 signal, decreased T1 signal, mild arterial phase hyperenhancement, and mild venous phase washout. A CT fluoroscopic guided core biopsy of the periportal soft tissue revealed plasma cell neoplasm. Subsequent bone marrow biopsy confirmed multiple myeloma
An 87-year-old male presents with history of diffuse osseous multiple myeloma who underwent routine surveillance imaging. Axial contrast-enhanced portal venous phase CT of the liver (a) demonstrates small scattered hypoattenuating masses. Subsequent, multiphasic MRI including T1 MR post-contrast arterial phase (b) and T1 MR post-contrast venous phase images (c) demonstrate hyper-enhancing nodules with washout on portal venous phase images (white arrows). A CT fluoroscopic guided core liver mass biopsy was performed, yielding metastatic myeloma. H&E stain at 200 magnification (d) of the liver biopsy shows complete replacement of liver parenchyma by numerous deep blue staining plasma cells
A 79-year-old male with a long-standing history of multiple myeloma, recurrence after two prior stem cell transplants and a history of multiple extraosseous plasmacytomas presented with new liver lesions on PET-CT. Axial (a) and coronal (b) fused 18-FDG PET-CT images demonstrate multiple hypermetabolic foci that were not biopsied and treated presumptively as hepatic myeloma (white arrows)
Spleen
Splenic involvement is seen in almost 30–45% of patients on autopsy [15]. It is most commonly seen in MM patients with splenomegaly and affects approximately two thirds of these patients [11, 19]. It is usually seen in the association of hepatic involvement. The literature on splenic involvement in MM is sparse. The most common imaging finding of splenic involvement is enlargement with diffuse infiltration and less commonly focal masses (Fig. 4) [15].
A 62-year-old female with new diagnosis of multiple myeloma confirmed by marrow biopsy with 100% replacement of the marrow by plasma cell myeloma demonstrates non-specific hepatic and splenic enlargement on non-contrast axial (a) and coronal (b) CT of the abdomen (white arrows). The patient expired several weeks later, and autopsy confirmed diffuse hepatic and splenic involvement by multiple myeloma
Renal
Renal involvement is estimated to occur in 10–30% according to autopsy reports with it being the most common site after the reticuloendothelial system [11, 19]. Symptoms of renal involvement are usually asymptomatic or may present with vague flank pain [22]. However, involvement of kidneys has been infrequently reported on imaging. Renal extraosseous myeloma presents as renal and/or perirenal masses that may be unilateral or bilateral. Limited literature demonstrate that the most common imaging finding of renal involvement of MM is a non-specific mass arising from the kidney, perirenal space, or retroperitoneum [20]. Less commonly when these masses become large, retroperitoneal masses may encase vessels and have a similar appearance to lymphoma [20]. In our experience, we have more commonly encountered infiltrative retroperitoneal masses encasing the kidney. On ultrasound renal myelomatous lesions are hypoechoic to anechoic with increased blood flow to the lesion on Doppler [15, 23] (Fig. 5). On CT, the masses are usually homogeneous and may show moderate to intense contrast enhancement [15, 22]. On MR, the masses have been described to demonstrate heterogeneous signal intensity on T2-weighted images with focal areas of high signal (Fig. 6) [20].
A 64-year-old female with a history of multiple myeloma presented to the emergency room with a history of hematuria and difficulty urinating. CT Urogram reveals an infiltrating mass in the left renal hilum extending into the retroperitoneum and a large left sacral mass extending toward the left bladder trigone (not shown). Axial contrast-enhanced nephrogram phase CT (a) shows a large infiltrating soft tissue mass centered in the left renal sinus which demonstrates mild enhancement (white arrow). Axial excretory phase CT (b) demonstrates compression of the renal collecting system by the mass without significant obstruction. Transverse US (c) on the same patient shows a hypoechoic renal sinus mass (white arrow) with loss of tissue planes with the parenchyma and mild hydronephrosis. Sacral and renal mass image-guided core biopsies were consistent with plasma cell myeloma. H&E stain at 200 magnification (d) of the renal biopsy shows numerous plasma cells replacing normal structures. The patient received initial treatment with nephrostomy
A 65-year-old female with a history of multiple myeloma and biopsy-proven urinary bladder extraosseous myeloma presented for suspected retroperitoneal bleed versus mass. Axial MR images: T2 fat-saturated (a), diffusion-weighted imaging b=600 (b), T1 pre-contrast, (c) and T1 fat-saturated post-contrast (d) through the kidneys show extensive abnormal left sided renal/perirenal soft tissue infiltrating much of the left retroperitoneum (white arrows) and extending across the midline toward the right renal hilum with vascular encasement. This abnormal soft tissue demonstrates mildly increased T2 signal with restricted diffusion and avid enhancement on post-gadolinium images. Image-guided core biopsy revealed plasma cell myeloma
Adrenal
Adrenal involvement of multiple myeloma is rare and mostly limited to case reports. Generally, patients are asymptomatic as majority of the reported cases were non-functioning tumors that were found incidentally on imaging [24, 25]. The most common imaging findings are heterogeneous, well-circumscribed, unilateral soft tissue density adrenal lesions (Fig. 7) [26]. Less commonly, there is bilateral adrenal involvement which has been reported in a case study by Li et al [27]. Larger lesions have been described to demonstrate central necrosis and heterogeneous enhancement [20]. MRI findings demonstrate heterogeneous signal intensity on both T1- and T2- weighted sequence with heterogeneous enhancement [20]. Unlike adrenal adenomas, these lesions do not demonstrate signal dropout on opposed phase imaging [21].
A 68-year-old female with longstanding history of osseous multiple myeloma and prior failed auto stem cell transplant had a CT Thorax for evaluation of pneumonia with new incidentally noted liver and adrenal lesions (not shown). These were subsequently evaluated with abdominal MR and PET-CT. Axial MR T2 fat-saturated image (a) and T1 fat-saturated image (b) demonstrate a T2 hyperintense and T1 isointense to muscle right adrenal mass (white arrow) which demonstrates avid enhancement on axial MR T1 fat-saturated post-contrast image (c). Axial fused 18-FDG PET-CT (d) shows intense hypermetabolism in the right adrenal mass (white arrow). Multiple hypermetabolic osseous lesions, liver lesions, and retroperitoneal masses were also present on the PET-CT (not shown). Repeat bone marrow aspirate confirmed recurrent plasma cell myeloma. Treatment was initiated and the adrenal mass and other abnormalities resolved on follow-up imaging (not shown)
Pancreas
Many autopsy studies demonstrate extraosseous involvement in the pancreas that range between 4 and 17% [11, 19]. Most patients are asymptomatic, but rarely may cause symptomatic obstructive jaundice when located in the pancreatic head (Fig. 8). Pancreatic involvement most commonly present as solitary well-defined soft tissue masses with rare reports of multifocal masses or diffuse involvement [20]. On ultrasound, lesions are heterogeneous, hypoechoic and may cause biliary obstruction when located in the pancreatic head [15]. On multiphase CT or MR, pancreatic masses have demonstrated avid enhancement on arterial phase, thus they have often been mistaken as neuroendocrine tumors of the pancreas [28]. Further MRI characteristics demonstrates these masses as hypointense to isointense on T1-weighted images and hyperintense on T2-weighted images relative to pancreatic parenchyma (Fig. 9) [29].
An 82-year-old male with a prior history of multiple myeloma presented with nausea, vomiting, weakness, and jaundice. Axial (a) and coronal (b) contrast-enhanced venous phase CT images of the upper abdomen show an enhancing ampullary mass (white arrows) causing dilation of the common bile duct and pancreatic duct (dashed arrows). Axial MRI T1 fat-saturated post-contrast (c) and coronal T2 image (d) through the pancreatic head demonstrate T2 hypointense and avidly enhancing ampullary mass extending into the pancreas (white arrows) with common duct dilatation (dashed arrows). Esophagogastroduodenoscopy, sphincterotomy with endoscopic ultrasound-guided biopsy was consistent with plasmacytoma
A 63-year-old female with a history of multiple myeloma, status post bone marrow transplant presented with dizziness and obstructive jaundice and disease progression based on bone marrow aspirate. Coronal T2 (a) and axial T1 post-contrast (b) images demonstrate a large enhancing peripancreatic mass that encases the distal common bile duct and portal splenic confluence (white arrows). Incidental note of gallstones. Endoscopic US biopsy was non-diagnostic. Patient entered hospice care and expired shortly afterwards
Lymph nodes
Tumor involvement of lymph nodes have been seen in 5–23% on autopsy studies [11, 19]. The most common sites of lymph node involvement were paratracheal, parasplenic, and supraclavicular nodes [11]. Imaging findings of lymph node involvement are enlarged discrete or conglomerate lymph nodes involving multiple nodal stations that enhance after contrast [28]. These are frequently T2 hyperintense similar to pathologic lymph nodes in other neoplasms but subtle low T2 signal have been reported on MR imaging [15]. Involved lymph nodes are hypermetabolic on 18-FDG PET-CT (Fig. 10).
A 68-year-old female presented with weight loss and jaundice. Axial contrast-enhanced venous phase CT (a) demonstrates mildly enlarged retroperitoneal lymph nodes (white arrow). On MRI, the lymph nodes (white arrows) are moderately hyperintense on axial T2 fat-saturated image (b), restrict diffusion on diffusion-weighted image b=600 (c) and moderately enhanced on post-contrast T1 fat-saturated image (d). The MR of the abdomen also showed hepatic involvement (Fig. 1). Myeloma was confirmed with a liver biopsy and bone marrow aspirate
Gastrointestinal
In order of most to least common site of myelomatous gastrointestinal involvement is the small bowel, stomach, colon, and esophagus. In autopsy series, fewer than 5% of patients have gastrointestinal tract involvement [11, 19]. Clinical symptoms, if present, are mainly dependent on the size of the lesion that can cause abdominal pain, obstruction, intussusception, bleeding, fistula formation, and perforation [30].
The most common imaging appearance that has been reported is a large mass with mural infiltration and wall thickening without causing significant luminal obstruction that mimics the appearance of lymphoma [15, 20] (Figs. 11,12). Less commonly, involvement of the bowel can appear as homogeneous soft tissue masses, which may be eccentric in location, lobulated, mural or intraluminal [13]. In our experience, we have most commonly encountered non-obstructing circumferential mural infiltration.
A 77-year-old female presented with a 12-pound weight loss and abdominal pain underwent a CT scan of the abdomen and pelvis. Axial (a) and coronal (b) contrast-enhanced venous phase CT images illustrate a soft tissue mass along the wall of the 1st and 2nd portion of duodenum with luminal narrowing (white arrows). Incidental note of gallstones. Esophagogastroduodenoscopy and biopsy following the CT was consistent with duodenal plasmacytoma. H&E stain at 200 magnification (c) of the duodenal biopsy shows expansion of the lamina propria with monotonous proliferation of numerous plasma cells. CD138 stain (d) has strongly positive membranous staining manifested as brown rim surrounding the cells (white arrow). Follow-up bone marrow biopsy revealed multiple myeloma
A 69-year-old female with a remote history of MGUS and subsequent diagnosis of multiple myeloma presented with abdominal pain and an upper gastrointestinal bleed. Axial (a) and coronal (b) contrast-enhanced venous phase CT images of the pelvis show two adjacent segments of small bowel wall thickening (white arrows) in the right lower quadrant without obstruction. Subsequent axial (c) and coronal (d) fused 18-FDG PET-CT images show hypermetabolic activity in the thickened small bowel loops (white arrows) in addition to metabolic abnormalities in the mediastinum and ribs (not shown). Incidental note of calcified uterine fibroids. CT-guided core biopsy of small bowel was consistent with plasma cell neoplasm
Testicular
Testicular involvement is extremely rare even on autopsy reports. Typically, it presents as painless unilateral or bilateral testicular swelling late in the course of the disease and is associated with a poor prognosis [31]. The most reported ultrasound findings of testicular involvement are diffuse enlargement then less commonly solitary or multiple testicular lesions [13, 28, 32]. Imaging findings can mimic other pathology such as lymphoma or sarcoidosis. In a few cases, diffuse enlargement or solitary or multiple hypoechoic lesions are seen on ultrasound (Fig. 13) [28, 32]. MR imaging of a reported case of testicular involvement showed enlarged testes with abnormal heterogeneous signal intensity [28].
49-year-old male with known multiple myeloma, presented with acute mental status changes, extensive central nervous system involvement including brain lesions and diffuse leptomeningeal spread, diagnosed by positive cerebral spinal fluid. Testicular US performed for testicular swelling. Grayscale US images of the right (a) and left (b) testes show enlarged, heterogeneous bilateral testes with multiple hypoechoic masses. These were clinically treated as myeloma given the disease burden elsewhere
Ovarian
Ovarian involvement by myeloma is quite rare presenting as a solid ovarian mass indistinguishable from an ovarian primary neoplasm (Fig. 14). The literature on plasma cell disorders involving the ovary is sparse and primarily consists of case reports of ovarian plasmacytoma and a single case of bilateral ovarian involvement in the setting of disseminated myeloma [33,34,35,36,37]. In the myeloma patient, the masses were relatively large measuring greater than 10 cm in diameter [36]. A similarly large size has been seen in the ovarian plasmacytoma patients [35]. There are limited descriptions of the imaging appearance but ovarian plasmacytomas have been described as being metabolic on 18-FDG PET-CT and large and heterogenous on CT scan [33, 37].
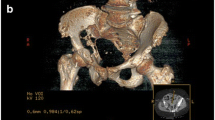
An 84-year-old female presented with several week history of weight loss and fatigue and acutely developed right leg swelling. US revealed a right lower extremity deep venous thrombosis. Subsequent contrast-enhanced CT scan of the abdomen and pelvis in venous phase axial (a) and coronal (b) images show a large, heterogeneously enhancing soft tissue mass in the right pelvis, involving the right ovary (white arrows). Patient underwent open resection. The ovarian mass was a plasmacytoma. Gross oophorectomy specimen (c) shows a 14 x 12 x 7.5 cm multicystic mass replacing the ovary. H&E stain (d) shows sheets of monotonously appearing plasma cells which efface the right ovary parenchyma. Bone marrow aspirate was negative for multiple myeloma
Subcutaneous/muscular
Myelomatous deposits may occur in the subcutaneous tissues or within the muscles. The skin is a frequent site that may manifest as subcutaneous palpable masses with a purplish hue with or without purpura [28]. Imaging findings usually demonstrate well-defined subcutaneous nodules/masses but may also be diffusely infiltrating lesions within the subcutaneous tissues/muscles that may infiltrate into adjacent organ structures (Fig. 15).
A 61-year-old female with a history of multiple myeloma presented with a 2-month history of a palpable subcutaneous abdominal masses. Axial fused 18-FDG PET-CT (a) and corresponding non-contrast axial CT images (b) through the lower abdomen show hypermetabolism within numerous infiltrative and nodular masses within the subcutaneous tissues of the abdominal wall. Open surgical biopsy was consistent with soft tissue myeloma
Conclusion
Multiple myeloma is an uncommon malignancy, primarily affecting the bone marrow. A small portion of these patients will develop extraosseous manifestations of their disease, usually in the form of an extraosseous mass. This is associated with a poorer prognosis and shorter survival time. The most common locations of extraosseous multiple myeloma include the liver, pancreas, mesentery, and retroperitoneum [20]. The imaging findings are variable, but generally consist of enhancing soft tissue masses [13]. It should be considered in the differential diagnosis when confronted with a patient with a history of MM presenting with an intra-abdominal mass. If the myeloma patient is presenting after stem cell transplantation the suspicion for extraosseous myeloma should be increased. Familiarity with the wide array of imaging appearances of extraosseous multiple myeloma in the abdomen and pelvis is useful when the radiologist is confronted with an unknown mass in the multiple myeloma patient.
References
Jemal A, Siegel R, Ward E, Hao Y, Xu J, Thun MJ (2009) Cancer statistics, 2009. CA Cancer J Clin 59 (4):225-249. https://doi.org/10.3322/caac.20006
Swerdlow SH (2008) WHO Classification of Tumours of Haematopoietic and Lymphoid Tissues. vol 2, 4th edn. IARC Press,
Amini B, Yellapragada S, Shah S, Rohren E, Vikram R (2016) State-of-the-Art Imaging and Staging of Plasma Cell Dyscrasias. Radiol Clin North Am 54 (3):581-596. https://doi.org/10.1016/j.rcl.2015.12.008
Kyle RA, Therneau TM, Rajkumar SV, Larson DR, Plevak MF, Offord JR, Dispenzieri A, Katzmann JA, Melton LJ, 3rd (2006) Prevalence of monoclonal gammopathy of undetermined significance. N Engl J Med 354 (13):1362-1369. https://doi.org/10.1056/nejmoa054494
Mangiacavalli S, Cocito F, Pochintesta L, Pascutto C, Ferretti V, Varettoni M, Zappasodi P, Pompa A, Landini B, Cazzola M, Corso A (2013) Monoclonal gammopathy of undetermined significance: a new proposal of workup. Eur J Haematol 91 (4):356-360. https://doi.org/10.1111/ejh.12172
Garcia-Sanz R, Montoto S, Torrequebrada A, de Coca AG, Petit J, Sureda A, Rodriguez-Garcia JA, Masso P, Perez-Aliaga A, Monteagudo MD, Navarro I, Moreno G, Toledo C, Alonso A, Besses C, Besalduch J, Jarque I, Salama P, Rivas JA, Navarro B, Blade J, Miguel JF, Spanish Group for the Study of Waldenstrom M, Pethema (2001) Waldenstrom macroglobulinaemia: presenting features and outcome in a series with 217 cases. Br J Haematol 115 (3):575-582. https://doi.org/10.1046/j.1365-2141.2001.03144.x
Knowling MA, Harwood AR, Bergsagel DE (1983) Comparison of extramedullary plasmacytomas with solitary and multiple plasma cell tumors of bone. J Clin Oncol 1 (4):255-262. https://doi.org/10.1200/jco.1983.1.4.255
Frassica DA, Frassica FJ, Schray MF, Sim FH, Kyle RA (1989) Solitary plasmacytoma of bone: Mayo Clinic experience. Int J Radiat Oncol Biol Phys 16 (1):43-48. https://doi.org/10.1016/0360-3016(89)90008-4
Siegel RL, Miller KD, Jemal A (2015) Cancer statistics, 2015. CA Cancer J Clin 65 (1):5-29. https://doi.org/10.3322/caac.21254
Kyle RA, Rajkumar SV (2009) Criteria for diagnosis, staging, risk stratification and response assessment of multiple myeloma. Leukemia 23 (1):3-9. https://doi.org/10.1038/leu.2008.291
Oshima K, Kanda Y, Nannya Y, Kaneko M, Hamaki T, Suguro M, Yamamoto R, Chizuka A, Matsuyama T, Takezako N, Miwa A, Togawa A, Niino H, Nasu M, Saito K, Morita T (2001) Clinical and pathologic findings in 52 consecutively autopsied cases with multiple myeloma. Am J Hematol 67 (1):1-5. https://doi.org/10.1002/ajh.1067
Varettoni M, Corso A, Pica G, Mangiacavalli S, Pascutto C, Lazzarino M (2010) Incidence, presenting features and outcome of extramedullary disease in multiple myeloma: a longitudinal study on 1003 consecutive patients. Ann Oncol 21 (2):325-330. https://doi.org/10.1093/annonc/mdp329
Hall MN, Jagannathan JP, Ramaiya NH, Shinagare AB, Van den Abbeele AD (2010) Imaging of extraosseous myeloma: CT, PET/CT, and MRI features. AJR Am J Roentgenol 195 (5):1057-1065. https://doi.org/10.2214/ajr.10.4384
Cerny J, Fadare O, Hutchinson L, Wang SA (2008) Clinicopathological features of extramedullary recurrence/relapse of multiple myeloma. Eur J Haematol 81 (1):65-69. https://doi.org/10.1111/j.1600-0609.2008.01087.x
Philips S, Menias C, Vikram R, Sunnapwar A, Prasad SR (2012) Abdominal manifestations of extraosseous myeloma: cross-sectional imaging spectrum. J Comput Assist Tomogr 36 (2):207-212. https://doi.org/10.1097/rct.0b013e318245c261
Bortnick A, Murre C (2016) Cellular and chromatin dynamics of antibody-secreting plasma cells. Wiley Interdiscip Rev Dev Biol 5 (2):136-149. https://doi.org/10.1002/wdev.213
Bataille R, Jego G, Robillard N, Barille-Nion S, Harousseau JL, Moreau P, Amiot M, Pellat-Deceunynck C (2006) The phenotype of normal, reactive and malignant plasma cells. Identification of “many and multiple myelomas” and of new targets for myeloma therapy. Haematologica 91 (9):1234-1240
Pellat-Deceunynck C, Barille S, Puthier D, Rapp MJ, Harousseau JL, Bataille R, Amiot M (1995) Adhesion molecules on human myeloma cells: significant changes in expression related to malignancy, tumor spreading, and immortalization. Cancer Res 55 (16):3647-3653
Kapadia SB (1980) Multiple myeloma: a clinicopathologic study of 62 consecutively autopsied cases. Medicine (Baltimore) 59 (5):380-392
Sedlic A, Chingkoe C, Lee KW, Duddalwar VA, Chang SD (2014) Abdominal extraosseous lesions of multiple myeloma: imaging findings. Can Assoc Radiol J 65 (1):2-8. https://doi.org/10.1016/j.carj.2011.12.010
Ooi GC, Chim JC, Au WY, Khong PL (2006) Radiologic manifestations of primary solitary extramedullary and multiple solitary plasmacytomas. AJR Am J Roentgenol 186 (3):821-827. https://doi.org/10.2214/ajr.04.1787
Monill J, Pernas J, Montserrat E, Perez C, Clavero J, Martinez-Noguera A, Guerrero R, Torrubia S (2005) CT features of abdominal plasma cell neoplasms. Eur Radiol 15 (8):1705-1712. https://doi.org/10.1007/s00330-005-2642-z
Birjawi GA, Jalbout R, Musallam KM, Tawil AN, Taher AT, Khoury NJ (2008) Abdominal manifestations of multiple myeloma: a retrospective radiologic overview. Clin Lymphoma Myeloma 8 (6):348-351. https://doi.org/10.3816/clm.2008.n.050
Kahara T, Nagai Y, Yamashita H, Nohara E, Kobayashi K, Takamura T (2001) Extramedullary plasmacytoma in the adrenal incidentaloma. Clin Endocrinol (Oxf) 55 (2):267-270. https://doi.org/10.1046/j.1365-2265.2001.01191.x
Rogers CG, Pinto PA, Weir EG (2004) Extraosseous (extramedullary) plasmacytoma of the adrenal gland. Arch Pathol Lab Med 128 (7):e86-88. https://doi.org/10.1043/1543-2165(2004)128%3ce86:eepota%3e2.0.co;2
Naymagon L, Abdul-Hay M (2019) Primary extramedullary plasmacytoma with diffuse lymph node involvement: a case report and review of the literature. J Med Case Rep 13 (1):153. https://doi.org/10.1186/s13256-019-2087-7
Li Y, Guo YK, Yang ZG, Ma ES, Min PQ (2007) Extramedullary plasmacytoma involving the bilateral adrenal glands on MR imaging. Korean J Radiol 8 (3):246-248. https://doi.org/10.3348/kjr.2007.8.3.246
Moulopoulos LA, Granfield CA, Dimopoulos MA, Kim EE, Alexanian R, Libshitz HI (1993) Extraosseous multiple myeloma: imaging features. AJR Am J Roentgenol 161 (5):1083-1087. https://doi.org/10.2214/ajr.161.5.8273615
Kazama T, Ng CS, Giralt SA (2005) Multiphasic CT and MRI appearances of extramedullary multiple myeloma involving the stomach, pancreas, and bladder. Clin Imaging 29 (4):263-265. https://doi.org/10.1016/j.clinimag.2004.11.002
Carlson HC, Breen JF (1986) Amyloidosis and plasma cell dyscrasias: gastrointestinal involvement. Semin Roentgenol 21 (2):128-138. https://doi.org/10.1016/0037-198x(86)90029-5
Garrido Abad P, Coloma Del Peso A, Bocardo Fajardo G, Jimenez Galvez M, Herranz Fernandez LM, Arellano Ganan R, Pereira Sanz I, Reina Duran T (2008) [Secondary bilateral testicular plasmacytoma. Case report and review of the literature]. Actas Urol Esp 32 (10):1039-1042. https://doi.org/10.1016/s0210-4806(08)73986-x
Patlas M, Khalili K, Dill-Macky MJ, Wilson SR (2004) Spectrum of imaging findings in abdominal extraosseous myeloma. AJR Am J Roentgenol 183 (4):929-932. https://doi.org/10.2214/ajr.183.4.1830929
Kazeminezhad B, Zare-Mirzaie A, Mirafsharieh A, Soleimantabar H, Zahedifard S (2013) Bilateral ovarian involvement: a rare presentation of disseminated multiple myeloma. J Obstet Gynaecol Res 39 (1):446-449. https://doi.org/10.1111/j.1447-0756.2012.01972.x
Zhong YP, Zhang JJ, Huang XN (2012) Multiple myeloma with rupture of ovarian plasmacytoma. Chin Med J (Engl) 125 (16):2948-2950
Emery JD, Kennedy AW, Tubbs RR, Castellani WJ, Hussein MA (1999) Plasmacytoma of the ovary: a case report and literature review. Gynecol Oncol 73 (1):151-154. https://doi.org/10.1006/gyno.1998.5246
Cook HT, Boylston AW (1988) Plasmacytoma of the ovary. Gynecol Oncol 29 (3):378-381. https://doi.org/10.1016/0090-8258(88)90239-9
Santhosh S, Mittal BR, Raveendran A, Jain V, Nijhawan R, Kumar R, Bhattacharya A, Sharma SC (2013) Plasmacytoma of the ovary: additional role of 18F-FDG PET/CT. Clin Nucl Med 38 (5):e230-232. https://doi.org/10.1097/rlu.0b013e318253212d
Funding
None.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
All authors declare that they have no conflict of interest.
Ethics approval
Institutional IRB approval was obtained.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Cho, R., Myers, D.T., Onwubiko, I.N. et al. Extraosseous multiple myeloma: imaging spectrum in the abdomen and pelvis. Abdom Radiol 46, 1194–1209 (2021). https://doi.org/10.1007/s00261-020-02712-2
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00261-020-02712-2