Abstract
Combined PET/MRI is a proposed imaging modality for rectal cancer, leveraging the advantages of MRI and 18F-fluorodeoxyglucose PET. Rectal cancer PET/MRI protocols typically include dedicated pelvis bed positions utilizing small field-of-view T2-weighted imaging. For staging of the primary tumor, PET/MRI can help delineate the extent of tumor better as well as the extent of tumor beyond the muscularis propria. PET uptake may help characterize small lymph nodes, and the use of hepatobiliary phase imaging can improve the detection of small hepatic metastases. The most beneficial aspect of PET/MRI may be in treatment response, although current data are limited on how to combine PET and MRI data in this setting. Limitations of PET/MRI include the inability to detect small pulmonary nodules and issues related to attenuation correction, although the development of new attenuation correction techniques may address this issue. Overall PET/MRI can improve the staging of rectal cancer, although this potential has yet to be fulfilled.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Combined PET/MRI is a proposed effective imaging modality for rectal cancer, leveraging the advantages of MRI, functional MRI, and 18F-fluorodeoxyglucose (FDG)-PET. These devices offer an appealing combination for imaging patients with rectal cancer, given the central role of MRI in rectal cancer staging and treatment response assessment. MRI is the standard imaging modality for staging primary rectal tumors, but is limited in its ability to detect metastatic disease, characterize lymph node involvement, and predict pathologic response after chemoradiation. Although there is promise for the role of PET/MRI in rectal cancer, the role of simultaneous evaluation of PET and MRI and how to use the information to improve the characterization of disease has yet to be defined. Below, we discuss the potential role of PET/MRI in patients with rectal cancer.
Proposed protocols
There are currently two FDA approved simultaneous PET/MRI devices in the United States: the mMR from Siemens and the Signa PET/MRI from GE Healthcare. Both systems have a 25-cm z-axis field of view and allow for the simultaneous acquisition of PET and MRI data. Protocols for rectal cancer PET/MRI include a dedicated MRI of the pelvis, which includes three-plane T2-weighted images (oblique axial, coronal, and sagittal) and T1-weighted 3D spoiled gradient echo images (LAVA or VIBE depending on the manufacturer) (Fig. 1). MR pulse sequence parameters used for these sequences in the setting of PET/MRI mirror that of what is acquired on stand-alone MRI systems have been previously described in depth [1]. The acquisition of diffusion-weighted imaging (DWI) and dynamic contrast-weighted imaging is not required, although many sites acquire DWI in the setting of response assessment. One hindrance in the setting of PET/MRI that is not encountered with routine MRI is the requirement that all MRI sequences on hybrid scanners must have their isocenter at the center of the PET field-of-view (FOV). Therefore, the initial selection of PET FOV placement is a crucial initial step. Additionally, in the setting of low-lying rectal tumors, it may not be possible to plan an oblique-coronal sequence through the anus, particularly if the PET FOV is too high relative to the anus.
In addition to the pelvis bed position, a whole-body PET acquisition is typically acquired. Most centers acquire T1-weighted images and T2-weighted images at each bed position, although there is significant variability in which T2-weighted images are acquired: single-shot half Fourier (SSFSE/HASTE) or fast spin-echo sequences (TSE/FSE). Although it is possible to combine the pelvis bed position with the whole-body acquisition, most centers perform each separately. DWI is often not used in the whole-body acquisition, as it rarely aids in detection of metastatic disease compared to FDG-PET [2].
Finally, it is possible to acquire a dedicated liver MRI in addition to the pelvis and whole-body acquisitions. This is not frequently performed, but can be beneficial given that the liver is a common location for metastatic lesions. If a dedicated liver MRI is being performed, hepatobiliary-specific contrast is typically used.
Benefits in imaging of the primary tumor
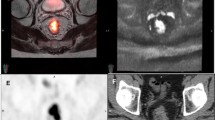
At initial staging, the high soft-tissue resolution provided by MRI allows for reliable assessment of tumor stage and the circumferential resection margin that is required for appropriate surgical planning [3, 4]. Although MRI is the reference standard for T-staging, the combination of FDG-PET with MRI can help in both lesion detection as well as margin delineation of the primary tumor [5]. Simultaneous evaluation of PET and MRI can improve reader confidence and help characterize extension of tumor beyond the muscularis propria (Fig. 2). Overall, the combined benefit of PET/MRI is limited for staging of primary tumors compared to MRI alone.
A 43-year-old man with newly diagnosed rectal adenocarcinoma. PET/MRI demonstrates a circumferential hypermetabolic rectal mass. On oblique axial (a–c) and coronal (d–f) T2-weighted and PET images, through the tumor soft-tissue extending from the primary tumor into the mesorectal fat is noted consistent with extramural vascular invasion (white and black arrows)
Benefits in imaging nodal disease
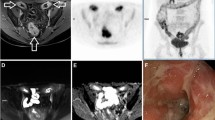
One of the central issues in rectal cancer MRI is the accurate characterization of small lymph nodes. Nodes measuring greater than 1 cm are easily characterized as malignant on MRI, but when nodes measure less than 1 cm and particularly less than 5 mm, the specificity of MRI falls [6, 7]. Although not studied as extensively as MRI for nodal characterization, hypermetabolism on PET appears to have a higher specificity than MRI, particularly in small nodes [8], therefore it is possible that combining PET with MRI can help better characterize small pelvic nodes (Fig. 3) [8]. Unfortunately DWI does not add value in characterization in pelvic nodes, and uptake on FDG-PET is more valuable for nodal characterization [9,10,11]. One other unexpected benefit of PET/MRI is that the longer PET acquisition that is simultaneous to the MRI sequences of the pelvis appears to result in a higher sensitivity for small perirectal nodes, particularly those that measure less than 5 mm [12]. Currently, it is not clear if there is an SUV cutoff for determining positive disease on FDG-PET in small perirectal nodes, but given the nodes’ small size and associated partial volume artifact, uptake greater than background is generally considered positive.
The use of ultra-small paramagnetic iron oxide (USPIOs) particles may play a role in staging of rectal cancer. The use of ferumoxtran-10 (Sinerem/Combidex) has been evaluated in one small series in rectal cancer patients, and demonstrated high signal intensity in involved nodes as expected [13]. Unfortunately, the future of this agent is unclear, and it is currently not available. Off-label use of ferumoxytol (Feraheme) has been studied preliminarily in the setting of prostate cancer and currently there is a Phase-1 study evaluating its role in rectal cancer [14, 15]. How the use of USPIOs in the setting of PET/MRI has yet to be explored.
Benefits in imaging metastatic disease
In the evaluation of metastatic disease, PET/MRI is particularly valuable for hepatic staging both given that the liver is a common site for metastatic disease and that MRI is the reference standard in liver imaging. Hepatobiliary phase (HBP) MRI of the liver is the preferred imaging modality to identify colon cancer liver metastasis [4]. Other investigators have shown added value of whole-body-integrated PET/MRI over contrast-enhanced CT alone in the detection and characterization of metastatic lesions [16, 17]. Combining HBP MRI in the setting of a rectal PET/MRI is feasible although care has to be taken to keep the total scan length to a reasonable time (Figs. 4, 5). Results of one retrospective study suggest PET/MRI may aid in the selection of more appropriate treatment strategies for colorectal cancer patients, with the treatment strategy changed in 21% of patients after PET/MRI added information to the CECT findings [18].
A 52-year-old man with a newly diagnosed rectal adenocarcinoma. A whole-body PET/MRI with dedicated rectal and liver bed positions was performed (a). In the rectal bed position, a 5-mm right perirectal node is seen which demonstrates hypermetabolism consistent with nodal metastatic disease (b circle, d and f black arrow). In the liver, a 6-mm caudate lobe lesion was visualized on hepatobiliary phase imaging (c), which demonstrated hypermetabolism (e and g, black circle) consistent with hepatic metastatic disease
A 65-year-old male status post abdominal peritoneal resection with a non-specific lesion on CT (a and d). At hepatobiliary phase MRI (b and e) and FDG-PET (c and f), no suspicious features are identified. This case demonstrates how PET/MRI provides improved characterizations of liver lesions compared to PET/CT
PET/MRI for evaluation of treatment response
Although MRI is frequently used for surgical planning after chemoradiation, MRI has many limitations as a predictor of treatment response. Changes in tumor/node size do not correlate well with response and DWI is often limited by artifact, therefore the combination with FDG-PET may improve response characterization (Figs. 6, 7). It has been demonstrated that percent change in tumor SUVmax and changes in DWI (apparent diffusion coefficient) post-neoadjuvant therapy are predictors of outcome. Although SUVmax is well established, it may not reflect the heterogeneous nature of the tumor. Volume-based PET parameters such as metabolic tumor volume (MTV) and total lesion glycolysis (TLG) have been developed to measure metabolic activity in the entire tumor mass. MTV represents the dual characteristics of tumor volume and extent of FDG uptake by tumor tissues. TLG has been proposed as a more accurate parameter as it takes into account SUVmean and MTV [19]. One preliminary study postulated that a higher SUV-to-ADC ratio indicates a more aggressive tumor [20], although given the tight inverse correlation between SUV and ADC it is unclear what the additive value of DWI and SUV is [2]. It may be that DWI correlates more with changes in fibrosis and extracellular space, while metabolism correlates with cellular density, although frequently these factors move inversely [21]. How one uses MRI and PET to improve characterization has yet to be defined, but in general one looks for changes in FDG-PET and changes in DWI/T2WI to determine if there has been a response to tumor. In many cases, there may be disagreement between changes in size/diffusion and changes in metabolism that might suggest a different response than based on MRI alone (Fig. 6).
A 72-year-old female with rectal adenocarcinoma before (a) and after chemoradiation (b). Post-therapy images suggest residual tumor on T2-weighted images, given that there was only a 24% reduction in tumor volume (b, white-dotted circle). FDG-PET shows a marked reduction in metabolic activity, with a reduction of the SUVmax from 13.7 to 4.5 (a 67% reduction). DWI images show a decrease in tumor that demonstrates restricted diffusion (c, white circle and d, white arrow). MRI indicated partial response on T2-weighted imaging, while PET imaging suggests potentially a complete response. At pathology this was confirmed a complete response
PET/MRI performed in a 53-year-old man three years after surgery with a rising CEA. Axial T2 (a) demonstrates circumferential soft-tissue thickening with heterogeneously mixed hyperintense and hypointense signal intensity within the surgical bed (arrow). Fused axial PET image (d) demonstrates intense FDG uptake within this circumferential soft-tissue mass consistent with local recurrence. Inferiorly in the same patient, the MRI appearance is similar with semicircular T2 signal hypointensity (b, arrow), but FDG-PET demonstrates an absence of hypermetabolism (e) consistent with fibrosis. At pathology, one can see both recurrent tumor (c and f, white arrows) as well as fibrosis surrounding the region of local recurrence (c and f, open arrows). The addition of FDG-PET increases reader confidence and sensitivity for local recurrence
FDG-PET is also valuable in identifying distant tumor recurrence, and in combination with MRI, may assist in differentiating post-therapy scar/desmoplastic reaction from residual tumor or local recurrence [3]. In the setting of recurrent tumor with suspicious lesions, PET/MRI had a sensitivity and specificity of 94% compared to pathology, although no comparison to MRI or PET interpretations was provided [22]. Non-operative management of rectal cancer is an emerging trend with growing interest. Therefore, evaluation of treatment response, and in particular the characterization of complete pathologic response, is key. Current clinical oncology guidelines do not include metabolic assessments in the evaluating response to therapy [23, 24]. It should be noted that there are competing technologies for determining the presence of residual tumor and approaches using circulating microparticles, including tumor DNA, may provide additional information [25].
Limitations of PET/MRI
One of the main challenges in oncologic imaging using PET/MRI is the limited ability of MRI to image pulmonary nodules compared to CT. This limitation is primarily due to two effects: [1] significant respiratory motion, and [2] inherent relatively low-proton density of lung parenchyma. Although conventional MRI sequences can typically image nodules greater than 1 cm, smaller lesions are frequently missed [26]. It should be noted that for hypermetabolic lesions seen on PET, an anatomic correlate was seen over 95% of the time on MRI, but the measurement accuracy of the visualized nodules is neither accurate nor reproducible with current imaging techniques. Recent approaches have used a combination of respiratory-gated techniques with ultrashort echo time (UTE) sequences, to overcome the short T2* of the lung. These have shown promise in detecting smaller pulmonary nodules [27], although they are not robust enough to use in routine clinical imaging.
Accuracy of PET quantification also remains a challenge in clinical imaging. Obtaining accurate measurements of uptake on PET imaging requires an attenuation map to correct for attenuation of photons. With PET/CT, attenuation maps are easily created by converting the CT images into an attenuation map, but attenuation map creation poses a unique challenge in PET/MRI, as MRI images reflect proton density rather than electron density. The standard approach for attenuation correction in the pelvis uses two-point Dixon approaches and can result in up to 20% error in pelvic lesions, with the highest error in bone lesions [28]. A number of novel approaches have been developed for pelvis applications that can minimize this error, including use of zero echo time (ZTE) and UTE-based imaging, as well as utilization of deep learning techniques to convert MRI images directly into density maps [29, 30].
Reimbursement is another controversial issue with regard to clinical PET/MRI imaging. Many insurance companies will not cover PET imaging once CT abdomen and pelvis has been acquired, and organizations such as the Society of Surgical Oncologists have argued against using PET imaging in rectal cancer [31]. Although PET has limited role in T1/2 rectal cancers, it can have a significant impact in patients at higher risk for metastatic disease [32].
One final limitation is that there currently are no agreed upon guidelines as to how to interpret PET and MRI simultaneously. PET, for example, can have false positives in the setting of inflammation, which may mimic recurrent disease [33]. How to decide when PET findings should drive the interpretation or MRI findings should be used has yet to be delineated.
Currently, there is no clear benefit in terms of diagnostic accuracy or tissue characterization for the simultaneous acquisition of PET/MRI, compared to sequentially acquired MRI and PET/CT. Simultaneous acquisitions do improve patient convenience and aid in fusion, but this may justify the expenditure required to acquire a PET/MRI. The sequential acquisition using PET/CT does provide improved imaging of pulmonary nodules.
Potential role for PET/MRI in clinical trials
The role of PET/MRI in evaluation of rectal cancer has yet to be defined. PET/MRI has the potential to develop into a staging and post-treatment imaging modality for rectal cancer, with the ability to provide prognostic information and potentially stratify patients for surgical versus non-surgical treatment. Currently, it is difficult to include PET/MRI in the setting of cooperative group or other national trials, given the limited availability of the technology. Nonetheless, it may be possible to incorporate PET/MRI into future prospective randomized studies at sites where it is available, in order to compare PET/MRI staging with standard of care imaging. Acquiring these data would also facilitate additional studies to evaluate the role of baseline and post-neoadjuvant therapy PET/MRI; parameters for predicting response to neoadjuvant therapy; and development of standardized criteria to differentiate responders from non-responders. As an initial step, the development of harmonized protocols across PET/MRI sites would be valuable to allow for the pooling of data across sites that may inform trial design in the future.
Conclusions
In rectal cancer, the role of PET/MRI has yet to be defined, although there is promise both in initial staging and in evaluation of treatment response. The addition of hepatobiliary imaging in addition to an abbreviated pelvic bed position can help increase the value of PET/MRI relative to other modalities, although impacts the workflow of the study. Overall, issues related to reimbursement, scanner availability, and inclusions into clinical trials may impact PET/MRIs role moving forward in rectal cancer staging.
References
Horvat N, Carlos Tavares Rocha C, Clemente de Oliveira B, Petkovska I, Gollub MJ. MRI of Rectal Cancer: Tumor Staging, Imaging Techniques, and Management. Radiographics. 2019;:180114.
Jeong JH, Cho IH, Chun KA, Kong EJ, Kwon SD, Kim JH. Correlation Between Apparent Diffusion Coefficients and Standardized Uptake Values in Hybrid (18)F-FDG PET/MR: Preliminary Results in Rectal Cancer. Nucl Med Mol Imaging. Springer Berlin Heidelberg. 2016;50:150–156.
Buchbender C, Heusner TA, Lauenstein TC, Bockisch A, Antoch G (2012) Oncologic PET/MRI, part 1: tumors of the brain, head and neck, chest, abdomen, and pelvis. J Nucl Med. 53:928–938
Park MJ, Kim SH, Lee SJ, Jang KM, Rhim H (2011) Locally advanced rectal cancer: added value of diffusion-weighted MR imaging for predicting tumor clearance of the mesorectal fascia after neoadjuvant chemotherapy and radiation therapy. Radiology. 260:771–780
Rosenkrantz AB, Friedman K, Chandarana H, et al. (2016) Current Status of Hybrid PET/MRI in Oncologic Imaging. American Journal of Roentgenology. 206:162–172
Langman G, Patel A, Bowley DM (2015) Size and distribution of lymph nodes in rectal cancer resection specimens. Dis Colon Rectum. 58:406–414
Lahaye MJ, Beets GL, Engelen SME, et al. Locally advanced rectal cancer: MR imaging for restaging after neoadjuvant radiation therapy with concomitant chemotherapy. Part II. What are the criteria to predict involved lymph nodes? Radiology. 2009;252:81–91.
Kim DJ, Kim JH, Ryu YH, et al. (2011) Nodal staging of rectal cancer: high-resolution pelvic MRI versus 18F-FDGPET/CT. J Comput Assist Tomogr. 35:531–534
Cerny M, Dunet V, Prior JO, et al. (2016) Initial Staging of Locally Advanced Rectal Cancer and Regional Lymph Nodes: Comparison of Diffusion-Weighted MRI With 18F-FDG-PET/CT. Clin Nucl Med. Clinical Nuclear Medicine. 41:289–295
Heijnen LA, Lambregts DMJ, Mondal D, et al. (2013) Diffusion-weighted MR imaging in primary rectal cancer staging demonstrates but does not characterise lymph nodes. European radiology. 23:3354–3360
Cho EY, Kim SH, Yoon J-H, et al. (2013) Apparent diffusion coefficient for discriminating metastatic from non-metastatic lymph nodes in primary rectal cancer. Eur J Radiol. 82:e662–e668
Bailey JJ, Jordan EJ, Burke C, et al. Does Extended PET Acquisition in PET/MRI Rectal Cancer Staging Improve Results? American Journal of Roentgenology. 2018;:1–5.
Koh D-M, Brown G, Temple L, et al. (2004) Rectal cancer: mesorectal lymph nodes at MR imaging with USPIO versus histopathologic findings–initial observations. Radiology. 231:91–99
Ferumoxytol-Enhanced MRI in Imaging Lymph Nodes in Patients With Locally Advanced Rectal Cancer. clinicaltrials.gov.https://clinicaltrials.gov/ct2/show/NCT03280277. Accessed May 22, 2019
Turkbey B, Agarwal HK, Shih J, et al. (2015) A Phase I Dosing Study of Ferumoxytol for MR Lymphography at 3 T in Patients With Prostate Cancer. American Journal of Roentgenology. 205:64–69
Hope TA, Pampaloni MH, Nakakura E, et al. Simultaneous (68)Ga-DOTA-TOC PET/MRI with gadoxetate disodium in patients with neuroendocrine tumor. Abdom Imaging. Springer US. 2015;40:1432–1440.
Sawicki LM, Deuschl C, Beiderwellen K, et al. Evaluation of (68)Ga-DOTATOC PET/MRI for whole-body staging of neuroendocrine tumours in comparison with (68)Ga-DOTATOC PET/CT. European radiology. Springer Berlin Heidelberg. 2017;26:3063–3069.
Kang B, Lee JM, Song YS, et al. (2016) Added Value of Integrated Whole-Body PET/MRI for Evaluation of Colorectal Cancer: Comparison With Contrast-Enhanced MDCT. American Journal of Roentgenology. 206:W10–W20
Avallone A, Aloj L, Pecori B, et al. 18F-FDG PET/CT Is an Early Predictor of Pathologic Tumor Response and Survival to Preoperative Radiochemotherapy with Bevacizumab in High Risk Locally Advanced Rectal Cancer. Journal of Nuclear Medicine. 2019;:jnumed.118.222604–jnumed.118.222629.
Rakheja R, Chandarana H, DeMello L, et al. (2013) Correlation between standardized uptake value and apparent diffusion coefficient of neoplastic lesions evaluated with whole-body simultaneous hybrid PET/MRI. American Journal of Roentgenology. 201:1115–1119
Cerny M, Dunet V, Rebecchini C, et al. (2019) Response of locally advanced rectal cancer (LARC) to radiochemotherapy: DW-MRI and multiparametric PET/CT in correlation with histopathology. Nuklearmedizin. 58:28–38
Plodeck V, Rahbari NN, Weitz J, et al. FDG-PET/MRI in patients with pelvic recurrence of rectal cancer: first clinical experiences. European radiology. Springer Berlin Heidelberg. 2019;29:422–428.
Altini C, Niccoli Asabella A, De Luca R, et al. Comparison of (18)F-FDG PET/CT methods of analysis for predicting response to neoadjuvant chemoradiation therapy in patients with locally advanced low rectal cancer. Abdom Imaging. Springer US. 2015;40:1190–1202.
Ippolito D, Monguzzi L, Guerra L, et al. (2012) Response to neoadjuvant therapy in locally advanced rectal cancer: assessment with diffusion-weighted MR imaging and 18FDG PET/CT. Abdom Imaging. 37:1032–1040
Tie J, Wang Y, Tomasetti C, et al. Circulating tumor DNA analysis detects minimal residual disease and predicts recurrence in patients with stage II colon cancer. Sci Transl Med. 2016;8:346ra92.
Chandarana H, Heacock L, Rakheja R, et al. (2013) Pulmonary nodules in patients with primary malignancy: comparison of hybrid PET/MR and PET/CT imaging. Radiology. 268:874–881
Burris NS, Johnson KM, Larson PEZ, et al. (2016) Detection of Small Pulmonary Nodules with Ultrashort Echo Time Sequences in Oncology Patients by Using a PET/MR System. Radiology. 278:239–246
Samarin A, Burger C, Wollenweber SD, et al. (2012) PET/MR imaging of bone lesions - implications for PET quantification from imperfect attenuation correction. Eur J Nucl Med Mol Imaging. 39:1154–1160
Leynes AP, Yang J, Shanbhag DD, et al. (2017) Hybrid ZTE/Dixon MR-based attenuation correction for quantitative uptake estimation of pelvic lesions in PET/MRI. Med Phys. 44:902–913
Leynes AP, Yang J, Wiesinger F, et al. (2018) Zero-Echo-Time and Dixon Deep Pseudo-CT (ZeDD CT): Direct Generation of Pseudo-CT Images for Pelvic PET/MRI Attenuation Correction Using Deep Convolutional Neural Networks with Multiparametric MRI. J Nucl Med. 59:852–858
Zukotynski K, Jadvar H, Hope TA, et al. (2017) SNMMI Comment on the 2016 Society of Surgical Oncology “Choosing Wisely” Recommendation on the Use of PET/CT in Colorectal Cancer. J Nucl Med. 58:11–12
Ozis SE, SOYDAL Ç, Akyol C, et al. The role of 18F-fluorodeoxyglucose positron emission tomography/computed tomography in the primary staging of rectal cancer. World J Surg Oncol. BioMed Central. 2014;12:26.
Rosenberg R, Herrmann K, Gertler R, et al. (2009) The predictive value of metabolic response to preoperative radiochemotherapy in locally advanced rectal cancer measured by PET/CT. Int J Colorectal Dis. Springer-Verlag. 24:191–200
Acknowledgements
T.A.H. was supported by the National Institutes of Health (Grant R01CA212148).
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
CME activity This article has been selected as the CME activity for the current month. Please visit https://ce.mayo.edu/node/85923 and follow the instructions to complete this CME activity.
Rights and permissions
About this article
Cite this article
Hope, T.A., Kassam, Z., Loening, A. et al. The use of PET/MRI for imaging rectal cancer. Abdom Radiol 44, 3559–3568 (2019). https://doi.org/10.1007/s00261-019-02089-x
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00261-019-02089-x