Abstract
Purpose
To compare the effects of gadoxetic acid and gadoteric acid on the image quality of single-breath-hold, triple (first, second, and third) arterial hepatic magnetic resonance imaging (MRI).
Methods
Two hundred and eleven patients were divided into two groups according to the contrast materials used (gadoxetic acid, 108 patients and gadoteric acid, 103 patients). All 3.0-T MR examinations included triple arterial phase acquisition using the 4D enhanced T1-weighted high-resolution isotropic volume examination (eTHRIVE) keyhole technique. The image qualities of the pre-contrast and triple arterial phases were assessed in terms of image artifacts, sharpness of the intrahepatic vessel and liver edge, and overall image quality with a 5-point scale for qualitative analysis.
Results
The image quality of gadoxetic acid-enhanced liver MRI in the triple arterial phases was significantly degraded compared with that of gadoteric acid-enhanced liver MRI, although better image scores were observed in the pre-contrast images in the gadoxetic acid group (P < 0.001). The overall image quality gradually improved from the first to the third arterial phases in both groups (P < 0.003).
Conclusions
Intravenous gadoxetic acid could have a detrimental effect on image quality of triple arterial phase MRI with the 4D eTHRIVE Keyhole technique. The third arterial phase images had the best image qualities; thus, they could be used as key scans.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Stepwise improvement in magnetic resonance imaging (MRI) speed has been achieved with the development of the parallel acquisition technique used to reduce scan time and enhance temporal resolution. Accordingly, several studies with high-speed MRI have described the value of sequential and multiple arterial phases in a single-breath-hold method for both the detection and differential diagnosis of hypervascular hepatic tumors [1,2,3]. Multiple arterial phase acquisition in contrast-enhanced MRI may allow appropriate selection of an arterial phase image with a small amount of contrast filling in the portal vein and can also reduce unintended portal venous contamination in the fixed arterial phase acquisition time [4]. However, the parallel acquisition technique has some limitations in that the numbers of imaging matrices and slices can be compromised because of the intrinsic trade-off with MRI parameters [5]. To overcome these limitations, Masayuki et al. performed a 2D high-spatial-resolution gadolinium-enhanced double-hepatic arterial phase liver MRI using a 2D-spoiled gradient-recalled echo (GRE) sequence with serial switching and reversed centric and centric k-space reordering [5]; however, this study had some limitations, including the reduced numbers of imaging matrices, obtainable slices, and through-plane spatial resolution, even when the parallel acquisition technique was employed. In several other studies using multiple arterial phases, more advanced and unique k-space filling strategies with GRE sequence have been introduced to reduce the MR acquisition time [5,6,7,8].
The THRIVE-CENTRA-keyhole technique is a combination of a 3D T1-weighted turbo GRE sequence, where a fat suppression pre-pulse is followed by the acquisition of several profiles [3D T1-weighted high-resolution isotropic volume examination (THRIVE)], with a segmented centric profile ordering technique [contrast-enhanced timing-robust angiography (CENTRA)]. Additionally, this profile ordering technique is combined with a 3D dynamic keyhole to accelerate data acquisition time during the arterial phase scanning [9]. Moreover, the 4D enhanced THRIVE (eTHRIVE) has been introduced for performing single-breath-hold multiple arterial phases in contrast-enhanced liver MRI [9].
Gadoxetic acid (Gd-EOB-DTPA), a liver-specific MRI contrast agent with perfusion and hepatocyte-selective properties, has been used for dynamic and hepatobiliary phase images [10]. However, adverse events such as nausea/vomiting, blood pressure decrease, and acute transient dyspnea after intravenous gadoxetic acid administration have been demonstrated in several studies [11, 12]. Notably, several techniques have been suggested to reduce truncation or respiratory artifacts, such as contrast agent dilution or respiratory triggering methods [13,14,15,16,17,18,19]. Nevertheless, severe image degradation with various artifacts (e.g., respiratory motion and truncation artifacts) in multiple arterial phases has often been experienced when gadoxetic acid is used. In fact, it is very important to reduce these artifacts because multiple arterial phases with the 4D eTHRIVE Keyhole technique are added to the standard MR protocol in contrast-enhanced liver MRI.
In this study, we hypothesized that severe image degradation of multiple arterial phases scanned with the 4D eTHRIVE Keyhole technique might be associated with the intravenous administration of gadoxetic acid. Accordingly, this study aimed to confirm our hypothesis and then identify the method that would help improve the multiple arterial phase image quality. Therefore, we conducted a retrospective study to evaluate the impact of Gd-EOB-DTPA on image degradation of a single-breath-hold, triple arterial dynamic MRI of the liver with the 4D eTHRIVE Keyhole technique, and we then compared our results with those of extracellular gadolinium contrast agent-enhanced MRI.
Materials and methods
Study population
The ethics committee of our institute approved this retrospective study and waived the requirement for informed consent. We conducted a computerized search in the medical records at our institution from April 2014 to September 2015, and a total of 563 patients who underwent liver MRI were consecutively registered as the study group (the gadoxetic acid group). At a similar period, from April 2014 to December 2015, a total of 480 patients who underwent MRI study of the pancreas, bile duct, or kidneys were recruited as the control group (the gadoteric acid group). Detailed inclusion and exclusion criteria are described in Fig. 1a, b.
Finally, 103 (35 women, [mean age, 58 years; range 29–84 years] and 68 men [mean age, 60 years; range 25–80 years]) were enrolled as the study group, and 103 (49 women [mean age, 55 years; range 19–83 years] and 54 men [mean age, 59 years; range 20–86 years]) were enrolled as the control group.
The choice of contrast agents was study dependent, not randomized; 108 administrations of gadoxetic acid in 103 patients (liver MRI, n = 108) and 103 administrations of gadoteric acid in 103 patients who had contrast-enhanced MRI of the pancreas (n = 50), MR cholangiopancreatography (MRCP, n = 51), or MRI of the kidneys (n = 2) were conducted.
Demographic data and risk factors of patients are summarized in Table 1. In the gadoxetic acid group, many patients had ascites due to underlying liver cirrhosis. The degrees of ascites, which were recorded through a blinded image review by one radiologist who had 4 years’ experience in abdomen MRI interpretation, were classified as Grade I (only detectable by imaging study), Grade II (moderate symmetrical distension of abdomen), or Grade III (large or gross ascites with marked abdominal distension) [20]. Other characteristics that could affect the image quality of the arterial phase were identified by a blinded review of the electronic medical records.
Contrast agents
The two gadolinium-based contrast agents (GBCAs) assessed in our study were gadoxetic acid (Gd-EOB-DTPA; Primovist®, Bayer Schering Pharma, Berlin, Germany) and gadoteric acid (Gd-DOTA; Dotarem®, Guerbet group, Roissy, France). At our institution, gadoxetic acid and gadoteric acid are always administered based on the patient’s body weight at the doses of 0.025 mmol/kg (or 0.1 mL/kg) and 0.1 mmol/kg (or 0.2 mL/kg), respectively [10]. This dose of gadoxetic acid has been recommended in prior studies to improve the arterial phase image quality [17, 21], although the lower approved dose (0.025 mmol/kg), which is one-quarter the dose of the traditional extracellular agents, may result in a weak arterial enhancement of the liver [11, 14, 22]. The two GBCAs were undiluted and were automatically administered intravenously at a rate of 1 mL/s (0.025 mmol/mL gadoxetic acid and 0.1 mmol/mL gadoteric acid) [13], followed by 25 mL of intravenous saline flush at a rate of 1 mL/s. Gadoxetic acid was mostly used in liver MRIs, and gadoteric acid was usually used in non-liver contrast-enhanced MRIs (such as pancreas MRI, MRCP, and kidney MRI) in our institution.
MR imaging techniques
All MR examinations were performed using a 3.0-T MR system (Achieva, Philips Health care, Best, the Netherlands with SENSE Torso/Cardiac coli 32 elements). Breath-holding instructions (verbal command from the technologist of image acquisition at end inspiration) and dynamic phase image timing were the same for both contrast agents. Triple (first, second, and third) arterial phase images were acquired using the 4D eTHRIVE Keyhole technique. In our keyhole technique, the central 30% of k-space data were collected for each phase, but the peripheral k-space data, used to obtain the reference dataset, were collected only once in the third arterial phase and were used to reconstruct the images of all arterial phases. The first arterial phase acquisition about 20–25 s after the contrast agent injection was initiated based on a real-time contrast bolus-triggering technique. The total scan duration of the triple arterial phase using the 4D eTHRIVE Keyhole technique was 10 s, with a dynamic reference scan of 4.7 s and each dynamic keyhole scan of 1.3 s (see Table 2 for more details),
Adverse events
The enrolled patients were interviewed after their MR examinations by an assigned technologist. Patients were asked how they experienced discomfort during the MR examinations. All seven technologists were not blinded to the contrast agents used. Patient complaints were recorded for each occurrence and were categorized according to discomfort types, such as dyspnea, nausea, or vomiting.
Image analysis
For each GBCA administration, the pre-contrast and triple arterial phase (first, second, and third arterial phase) contrast-enhanced T1-weighted images were evaluated on a picture archiving and communicating system (INFINITT PACS version 3.0; INFINITT Healthcare Co., Seoul, Korea). Other phase images (e.g., portal or delayed phase images) were not included in the analysis to prevent inadvertent unblinding. All MR images were independently assessed blindly by two gastrointestinal radiologists who had 6 years’ [reviewer 1] or 15 years’ [reviewer 2] experience in liver MRI interpretation. They were blinded to the patients’ clinical history and contrast agents used. The image quality was assessed using a five-point score rating in terms of degrees of artifacts (including motion artifact, pulsation artifact, absence of B1 inhomogeneity artifact, and truncation artifact), the sharpness of intrahepatic vessel and liver edge, and subjective determination of overall image quality of arterial phases. The degrees of “artifact” were rated as 0 = severe image artifact (non-diagnostic); 1 = moderate to severe image artifact (between scores 0 and 2, but still diagnostic); 2 = moderate image artifact, considerably obscured anatomy; 3 = minimal image artifact, slightly affected visualization of anatomy; or 4 = no image artifact. The degrees of “sharpness of intrahepatic vessel” and “sharpness of liver edge” were rated as 0 = unacceptable; 1 = poor and severely blurred; 2 = moderate (between scores 1 and 3); 3 = clearly depicted with slight blurring; or 4 = excellently depicted without blurring. The evaluation of the overall image quality was ranked as 0 = non-diagnostic; 1 = poor; 2 = fair; 3 = good; or 4 = excellent. The five scores for each phase were averaged to produce a mean score for that phase and administration. After independent analysis, the image scores of the two reviewers were averaged, and the score difference between the two study groups was compared. The best image quality phase was selected among the three arterial phases.
Statistical analyses
The Mann–Whitney test was used to determine significant differences between images acquired using the two GBCAs. The Wilcoxon signed-ranks test was used to determine image quality differences between each image phases (e.g., second arterial phase vs. first arterial phase, third arterial phase vs. second arterial phase, and third arterial phase vs. pre-contrast phase). Inter-reviewer agreement was assessed with the weighted kappa test. Weight kappa values were interpreted as follows: < 0.20, poor agreement; 0.21–0.40, fair agreement; 0.41–0.60, moderate agreement; 0.61–0.80, good agreement; and > 0.81, excellent agreement. Chi-square test was used to determine image quality differences based on risk factors and demographics of patients.
Underlying demographic data and patient risk factors were compared between the two GBCAs using Fisher’s exact test. Qualitative image quality and patient characteristics were compared between the two GBCAs using the Mann–Whitney test. In addition, the χ2 test was used to determine image quality differences by MRI technologists. All statistical analyses were performed using SPSS 20.0 Software (Chicago, Illinois). A P value < 0.05 indicated a statistically significant difference.
Results
Patients receiving gadoxetic acid were statistically more likely to be cirrhotic and have ascites and other variable comorbid malignancies, which could lead to more degraded image qualities (P < 0.05), than those receiving gadoteric acid (Table 1). Nevertheless, the overall image quality was significantly higher in the pre-contrast phase in the gadoxetic acid group than in the gadoteric acid group, and mean image scores were 2.83 in the gadoxetic acid group and 2.55 in the gadoteric acid group (Table 3, P < 0.003). However, in the post-contrast phase, the overall image quality was significantly lower in the gadoxetic acid group than in the gadoteric acid group, and mean image scores were 2.40 in the gadoxetic acid group and 2.94 in the gadoteric acid (Table 3, P < 0.001).
Significant differences in the BMI between the two groups were observed (Table 1). However, patients whose height could not be identified in the medical record were excluded from the analysis: 4 patients were excluded from the gadoxetic acid group and 28 from the gadoteric acid group.
The specific image quality between each arterial phase assessed using the 4D eTHRIVE Keyhole technique significantly improved with an increasing trend from the first to the third arterial phases in both groups (Table 3, Figs. 2, 3), and the third arterial phase images had the best image qualities among the three arterial phases (Table 4) (P < 0.001).
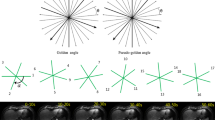
Averaged image quality scores of three evaluation factors from two reviewers. Artifacts (a), degrees of sharpness of hepatic vessels (b), and degrees of sharpness of hepatic edge (c). Overall scores were higher in the gadoteric acid group than in the gadoxetic acid group. (The exact statistical value correlates with Table 3.) The image quality between each arterial phase using the 4D enhanced T1-weighted high-resolution isotropic volume examination (eTHRIVE) keyhole technique significantly improved from the first to the third arterial phases
MR images of a 61-year-old man with hepatocellular carcinoma who had five transcatheter arterial chemoembolizations and one radiofrequency ablation previously. Image quality between each arterial phase using the 4D eTHRIVE Keyhole technique significantly improved from the first to the third arterial phases. The artifact scores were assigned as 3 (minimal) in the pre-contrast image (a), 1 (moderate to severe) in the first arterial phase (b), 2 (moderate) in the second arterial phase (c), and 3 (minimal) in the third arterial phase (d)
In the qualitative analyses of images, all rated scores were significantly higher in the gadoteric acid group. Accordingly, gadoxetic acid-enhanced liver MRI showed poorer image qualities in all arterial phases than did gadoteric acid-enhanced MRI.
Six patients in the gadoxetic acid group and three patients in the gadoteric acid group complained of dyspnea, although no statistical differences in the incidence of dyspnea were observed between the two groups (P = 0.499). Four patients from both groups had a past medical history of allergies: one nickel allergy and one CT contrast agent (Iodine) allergy in the gadoxetic acid group, and one hair dye allergy and one atopic dermatitis in the gadoteric acid group. However, these patients did not complain about any adverse events during the MRI study.
Image quality scores rated as 0 (non-diagnostic image) were mostly artifact scores and more common in the first arterial phase (Fig. 4). For example, in first arterial phase, 25 patients were rated as artifact score 0, 2 patients were rated as vessel score 0, and 2 patients were rated as edge score 0 by reviewer 2 (Table 5).
MR images of a 72-year-old man with liver cirrhosis in the gadoxetic acid group. The artifact score was assigned as 2 (moderate) or 3 (minimal) in the pre-contrast image (a). The artifact score was assigned as 0 (non-diagnostic) in the first arterial phase image by two reviewers (b). Pre-contrast MR images had adequate quality. However, severe artifacts, which were thought to be the cause of respiratory failure, were found in the first arterial phase images taken after contrast administration
The weighted kappa test showed that some of the inter-reviewer reliabilities were moderate, while others were good. There was a moderate agreement in the pre-contrast images (0.56–0.61) and the first arterial phase (0.57–0.59) and a good agreement in the second arterial phase (0.61–0.72, third arterial phase (0.65–0.73), and overall quality (0.78) (Table 6). No significant differences in MR image quality were reported by the MRI technologists (P = 0.21).
Discussion
The first principal finding of our study is that intravenous gadoxetic acid administration led to significantly degraded multiple arterial hepatic images than did intravenous gadoteric acid administration. Despite the differences in patient characteristics, the image quality of the pre-contrast scan of the gadoxetic acid group was significantly higher than that of the gadoteric acid group. Gadoxetic acid-enhanced liver MRI has been increasingly used, and the detection of arterial hyperenhancement within a liver lesion is of cardinal importance, particularly in patients with cirrhosis; however, image quality degradation in the arterial phase can be a disadvantage.
Several studies have found “acute transient dyspnea” or “transient motion artifact” after intravenous gadoxetic acid administration during the arterial phase, and this phenomenon can cause image quality degradation [23,24,25]. Thus, gadoxetic acid may degrade the image quality of the arterial phase when compared with other extracellular contrast agents. In our study, only a small number of patients in both groups (5.6% in the gadoxetic acid group and 2.9% in the gadoteric acid group) complained of overt transient dyspnea, and no significant difference was found between the two groups. Therefore, we can hypothesize that subclinical dyspnea was a greater influence on image quality degradation than overt breath-hold failure.
The second principal finding of our study is that the third arterial phase images had the best image quality regardless of the contrast agents used. We found a high prevalence of severe artifacts in the first arterial phase in the gadoxetic acid group (Table 5). Although we did not subcategorize specific artifact types, most artifacts were ringing artifacts, while some were respiratory motion artifacts.
In the keyhole technique, the central k-space portion is acquired in the initial phase of the multiple arterial phase scanning and after the complete filling of the central portion, and the k-space filling is then continued toward the peripheral k-space portion (reference image) [9]. In the last arterial phase image (the third arterial phase in our study), peripheral k-space and central k-space are acquired at the same time; accordingly, the last arterial phase image is not a reconstructed image, and it has a low chance to be interrupted by respiratory motion and other artifacts. The adverse effects of transient dyspnea during the initial gadoxetic acid injection may have degraded the quality of the first arterial phase but not that of the third arterial phase. It has been indicated that the image quality of the multiple arterial phases can be improved if the peripheral k-space is obtained immediately after injection of the contrast agent and the reference scan is conducted in the early phase [9]. However, we speculate that the reference scan using the keyhole technique in gadoxetic acid-enhanced liver MRI should be taken at the last arterial phase to obtain better results. The image quality of every phase may be affected if the reference scan during the early phase is compromised by respiratory motion or ringing artifacts [11, 16].
Regarding the concentration of contrast agent used, gadoxetic acid (Primovist®, 0.025 mmol/kg) was a quarter times more diluted than other extracellular agents such as gadoteric acid (Dotarem, 0.1 mmol/kg) and gadobenate dimeglumine (MultiHance; Bracco Diagnostics, Princeton, NJ; 0.1 mmol/kg). This low approved dose of gadoxetic acid may have resulted in the difficult timing of the peak signal intensity of the multiple arterial phase imaging.
Gadoxetic acid has a higher relaxivity than other extracellular contrast agents; however, based on our experiences, gadoxetic acid-associated contrast enhancement tends to decrease slightly in the arterial phase when compared with gadoteric acid-associated contrast enhancement. Moreover, because the concentration of gadoxetic acid was low, the signal-to-noise ratio was lower than those pertaining to other extracellular contrast agents. In addition, the low dose of gadoxetic acid may lead to lesser contrast enhancement in the first phase of the multiple arterial phases and degraded image quality. Therefore, the image quality would improve in the last phase when the contrast agent is fully filled. Furthermore, the recently introduced dilution contrast injection method is thought to prevent truncation artifacts in the early arterial phase, resulting in rapid changes of contrast levels in soft tissues [15, 19].
The third principal finding of our study is that among the three factors evaluated, the “artifact” had the lowest scores in both contrast agents. The sharpness of the intrahepatic vessel and the liver edge had relatively high scores. These two evaluated factors seemed to have helped to assess the appropriateness of the image quality that was less affected by the artifact. This is possibly due to single-breath-hold image acquisition in our study rather than respiratory triggering. Liver edge blurring and poor depiction of intrahepatic vessels are usually used to assess the quality of images obtained by respiratory-triggered acquisition [26]. Compared with the artifacts in the pre-contrast image and each arterial phase image, those in the first arterial phase with gadoxetic acid were more severe, with statistically significant differences. Despite the relatively prominent image degradation caused by artifacts, other evaluation factors and the overall image quality were still acceptable.
In our study, we did not subcategorize specific artifact types; most of the artifacts were similar and they were motion or ringing artifacts. These findings are consistent with those of Davenport MS et al., who found that intravenous gadoxetate disodium resulted in acute self-limiting dyspnea, which had a deleterious effect on the arterial phase MR image quality, mainly manifesting as respiratory motion artifacts [11]. Tanimoto et al. showed that ringing artifacts derived from truncation artifacts in a narrow sense and phase ghost from organs and vessels were more susceptible in gadoxetic acid-enhanced MRIs than in other GBCA-enhanced MRIs [16].
The image quality of MRI and computed tomography (CT) can be affected by hardware, software, and patients’ factors. Compared with CT, MRI has a longer scan time and many sequences; in addition, the proficiency of the MRI technologist is also likely to affect image quality; therefore, we also evaluated the differences in the quality of images obtained by MRI technologists. Seven technologists performed a total of 211 consecutive MR examinations, and no differences in the quality of images acquired by them were observed.
Our study has several limitations. First, although the reviewers were blinded to the administered contrast agents, the GBCA subgroup was not randomized, and most baseline risk factors between the two groups were not similar. Generally, more compromised general medical conditions were observed in the gadoxetic acid group, and breath-hold during the arterial phase was more difficult, thus causing image quality degradation. However, better image quality was observed in the pre-contrast phase in the gadoxetic acid group. This finding suggests that the baseline risk factor differences in the two groups do not influence the study results. Second, a recent study has shown that end-expiration breath-hold methods reduce respiratory motion artifacts over end-inspiration breath-hold methods in T1WI abdominal MRI [27]. Also, the recently introduced dilution contrast injection method is thought to prevent truncation artifacts in the early arterial phase [15, 19]. However, the MR acquisition protocol utilized the end-inspiration method, and contrast dilution was not used in the current study; thus, these factors were thought to have influenced our results. Third, only the subjective qualitative analysis was performed, not quantitative analysis. Moreover, we did not assess the overall performance of the technique in the diagnosis of focal hepatic lesions. Fourth, the intra-reviewer agreement was not assessed for image quality.
In conclusions, intravenous gadoxetic acid can have a detrimental effect on the image quality of triple arterial phase MR imaging with the 4D eTHRIVE Keyhole technique. The third arterial phase images had the best image quality regardless of the contrast media used; therefore, this phase could be used as a key scan among degraded images.
References
Yoshioka H, Takahashi N, Yamaguchi M, Lou D, Saida Y, Itai Y (2002) Double arterial phase dynamic MRI with sensitivity encoding (SENSE) for hypervascular hepatocellular carcinomas. J Magn Reson Imaging 16:259-266. https://doi.org/10.1002/jmri.10146
Taouli B, Martin AJ, Qayyum A, Merriman RB, Vigneron D, Yeh BM, Coakley FV (2004) Parallel imaging and diffusion tensor imaging for diffusion-weighted MRI of the liver: preliminary experience in healthy volunteers. AJR Am J Roentgenol 183:677-680. https://doi.org/10.2214/ajr.183.3.1830677
Ito K, Fujita T, Shimizu A, Koike S, Sasaki K, Matsunaga N, Hibino S, Yuhara M (2004) Multiarterial phase dynamic MRI of small early enhancing hepatic lesions in cirrhosis or chronic hepatitis: differentiating between hypervascular hepatocellular carcinomas and pseudolesions. AJR Am J Roentgenol 183:699-705. https://doi.org/10.2214/AJR.183.3.1830699
Beck GM, De Becker J, Jones AC, von Falkenhausen M, Willinek WA, Gieseke J (2008) Contrast-enhanced timing robust acquisition order with a preparation of the longitudinal signal component (CENTRA plus) for 3D contrast-enhanced abdominal imaging. J Magn Reson Imaging 27:1461-1467. https://doi.org/10.1002/jmri.21393
Kanematsu M, Goshima S, Kondo H, Yokoyama R, Kajita K, Hoshi H, Onozuka M, Nozaki A, Hirano M, Shiratori Y, Moriyama N (2006) Double hepatic arterial phase MRI of the liver with switching of reversed centric and centric K-space reordering. AJR Am J Roentgenol 187:464-472. https://doi.org/10.2214/AJR.05.0522
Low RN, Bayram E, Panchal NJ, Estkowski L (2010) High-resolution double arterial phase hepatic MRI using adaptive 2D centric view ordering: initial clinical experience. AJR Am J Roentgenol 194:947-956. https://doi.org/10.2214/AJR.09.2507
McKenzie CA, Lim D, Ransil BJ, Morrin M, Pedrosa I, Yeh EN, Sodickson DK, Rofsky NM (2004) Shortening MR image acquisition time for volumetric interpolated breath-hold examination with a recently developed parallel imaging reconstruction technique: clinical feasibility. Radiology 230:589-594. https://doi.org/10.1148/radiol.2302021230
Rofsky NM, Lee VS, Laub G, Pollack MA, Krinsky GA, Thomasson D, Ambrosino MM, Weinreb JC (1999) Abdominal MR imaging with a volumetric interpolated breath-hold examination. Radiology 212:876-884. https://doi.org/10.1148/radiology.212.3.r99se34876
Hong HS, Kim HS, Kim MJ, De Becker J, Mitchell DG, Kanematsu M (2008) Single breath-hold multiarterial dynamic MRI of the liver at 3T using a 3D fat-suppressed keyhole technique. J Magn Reson Imaging 28:396-402. https://doi.org/10.1002/jmri.21442
Tanimoto A, Lee JM, Murakami T, Huppertz A, Kudo M, Grazioli L (2009) Consensus report of the 2nd International Forum for Liver MRI. Eur Radiol 19 Suppl 5:S975-S989. https://doi.org/10.1007/s00330-009-1624-y
Davenport MS, Viglianti BL, Al-Hawary MM, Caoili EM, Kaza RK, Liu PS, Maturen KE, Chenevert TL, Hussain HK (2013) Comparison of acute transient dyspnea after intravenous administration of gadoxetate disodium and gadobenate dimeglumine: effect on arterial phase image quality. Radiology 266:452-461. https://doi.org/10.1148/radiol.12120826
Bayer Inc. (2017) PRIMOVIST product Monograph: part III Consumer information. http://www.bayer.ca/omr/online/primovist-pm-pt3-en.pdf. Accessed on March 28 2019.
Haradome H, Grazioli L, Tsunoo M, Tinti R, Frittoli B, Gambarini S, Morone M, Motosugi U, Colagrande S (2010) Can MR fluoroscopic triggering technique and slow rate injection provide appropriate arterial phase images with reducing artifacts on gadoxetic acid-DTPA (Gd-EOB-DTPA)-enhanced hepatic MR imaging? J Magn Reson Imaging 32:334-340. https://doi.org/10.1002/jmri.22241
Feuerlein S, Boll DT, Gupta RT, Ringe KI, Marin D, Merkle EM (2011) Gadoxetate disodium-enhanced hepatic MRI: dose-dependent contrast dynamics of hepatic parenchyma and portal vein. AJR Am J Roentgenol 196:W18-W24. https://doi.org/10.2214/AJR.10.4387
Motosugi U, Ichikawa T, Sou H, Sano K, Ichikawa S, Tominaga L, Araki T (2009) Dilution method of gadolinium ethoxybenzyl diethylenetriaminepentaacetic acid (Gd-EOB-DTPA)-enhanced magnetic resonance imaging (MRI). J Magn Reson Imaging 30:849-854. https://doi.org/10.1002/jmri.21913
Tanimoto A, Higuchi N, Ueno A (2012) Reduction of ringing artifacts in the arterial phase of gadoxetic acid-enhanced dynamic MR imaging. Magn Reson Med Sci 11:91-97. https://doi.org/10.2463/mrms.11.91
Ringe KI, Husarik DB, Sirlin CB, Merkle EM (2010) Gadoxetate disodium-enhanced MRI of the liver: part 1, protocol optimization and lesion appearance in the noncirrhotic liver. AJR Am J Roentgenol 195:13-28. https://doi.org/10.2214/AJR.10.4392
Zech CJ, Vos B, Nordell A, Urich M, Blomqvist L, Breuer J, Reiser MF, Weinmann HJ (2009) Vascular enhancement in early dynamic liver MR imaging in an animal model: comparison of two injection regimen and two different doses Gd-EOB-DTPA (gadoxetic acid) with standard Gd-DTPA. Invest Radiol 44:305-310. https://doi.org/10.1097/RLI.0b013e3181a24512
Kim YK, Lin WC, Sung K, Raman SS, Margolis D, Lim Y, Gu S, Lu D (2016) Reducing Artifacts during Arterial Phase of Gadoxetate Disodium-enhanced MR Imaging: Dilution Method versus Reduced Injection Rate. Radiology 283:429-437. https://doi.org/10.1148/radiol.2016160241
Moore KP, Wong F, Gines P, Bernardi M, Ochs A, Salerno F, Angeli P, Porayko M, Moreau R, Garcia-Tsao G, Jimenez W, Planas R, Arroyo V (2003) The management of ascites in cirrhosis: report on the consensus conference of the International Ascites Club. Hepatology 38:258-266. https://doi.org/10.1053/jhep.2003.50315
Cruite I, Schroeder M, Merkle EM, Sirlin CB (2010) Gadoxetate disodium-enhanced MRI of the liver: part 2, protocol optimization and lesion appearance in the cirrhotic liver. AJR Am J Roentgenol 195:29-41. https://doi.org/10.2214/AJR.10.4538
Tamada T, Ito K, Sone T, Yamamoto A, Yoshida K, Kakuba K, Tanimoto D, Higashi H, Yamashita T (2009) Dynamic contrast-enhanced magnetic resonance imaging of abdominal solid organ and major vessel: comparison of enhancement effect between Gd-EOB-DTPA and Gd-DTPA. J Magn Reson Imaging 29:636-640. https://doi.org/10.1002/jmri.21689
Pietryga JA, Burke LM, Marin D, Jaffe TA, Bashir MR (2014) Respiratory motion artifact affecting hepatic arterial phase imaging with gadoxetate disodium: examination recovery with a multiple arterial phase acquisition. Radiology 271:426-434. https://doi.org/10.1148/radiol.13131988
Motosugi U, Bannas P, Bookwalter CA, Sano K, Reeder SB (2016) An investigation of transient severe motion related to gadoxetic acid-enhanced MR imaging. Radiology 279:93-102. https://doi.org/10.1148/radiol.2015150642
Kim SY, Park SH, Wu EH, Wang ZJ, Hope TA, Chang WC, Yeh BM (2015) Transient respiratory motion artifact during arterial phase MRI with gadoxetate disodium: risk factor analyses. AJR Am J Roentgenol 204:1220-1227. https://doi.org/10.2214/AJR.14.13677
Hirokawa Y, Isoda H, Maetani YS, Arizono S, Shimada K, Togashi K (2008) MRI artifact reduction and quality improvement in the upper abdomen with PROPELLER and prospective acquisition correction (PACE) technique. AJR Am J Roentgenol 191:1154-1158. https://doi.org/10.2214/AJR.07.3657
Vu KN, Haldipur AG, Roh AT, Lindholm P, Loening AM (2019) Comparison of End-Expiration Versus End-Inspiration Breath-Holds With Respect to Respiratory Motion Artifacts on T1-Weighted Abdominal MRI. AJR Am J Roentgenol 212:1024-1029. https://doi.org/10.2214/AJR.18.20239
Funding
No funding was received for this study.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki Declaration and its later amendments or comparable ethical standards.
Informed consent
The Institutional Review Board waived the requirement for informed consent for this retrospective study.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Sim, K.C., Park, B.J., Han, N.Y. et al. Effects of gadoxetic acid on image quality of arterial multiphase magnetic resonance imaging of liver: comparison study with gadoteric acid-enhanced MRI. Abdom Radiol 44, 4037–4047 (2019). https://doi.org/10.1007/s00261-019-02202-0
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00261-019-02202-0