Abstract
Objective
Since the time of inception of autoimmune pancreatitis (AIP), our knowledge of autoimmune pancreatitis has expanded significantly. The aim of this review is to provide an update on clinical manifestations, diagnosis, imaging features, and treatment of AIP.
Background and clinical significance
Type 1 AIP is the pancreatic manifestation of IgG4-related systemic disease, which can be diagnosed using a combination of clinical, histopathological, pancreatic imaging findings in conjunction with manifestation in other organs, as well of responsiveness to steroid treatment. It is vital to differentiate AIP from pancreatic cancer since both can mimic each other clinically and radiologically. Type 2 AIP is a rare but distinct subtype of AIP which occurs mostly in the younger patient.
Conclusion
AIP is steroid-responsive chronic pancreatitis with distinct manifestations on imaging.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
There is an increasing understanding of autoimmune pancreatitis (AIP) since Sarles et al. first described a case of chronic sclerosing pancreatitis with hypergammaglobulinaemia in 1961 [1]. Yoshida et al. coined the term AIP for a steroid-responsive mass-forming pancreatitis syndrome with elevated autoantibodies in 1995 [2]. AIP is a distinct form of pancreatitis which may be associated with IgG4 laden lymphoplasmacytic infiltration and fibrosis in multiple organs and often respond to steroid or other immunomodulatory therapy [3]. AIP commonly presents as painless jaundice, mild abdominal pain, or recurrent acute pancreatitis [4]. AIP sometimes is confused clinically and radiologically with pancreatic ductal adenocarcinoma (PDAC) [5]. It is vital to distinguish these two entities as treatment and prognosis are dramatically different. In this article, we review the recent advances in understanding, diagnosis, and management of AIP and extrapancreatic IgG4-related diseases.
Epidemiology
Exact incidence and prevalence of AIP is unknown. AIP accounted for 5–6% of all patients with chronic pancreatitis [6]. Data from a nationwide survey in Japan where AIP is much more common than the western world indicated that autoimmune pancreatitis has an overall prevalence of 4.6 per 100,000 and an incidence of 1.4 per 100,000 people [7]. Approximately 4% of patients undergoing pancreaticoduodenectomy for pancreatic head mass between 1992 and 2005 were due to AIP [8]. AIP was identified in 11% of cases of 245 pancreatic resections performed for tumefactive chronic pancreatitis [9].
AIP clinical subtypes and histopathology
AIP is now clearly divided into type 1 and type 2 subtypes, which represent the clinical profiles associated with corresponding distinct histological subtypes lymphoplasmacytic sclerosing pancreatitis (LPSP) and idiopathic duct-centric pancreatitis (IDCP), respectively [10]. LPSP is characterized by the infiltration of tissues with IgG4-positive plasma cells along with storiform fibrosis and obliterative phlebitis (Fig. 1) [11]. Patients with type 1 AIP (LPSP) commonly presents with painless obstructive jaundice and represents the pancreatic manifestation of IgG4-associated systemic disease. A typical patient is over 60 years, and males are three times more commonly affected than females. Other clinical features include chronic or recurrent abdominal pain, weight loss, and steatorrhea. Abnormalities of the pancreatic exocrine function are seen in up to 85% of patients with AIP and diabetes mellitus in up to 78% [12]. The diabetes mellitus can occur before (33%), simultaneously (52%), or after steroid treatment (14%) of AIP [13]. Extrapancreatic involvement affecting almost all organ systems, including the liver, biliary tree, kidneys, prostate gland, testicles, the peritoneum and the retroperitoneum, salivary and lacrimal glands, orbital tissues, pituitary gland, thyroid gland, lungs, lymph nodes, breasts, and vascular structures have been reported with LPSP [14].
Type 2 AIP accounts for 20% to 40% of all AIP cases in the USA [15, 16]. The histologic pattern in type 2 AIP is characterized as IDCP the hallmarks of which are granulocytic epithelial lesions (Fig. 2) [17]. ICDP is characterized mainly by a younger age (mean age, 43 years) at presentation, the absence of extrapancreatic involvement seen in type 1 AIP and lack of IgG4 elevation. Type 2 AIP is associated with inflammatory bowel disease (up to 30%). Type 2 has roughly equal gender distribution, more common in the western population, and presentation with acute pancreatitis is more commonly seen.
Serological markers
Type 1 AIP is associated with elevated titers of IgG (≥ 1800 mg/dL) and its subset IgG4 (≥ 140 mg/dL) [18]. Serum IgG4 levels greater than 140 mg/dL are considered to be 86% sensitive and 90–96% specific for the diagnosis of AIP [19, 20]. Mild elevation of IgG4 can be seen in 10% of pancreatic cancer [20]. Values greater than twofold of normal (> 280 mg/dL) are highly specific for AIP and are used as level 1 criteron in international consensus diagnostic criteria (ICDC) [21]. Serum IgG4 levels are normal in type 2 AIP. Several other serological markers have been reported to be elevated in AIP such as γ-globulin (> 2.0 g/dL), rheumatoid factor (20-30%), and antinuclear antibody (60%) but they are nonspecific [10].
Imaging: pancreas
AIP is often first recognized on imaging which can accurately characterize pancreatic parenchyma and main pancreatic duct. The diagnosis of AIP should be considered once a thorough workup for underlying PDAC is negative as PDAC is a far more common entity and must be excluded. Various imaging modalities have been investigated for the evaluation of AIP and discussed below.
CT and MR
CT with intravenous contrast obtained in pancreatic parenchymal phase (typically 40–45 s delay) and portal venous phase (typically 60–70 s delay) are adequate for assessment of changes of AIP. We use CT parameters as CT rotation time = 0.5 s, pitch = 0.75, kVp = 120, and quality reference mAs = 350. Delayed, 3-min phase, can be added if clinical or initial imaging assessment is suspicious for AIP.
For MRI, T1-weighted, T2-weighted, and diffusion-weighted (DWI B500 and 1000) and post-contrast imaging (arterial, venous, and 5-minute delay) are used to assess. The biliary tree is best evaluated on MRCP. Post-contrast imaging (arterial, venous, and 5-minute delay) is beneficial for accurate assessment of enhancement characteristics of AIP. We use phased-array torso coil for pancreatic imaging.
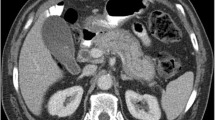
Three distinct morphologic patterns of involvement have been described: diffuse (up to 50–70%), focal (up to 30% in type 1 and 80% in type 2), and multifocal forms (5%) [3, 22,23,24,25]. Diffuse enlargement of the pancreas, also known as sausage-shaped pancreas with delayed enhancement, is the most characteristic finding in AIP (Fig. 3). Pancreatic inflammation and swelling results in loss of lobulation (featureless pancreas). On MRI, the affected gland may show relative hypointensity on T1- weighting and mild hyperintensity on T2-weighting [26]. A low-attenuating rim of soft tissue (capsule rim sign) may be seen in only about 30–40% patients but is very specific for AIP and thought to be secondary to the infiltration of inflammatory cells and fibrosis [27]. The rim appears as T1 and T2 hypointense with delayed enhancement on MRI (Figs. 4, 5). Case must be taken not to mistake peripancreatic fluid as capsule rim. Peripancratic fluid often has irregular border, T2 hyperintense, and does not enhance. Pancreatic parenchyma may show arterial and venous phase hypoattenuation that becomes iso-attenuating (or hyperattenuating, due to fibrosis) in the delayed phase (Fig. 5) [28]. The segmental form of the disease affects an entire segment of the gland, such as tail or body (Fig. 6). The focal form of AIP often involves the head or uncinate process of pancreas and is seen as a localized enlargement or a masslike appearance of the pancreas with delayed enhancement after administration of contrast agent. Sometimes AIP can present with multifocal masses (Fig. 7).
45-year-old female with type 1 AIP presenting with mild abdominal discomfort. Axial T1-weighted images pre-contrast (a), arterial phase (b), portal phase (c), and delayed phase (d) show characteristic diffuse enlargement of pancreas. The body of pancreas shows low T1 signal before contrast with delayed enhancement. The tail of pancreas shows relatively normal signal although surrounded by capsule-like rim with delayed enhancement (arrow). The biopsy confirmed LPSP
81-year-old female with segmental form of type 1 AIP. Axial and coronal CT images show segmental enlargement of body and tail of pancreas with capsule-like rim (white arrow). Note sparing of pancreatic head and no significant pancreatic duct dilation. Coronal image also shows bile duct wall enhancement at hilum suggestive of associated IgG4-related biliary disease (black arrow). EUS-guided biopsy showed LPSP
24-year-old female with type 2 AIP and history of ulcerative colitis. Axial post-contrast T1-weighted image in portal phase (a, b) show multiple focal pancreatic masses with decreased enhancement (arrows). Initial hypoenhancement and delayed hyperenhancement are characteristic of AIP. Subsequent biopsy confirmed IDCP
Pancreatic ducts are either small and nondilated or diffusely narrowed due to compression from the swollen gland. While mild ductal dilation proximal to the area of pancreatic enlargement may be secondary to a focal inflammatory mass, significant dilation of the pancreatic duct with an abrupt cutoff is rare in AIP and PDAC should be suspected [29]. Magnetic resonance cholangiopancreatography (MRCP) is a noninvasive nonionizing technique highly useful for identifying ductal changes of AIP. MRCP can show the presence of a long stricture (more than one-third of the pancreatic duct), absence of upstream dilatation (Fig. 8), presence of multiple strictures, and presence of strictures that result in side branch ectasia [30,31,32]. A focal stricture of the pancreatic portion of the common bile duct (CBD) with thickening and enhancement may be found, usually signifying associated IgG4-related sclerosing cholangitis. Penetrating duct sign or icicle sign (smoth taped narrowing of the pancreatic duct)favors benign stricture of AIP [33]. Normalization of duct caliber on follow-up is an early imaging finding of response to steroid therapy [3]. Diffusion-weighted imaging may show restricted diffusion with low ADC values (Fig. 9) [34]. ADC values can be related to clinical features, follow-up status, and prior corticosteroid treatment and often have values overlapping between PDAC and AIP [26, 29, 34, 35]. It should be noted that a normal-appearing pancreas on CT or MRI does not rule out AIP [14].
74-year-old male with type 1 AIP. The pancreas is diffusely enlarged (not shown). The main pancreatic duct is diffusely narrowed due to compression from swollen gland and not visible except for a short segment of mildly dilated duct in the pancreatic neck (white arrow). Common bile duct is narrowed within the pancreatic head (dashed white arrow)
Sometimes AIP is complicated by pseudocysts formation or vascular complications such as compression, encasement, or thrombosis of splenic or mesenteric vessels [3, 36, 37]. Rarely AIP is complicated by the formation of parenchymal or ductal calculi (7% of type 1) on follow-up (Fig. 10) [38].
Both type 1 and type 2 AIP appear similar on imaging [39]. Few recent studies have shown that type 2 tends to be more focal (up to 85% times) and may have distal shortening of pancreas owing to ductal injury [16]. A higher degree of dilation of the MPD has also been noted in Type 1 AIP possibly because the inflammatory infiltrate and the fibrosis tend to compress the MPD [39]. Risk factors for pancreatic calculi include excessive alcohol consumption and recurrent AIP relapses [40].
Differentiation of AIP with PDAC
One of the major challenges in accurately diagnosing AIP is its differentiation from PDAC. The presentation with painless jaundice in an elderly patient with unreliable tumor markers may make clinical picture confusing. Patients with AIP show higher levels of serum IgG4 (a more than twofold elevation or a value > 280 mg/dL is 99% specific for AIP) compared with patients with pancreatic adenocarcinoma [3]. On the contrary, CA 19-9 is considered a standard biomarker for pancreatic cancer. Reported pooled sensitivity and specificity of CA 19-9 for the diagnosis of pancreatic cancer are 79–81% and 82–90%, respectively [41]. While the diffuse form of AIP may be easier to diagnose on imaging, the focal pancreatic involvement may require biopsy confirmation for a definite diagnosis. Sometimes the segmental form of enlargement may be secondary to small cancer with upstream pancreatitis or diffuse infiltrating tumor. High-density rim sign of pancreatic cancer may be helpful to distinguish it from AIP where the hyperattenuating rim around the tumor is hyperdense on the noncontrast study and shows hyperenhancement compared to pancreatic parenchyma on portal venous phase [42]. The major distinguishing features between AIP and pancreatic adenocarcinoma are summarized in Table 1.
Endoscopic ultrasound (EUS) and Endoscopic retrograde cholangiopancreatography (ERCP)
Typical AIP appears as diffusely enlarged hypoechoic pancreas with echogenic interlobular septa and narrowing of the main pancreatic duct on EUS. An additional advantage of endoscopic ultrasound is the feasibility of obtaining an ultrasound-guided biopsy for definitive histologic diagnosis [3, 43]. EUS-guided FNAC alone has low sensitivity (up to 40%) when compared to combination of FNAC and Trucut biopsy (accuracy up to 85%) [3]. Even though ERCP can accurately depict characteristic ductal changes of AIP, ERCP is typically reserved for the cases where therapeutic intervention for ductal obstruction is needed [31, 44].
Positron emission tomography (PET)
PET is not required for diagnosis [45]. When performed due to suspicion for underlying pancreatic cancer and staging, it may show intense, diffuse, or focal fluorine-18 fluorodeoxyglucose (FDG) uptake in the inflamed areas of the pancreas in 90–100% of cases (Fig. 11), but not useful for differentiation from PDAC [46, 47]. The presence of FDG uptake in the organs typical for type 1 AIP may aid in the differential diagnosis. There may be a role in the monitoring of early response in the steroid trial.
52-year-old female with type 1 AIP presented with abdominal pain. Axial post-contrast CT images (a, b) show hypoattenuating masses in the head and tail of pancreas (arrow). Heterogenous enhancement of head lesion with moderate pancreatic duct dilatation in the neck was atypical findings of AIP. PET–CT (c, d) shows increased FDG uptake (arrow) with SUV max of 10.7. Subsequent biopsy confirmed LPSP
Imaging: extrapancreatic findings
As type 1 AIP is the pancreatic manifestations of IgG4-related disease, it is often associated with IgG4-related lesions involving various extrapancreatic organs. The most common sites of involvement include biliary tree, kidneys, retroperitoneum, orbits, and salivary gland. Presence of the extrapancreatic lesions could provide a clue for the diagnosis of AIP.
Bile duct, kidney, aorta/artery, and retroperitoneum are common sites of involvement in the abdomen. Biliary involvement may occur up to 90% of patients with AIP. It may involve anywhere in the biliary tree, but intrapancreatic bile duct is most commonly affected. The affected duct typically shows diffuse wall thickening with or without proximal bile duct dilatation (Fig. 12). Wall thickening is usually circumferential and concentric. On MRCP or ERCP, biliary strictures are often smooth and long compared to those seen in primary sclerosing cholangitis. The extent of bile duct wall thickening is usually longer than extent of biliary stricture. Occasionally, biliary involvement may mimic primary sclerosing cholangitis or cholangiocarcinoma in the absence of pancreatic involvement.
73-year-old male with type 1 AIP presented with painless jaundice. Axial (a) and coronal (b) post-contrast CT images show moderate intrahepatic bile duct dilatation and mild diffuse wall thickening of the extrahepatic bile duct (white arrow). Retrograde cholangiogram demonstrate multifocal smooth strictures of the both intrahepatic and extrahepatic bile ducts (black arrow). Proximal intrahepatic bile ducts are mildly dilated. Findings are consistent with biliary involvement of IgG4-related disease
Renal involvement may occur in up to 35% of patients with AIP. Typical imaging findings of the kidney involvement include multiple low-density areas predominantly distributed in the renal cortex, commonly bilateral (Fig. 13a) [48]. Renal pelvic wall thickening may occur.
Various extrapancreatic manifestation of AIP. a 71-year-old male with type 1 AIP. CT of abdomen shows multiple wedge-shaped low-density areas in bilateral renal cortex (arrows) consistent with IgG4-related renal disease. b 69-year-old male with type 1 AIP. CT of abdomen shows circumferential wall thickening of the abdominal aorta (arrows) suggestive of periaortic IgG4-related disease. c 81-year-old male with type 1 AIP. Ultrasonography of the submandibular gland demonstrates enlarged gland with multiple low-echoic areas surrounded by linear hyperechoic structures. d 62-year-old male with type 1 AIP and extrapancreatic involvement of lacrimal and infraorbital nerves. MRI (T2WI coronal) shows bilateral lacrimal glands enlargement (arrows). Bilateral infraorbital nerves are also swollen (dashed arrows). e 50-year-old female with type 1 AIP. Chest CT shows bronchovascular thickening in both lungs (arrows). Lung involvement is often nonspecific with IgG4-related disease. f 91-year-old male with type 1 AIP. Chest CT shows band-like soft tissue in right lateral aspect of lower thoracic vertebra (arrow)
Aortic involvement usually present with circumferential soft tissue thickening around the aorta. Though it could involve any part of the aorta, the infrarenal abdominal aorta is frequently affected and sometimes could lead to luminal dilatation (inflammatory abdominal aortic aneurysm) (Fig. 13b) [49]. Retroperitoneal fibrosis may involve other parts of the body and manifest as band-like or sheet-like area of soft tissue thickening. Aortic lesions or retroperitoneal fibrosis sometimes causes urinary tract obstruction.
Salivary and lacrimal glands, pituitary gland, meninges, and cranial nerve are common sites of involvement in the head and neck region. Of these manifestations, salivary and lacrimal glands are most prevalent. Typical imaging finding is well-demarcated bilateral enlargement of the involved glands. On the US, the enlarged glands demonstrate multiple low-echoic areas surrounded by linear hyperechoic structures (Fig. 13c) [50]. MRI may show perineural involvement, especially in branches of trigeminal nerves, associated with lacrimal gland enlargement (Fig. 13d) [51].
Lung, paravertebral, and aortic/arterial are common sites of involvement in the thorax. Though lung lesions show various imaging findings from inflammatory pseudotumor to interstitial pneumonia, typical CT findings are diffuse thickening of the bronchovascular bundle and interlobular septa associated with ground-glass opacities (Fig. 13e) [52]. Paravertebral lesions are recently recognized manifestations of IgG4-RD and they present as band-like soft tissue mainly on the right side of lower thoracic vertebrae (Fig. 13f) [53].
Diagnostic criteria
Several diagnostic criteria were proposed from various countries between 2002 and 2009. These diagnostic criteria varied reflecting the differences in the diagnostic approach and patient population (proportion of type 1 and type 2 AIP). In 2011, the International Consensus Diagnostic Criteria (ICDC) for AIP were developed [21]. The criteria emphasize five cardinal features of AIP: Histology, Imaging (pancreatic parenchyma and pancreatic duct), Serum IgG4 level, Other organ involvement, and Response to steroid Therapy. These features (except for response to therapy) are categorized as level 1 and 2 findings, depending on the specificity of the findings. Two distinct subtypes are recognized in the ICDC classification. The diagnosis of type 1 and type 2 AIP can be described as either definitive or probable. Similar features with minor differences are used in Mayo HISORt criteria. The complete description of variety of these criteria are beyond the scope of this review.
Treatment, prognosis and imaging surveillance
High-dose corticosteroids (prednisolone 0.6 mg/kg/day or prednisone 40 mg once daily × 4 weeks) with gradual taper is the most common treatment regimens for patients with AIP. The treatment is aimed at symptomatic relief and preservation the function of the organs involved. Imaging changes of therapeutic response can occur as early as 2 weeks, although repeat imaging is often performed after 2 months [3, 14]. The pancreatic swelling, surrounding halo, extrapancreatic disease, and ductal strictures resolve, suggesting favorable treatment response. Glandular atrophy secondary to fibrous changes may occur and it is permanent. Both endocrine and exocrine insufficiency may result from AIP despite prompt treatment. Nonresolution of the clinical symptoms or radiological abnormality should prompt the search for the alternative diagnosis and pancreatic cancer must be excluded. Presence of a focal mass and strictures may suggest refractory disease. Relapse may occur in 53% of patients after steroid withdrawal [54]. Relapse is far more common in type 1 AIP (60%) compared to type 2 AIP (5%) [23]. Immunomodulators or rituximab are used in the patient with relapse or steroid-resistant disease [55]. Possible association of AIP (IgG4-related disease) and pancreatic cancer and other malignancy is still debatable [38, 55]. Rarely, pancreatic duct stone formation may be a sequela of relapsing on the recurrent disease.
Conclusion
The term AIP comprises of two clinically and histopathologically distinct form of steroid-responsive pancreatitis, LPSP, and IDCP. Imaging features of two subtypes are overlapping and often indistingushible. Imaging plays quintessential role in the diagnosis of AIP and distinguishing AIP from PDAC. Rituximab is emerging as promising alternative treatment to steroid.
References
Sarles H, Sarles JC, Muratore R, Guien C (1961) Chronic inflammatory sclerosis of the pancreas--an autonomous pancreatic disease? The American journal of digestive diseases 6:688-698
Yoshida K, Toki F, Takeuchi T, Watanabe S, Shiratori K, Hayashi N (1995) Chronic pancreatitis caused by an autoimmune abnormality. Proposal of the concept of autoimmune pancreatitis. Digestive diseases and sciences 40 (7):1561-1568
KKhandelwal A, Shanbhogue AK, Takahashi N, Sandrasegaran K, Prasad SR (2014) Recent advances in the diagnosis and management of autoimmune pancreatitis. AJR American journal of roentgenology 202 (5):1007-1021. https://doi.org/10.2214/ajr.13.11247
Nagpal SJS, Sharma A, Chari ST (2018) Autoimmune Pancreatitis. The American journal of gastroenterology 113 (9):1301.https://doi.org/10.1038/s41395-018-0146-0
Chari ST, Takahashi N, Levy MJ, Smyrk TC, Clain JE, Pearson RK, Petersen BT, Topazian MA, Vege SS (2009) A diagnostic strategy to distinguish autoimmune pancreatitis from pancreatic cancer. Clinical gastroenterology and hepatology : the official clinical practice journal of the American Gastroenterological Association 7 (10):1097-1103. https://doi.org/10.1016/j.cgh.2009.04.020
Nishimori I, Tamakoshi A, Otsuki M (2007) Prevalence of autoimmune pancreatitis in Japan from a nationwide survey in 2002. Journal of gastroenterology 42 Suppl 18:6-8. https://doi.org/10.1007/s00535-007-2043-y
Kanno A, Masamune A, Okazaki K, Kamisawa T, Kawa S, Nishimori I, Tsuji I, Shimosegawa T (2015) Nationwide epidemiological survey of autoimmune pancreatitis in Japan in 2011. Pancreas 44 (4):535-539. https://doi.org/10.1097/mpa.0000000000000325
de Castro SM, de Nes LC, Nio CY, Velseboer DC, ten Kate FJ, Busch OR, van Gulik TM, Gouma DJ (2010) Incidence and characteristics of chronic and lymphoplasmacytic sclerosing pancreatitis in patients scheduled to undergo a pancreatoduodenectomy. HPB : the official journal of the International Hepato Pancreato Biliary Association 12 (1):15-21. https://doi.org/10.1111/j.1477-2574.2009.00112.x
Yadav D, Notahara K, Smyrk TC, Clain JE, Pearson RK, Farnell MB, Chari ST (2003) Idiopathic tumefactive chronic pancreatitis: clinical profile, histology, and natural history after resection. Clinical gastroenterology and hepatology : the official clinical practice journal of the American Gastroenterological Association 1 (2):129-135. https://doi.org/10.1053/cgh.2003.50016
Khandelwal A, Saboo SS (2013) Re: Autoimmune pancreatitis: an illustrated guide to diagnosis. Clinical radiology 68 (9):e518. https://doi.org/10.1016/j.crad.2013.03.013
Kamisawa T, Chari ST, Giday SA, Kim MH, Chung JB, Lee KT, Werner J, Bergmann F, Lerch MM, Mayerle J, Pickartz T, Lohr M, Schneider A, Frulloni L, Webster GJ, Reddy DN, Liao WC, Wang HP, Okazaki K, Shimosegawa T, Kloeppel G, Go VL (2011) Clinical profile of autoimmune pancreatitis and its histological subtypes: an international multicenter survey. Pancreas 40 (6):809-814. https://doi.org/10.1097/MPA.0b013e3182258a15
Ito T, Nakamura T, Fujimori N, Niina Y, Igarashi H, Oono T, Uchida M, Kawabe K, Takayanagi R, Nishimori I, Otsuki M, Shimosegawa T (2011) Characteristics of pancreatic diabetes in patients with autoimmune pancreatitis. Journal of digestive diseases 12 (3):210-216. https://doi.org/10.1111/j.1751-2980.2011.00498.x
Nishimori I, Tamakoshi A, Kawa S, Tanaka S, Takeuchi K, Kamisawa T, Saisho H, Hirano K, Okamura K, Yanagawa N, Otsuki M (2006) Influence of steroid therapy on the course of diabetes mellitus in patients with autoimmune pancreatitis: findings from a nationwide survey in Japan. Pancreas 32 (3):244-248. https://doi.org/10.1097/01.mpa.0000202950.02988.07
Sandrasegaran K, Menias CO (2018) Imaging in Autoimmune Pancreatitis and Immunoglobulin G4-Related Disease of the Abdomen. Gastroenterology clinics of North America 47 (3):603-619. https://doi.org/10.1016/j.gtc.2018.04.007
Chari ST, Kloeppel G, Zhang L, Notohara K, Lerch MM, Shimosegawa T (2010) Histopathologic and clinical subtypes of autoimmune pancreatitis: the Honolulu consensus document. Pancreas 39 (5):549-554. https://doi.org/10.1097/MPA.0b013e3181e4d9e5
Sah RP, Chari ST, Pannala R, Sugumar A, Clain JE, Levy MJ, Pearson RK, Smyrk TC, Petersen BT, Topazian MD, Takahashi N, Farnell MB, Vege SS (2010) Differences in clinical profile and relapse rate of type 1 versus type 2 autoimmune pancreatitis. Gastroenterology 139 (1):140-148; quiz e112-143. https://doi.org/10.1053/j.gastro.2010.03.054
Shinagare S, Shinagare AB, Deshpande V (2012) Autoimmune pancreatitis: a guide for the histopathologist. Seminars in diagnostic pathology 29 (4):197-204. https://doi.org/10.1053/j.semdp.2012.07.007
Okazaki K, Kawa S, Kamisawa T, Naruse S, Tanaka S, Nishimori I, Ohara H, Ito T, Kiriyama S, Inui K, Shimosegawa T, Koizumi M, Suda K, Shiratori K, Yamaguchi K, Yamaguchi T, Sugiyama M, Otsuki M (2006) Clinical diagnostic criteria of autoimmune pancreatitis: revised proposal. Journal of gastroenterology 41 (7):626-631. https://doi.org/10.1007/s00535-006-1868-0
Choi EK, Kim MH, Lee TY, Kwon S, Oh HC, Hwang CY, Seo DW, Lee SS, Lee SK (2007) The sensitivity and specificity of serum immunoglobulin G and immunoglobulin G4 levels in the diagnosis of autoimmune chronic pancreatitis: Korean experience. Pancreas 35 (2):156-161. https://doi.org/10.1097/MPA.0b013e318053eacc
Ghazale A, Chari ST, Smyrk TC, Levy MJ, Topazian MD, Takahashi N, Clain JE, Pearson RK, Pelaez-Luna M, Petersen BT, Vege SS, Farnell MB (2007) Value of serum IgG4 in the diagnosis of autoimmune pancreatitis and in distinguishing it from pancreatic cancer. The American journal of gastroenterology 102 (8):1646-1653. https://doi.org/10.1111/j.1572-0241.2007.01264.x
Shimosegawa T, Chari ST, Frulloni L, Kamisawa T, Kawa S, Mino-Kenudson M, Kim MH, Kloppel G, Lerch MM, Lohr M, Notohara K, Okazaki K, Schneider A, Zhang L (2011) International consensus diagnostic criteria for autoimmune pancreatitis: guidelines of the International Association of Pancreatology. Pancreas 40 (3):352-358. https://doi.org/10.1097/MPA.0b013e3182142fd2
Majumder S, Takahashi N, Chari ST (2017) Autoimmune Pancreatitis. Digestive diseases and sciences 62 (7):1762-1769. https://doi.org/10.1007/s10620-017-4541-y
CCrosara S, D'Onofrio M, De Robertis R, Demozzi E, Canestrini S, Zamboni G, Pozzi Mucelli R (2014) Autoimmune pancreatitis: Multimodality non-invasive imaging diagnosis. World journal of gastroenterology 20 (45):16881-16890. https://doi.org/10.3748/wjg.v20.i45.16881
Morse B, Centeno B, Vignesh S (2014) Autoimmune pancreatitis: updated concepts of a challenging diagnosis. The American journal of medicine 127 (10):1010.e1011-1019. https://doi.org/10.1016/j.amjmed.2014.04.033
RRehnitz C, Klauss M, Singer R, Ehehalt R, Werner J, Buchler MW, Kauczor HU, Grenacher L (2011) Morphologic patterns of autoimmune pancreatitis in CT and MRI. Pancreatology : official journal of the International Association of Pancreatology (IAP) [et al] 11 (2):240-251. https://doi.org/10.1159/000327708
Manfredi R, Frulloni L, Mantovani W, Bonatti M, Graziani R, Pozzi Mucelli R (2011) Autoimmune pancreatitis: pancreatic and extrapancreatic MR imaging-MR cholangiopancreatography findings at diagnosis, after steroid therapy, and at recurrence. Radiology 260 (2):428-436. https://doi.org/10.1148/radiol.11101729
Takahashi N, Fletcher JG, Hough DM, Fidler JL, Kawashima A, Mandrekar JN, Chari ST (2009) Autoimmune pancreatitis: differentiation from pancreatic carcinoma and normal pancreas on the basis of enhancement characteristics at dual-phase CT. AJR American journal of roentgenology 193 (2):479-484. https://doi.org/10.2214/ajr.08.1883
Kwon JH, Kim JH, Kim SY, Byun JH, Kim HJ, Lee MG, Lee SS (2019) Differentiating focal autoimmune pancreatitis and pancreatic ductal adenocarcinoma: contrast-enhanced MRI with special emphasis on the arterial phase. European radiology. https://doi.org/10.1007/s00330-019-06200-0
Choi SY, Kim SH, Kang TW, Song KD, Park HJ, Choi YH (2016) Differentiating Mass-Forming Autoimmune Pancreatitis From Pancreatic Ductal Adenocarcinoma on the Basis of Contrast-Enhanced MRI and DWI Findings. AJR American journal of roentgenology 206 (2):291-300. https://doi.org/10.2214/ajr.15.14974
Park SH, Kim MH, Kim SY, Kim HJ, Moon SH, Lee SS, Byun JH, Lee SK, Seo DW, Lee MG (2010) Magnetic resonance cholangiopancreatography for the diagnostic evaluation of autoimmune pancreatitis. Pancreas 39 (8):1191-1198. https://doi.org/10.1097/MPA.0b013e3181dbf469
Kamisawa T, Tu Y, Egawa N, Tsuruta K, Okamoto A, Kodama M, Kamata N (2009) Can MRCP replace ERCP for the diagnosis of autoimmune pancreatitis? Abdominal imaging 34 (3):381-384. https://doi.org/10.1007/s00261-008-9401-y
Sahani DV, Kalva SP, Farrell J, Maher MM, Saini S, Mueller PR, Lauwers GY, Fernandez CD, Warshaw AL, Simeone JF (2004) Autoimmune pancreatitis: imaging features. Radiology 233 (2):345-352. https://doi.org/10.1148/radiol.2332031436
Kim HJ, Kim YK, Jeong WK, Lee WJ, Choi D (2015) Pancreatic duct "Icicle sign" on MRI for distinguishing autoimmune pancreatitis from pancreatic ductal adenocarcinoma in the proximal pancreas. European radiology 25 (6):1551-1560 https://doi.org/10.1007/s00330-014-3548-4
Kamisawa T, Takuma K, Anjiki H, Egawa N, Hata T, Kurata M, Honda G, Tsuruta K, Suzuki M, Kamata N, Sasaki T (2010) Differentiation of autoimmune pancreatitis from pancreatic cancer by diffusion-weighted MRI. The American journal of gastroenterology 105 (8):1870-1875. https://doi.org/10.1038/ajg.2010.87
Klauss M, Maier-Hein K, Tjaden C, Hackert T, Grenacher L, Stieltjes B (2016) IVIM DW-MRI of autoimmune pancreatitis: therapy monitoring and differentiation from pancreatic cancer. European radiology 26 (7):2099-2106. https://doi.org/10.1007/s00330-015-4041-4
Raina A, Yadav D, Krasinskas AM, McGrath KM, Khalid A, Sanders M, Whitcomb DC, Slivka A (2009) Evaluation and management of autoimmune pancreatitis: experience at a large US center. The American journal of gastroenterology 104 (9):2295-2306. https://doi.org/10.1038/ajg.2009.325
Kawamoto S, Siegelman SS, Hruban RH, Fishman EK (2008) Lymphoplasmacytic sclerosing pancreatitis (autoimmune pancreatitis): evaluation with multidetector CT. Radiographics : a review publication of the Radiological Society of North America, Inc 28 (1):157-170. https://doi.org/10.1148/rg.281065188
Ikeura T, Miyoshi H, Shimatani M, Uchida K, Takaoka M, Okazaki K (2016) Long-term outcomes of autoimmune pancreatitis. World journal of gastroenterology 22 (34):7760-7766. https://doi.org/10.3748/wjg.v22.i34.7760
Negrelli R, Boninsegna E, Avesani G, Zamboni GA, Brozzi L, Frulloni L, Manfredi R, Pozzi Mucelli R (2018) Type 1 and Type 2 Autoimmune Pancreatitis: Distinctive Clinical and Pathological Features, But Are There Any Differences at Magnetic Resonance? Experience From a Referral Center. Pancreas 47 (9):1115-1122. https://doi.org/10.1097/mpa.0000000000001142
Maruyama M, Arakura N, Ozaki Y, Watanabe T, Ito T, Yoneda S, Maruyama M, Muraki T, Hamano H, Matsumoto A, Kawa S (2012) Risk factors for pancreatic stone formation in autoimmune pancreatitis over a long-term course. Journal of gastroenterology 47 (5):553-560. https://doi.org/10.1007/s00535-011-0510-y
Ballehaninna UK, Chamberlain RS (2012) The clinical utility of serum CA 19-9 in the diagnosis, prognosis and management of pancreatic adenocarcinoma: An evidence based appraisal. Journal of gastrointestinal oncology 3 (2):105-119. https://doi.org/10.3978/j.issn.2078-6891.2011.021
Choi YJ, Byun JH, Kim JY, Kim MH, Jang SJ, Ha HK, Lee MG (2008) Diffuse pancreatic ductal adenocarcinoma: characteristic imaging features. European journal of radiology 67 (2):321-328. https://doi.org/10.1016/j.ejrad.2007.07.010
Levy MJ, Reddy RP, Wiersema MJ, Smyrk TC, Clain JE, Harewood GC, Pearson RK, Rajan E, Topazian MD, Yusuf TE, Chari ST, Petersen BT (2005) EUS-guided trucut biopsy in establishing autoimmune pancreatitis as the cause of obstructive jaundice. Gastrointestinal endoscopy 61 (3):467-472
Nishino T, Oyama H, Toki F, Shiratori K (2010) Differentiation between autoimmune pancreatitis and pancreatic carcinoma based on endoscopic retrograde cholangiopancreatography findings. Journal of gastroenterology 45 (9):988-996. https://doi.org/10.1007/s00535-010-0250-4
Nakajo M, Jinnouchi S, Fukukura Y, Tanabe H, Tateno R, Nakajo M (2007) The efficacy of whole-body FDG-PET or PET/CT for autoimmune pancreatitis and associated extrapancreatic autoimmune lesions. European journal of nuclear medicine and molecular imaging 34 (12):2088-2095. https://doi.org/10.1007/s00259-007-0562-7
Lee TY, Kim MH, Park DH, Seo DW, Lee SK, Kim JS, Lee KT (2009) Utility of 18F-FDG PET/CT for differentiation of autoimmune pancreatitis with atypical pancreatic imaging findings from pancreatic cancer. AJR American journal of roentgenology 193 (2):343-348. https://doi.org/10.2214/ajr.08.2297
Kamisawa T, Takum K, Anjiki H, Egawa N, Kurata M, Honda G, Tsuruta K (2010) FDG-PET/CT findings of autoimmune pancreatitis. Hepato-gastroenterology 57 (99-100):447-450
Takahashi N, Kawashima A, Fletcher JG, Chari ST (2007) Renal involvement in patients with autoimmune pancreatitis: CT and MR imaging findings. Radiology 242 (3):791-801. https://doi.org/10.1148/radiol.2423060003
Inoue D, Zen Y, Abo H, Gabata T, Demachi H, Yoshikawa J, Miyayama S, Nakanuma Y, Matsui O (2011) Immunoglobulin G4-related periaortitis and periarteritis: CT findings in 17 patients. Radiology 261 (2):625-633. https://doi.org/10.1148/radiol.11102250
Sakamoto M, Moriyama M, Shimizu M, Chinju A, Mochizuki K, Munemura R, Ohyama K, Maehara T, Ogata K, Ohta M, Yamauchi M, Ishiguro N, Matsumura M, Ohyama Y, Kiyoshima T, Nakamura S (2019) The diagnostic utility of submandibular gland sonography and labial salivary gland biopsy in IgG4-related dacryoadenitis and sialadenitis: Its potential application to the diagnostic criteria. Modern rheumatology:1-6. https://doi.org/10.1080/14397595.2019.1576271
Inoue D, Zen Y, Sato Y, Abo H, Demachi H, Uchiyama A, Gabata T, Matsui O (2012) IgG4-Related Perineural Disease. International journal of rheumatology 2012:401890. https://doi.org/10.1155/2012/401890
Inoue D, Zen Y, Abo H, Gabata T, Demachi H, Kobayashi T, Yoshikawa J, Miyayama S, Yasui M, Nakanuma Y, Matsui O (2009) Immunoglobulin G4-related lung disease: CT findings with pathologic correlations. Radiology 251 (1):260-270. https://doi.org/10.1148/radiol.2511080965
IInoue D, Zen Y, Komori T, Yoshida K, Yoneda N, Kitao A, Kozaka K, Izumozaki A, Matsumoto J, Toshima F, Kobayashi S, Gabata T (2019) CT Findings of Thoracic Paravertebral Lesions in IgG4-Related Disease. AJR American journal of roentgenology:1-6. https://doi.org/10.2214/ajr.18.20834
Ghazale A, Chari ST, Zhang L, Smyrk TC, Takahashi N, Levy MJ, Topazian MD, Clain JE, Pearson RK, Petersen BT, Vege SS, Lindor K, Farnell MB (2008) Immunoglobulin G4-associated cholangitis: clinical profile and response to therapy. Gastroenterology 134 (3):706-715. https://doi.org/10.1053/j.gastro.2007.12.009
HHart PA, Kamisawa T, Brugge WR, Chung JB, Culver EL, Czako L, Frulloni L, Go VL, Gress TM, Kim MH, Kawa S, Lee KT, Lerch MM, Liao WC, Lohr M, Okazaki K, Ryu JK, Schleinitz N, Shimizu K, Shimosegawa T, Soetikno R, Webster G, Yadav D, Zen Y, Chari ST (2013) Long-term outcomes of autoimmune pancreatitis: a multicentre, international analysis. Gut 62 (12):1771-1776. https://doi.org/10.1136/gutjnl-2012-303617
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Khandelwal, A., Inoue, D. & Takahashi, N. Autoimmune pancreatitis: an update. Abdom Radiol 45, 1359–1370 (2020). https://doi.org/10.1007/s00261-019-02275-x
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00261-019-02275-x