Abstract
Purpose
To evaluate factors that may affect successful ultrasound-guided percutaneous thrombin injection of iatrogenic femoral artery pseudoaneurysms (PSA).
Materials and methods
This was an IRB-approved, HIPAA-compliant retrospective study of 326 consecutive subjects (138 males, 188 females; mean age 68 years, range 18–95) who underwent thrombin injection for treatment of femoral PSA; follow-up ultrasound was available in 145 subjects. The number of PSA lobes and dimensions, pre-procedure laboratory values (international normalized ratio [INR], activated partial thromboplastin time [aPTT], platelet count), and concomitant anticoagulation therapy were recorded.
Results
Technical success was achieved in 98.2% (320/326) of subjects. Primary effectiveness (complete thrombosis at 24 h) was achieved in 74.5% (108/145). Twenty-five subjects underwent repeat thrombin injection, successful in 21 subjects, for a total effectiveness rate of 97.0% (129/133). No imaging factor was associated with technique failure, including number of lobes (p = 0.898), largest dimension (p = 0.344), or volume (p = 0.697). No statistically significant difference in pre-procedure INR, aPTT, or platelet count was found between subjects with CT and those with IT (p > 0.138). Anticoagulation therapy was associated with incomplete thrombosis (35.5% [38/107] for CT vs. 63.9% [23/26] for IT; p = 0.002).
Conclusion
Imaging-guided percutaneous thrombin injection has high technical success and effectiveness rates for the treatment of iatrogenic femoral artery PSA. Anticoagulation therapy was the only factor associated with incomplete thrombosis.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
A pseudoaneurysm is a false aneurysm representing contained extravascular blood flow that communicates with an artery. Pseudoaneurysms can be seen following penetrating injury and are an uncommon but well-recognized complication of endovascular procedures requiring arterial puncture, reported in up to 8% of post-procedural patients [1,2,3]. Physical examination of a patient with a pseudoaneurysm may demonstrate overlying ecchymosis, soft tissue swelling, or bruit on auscultation. Ultrasonographic evaluation may demonstrate a complex fluid collection within the area of puncture, frequently the groin, composed of a single or multiple loculations; swirling of internal blood flow on color Doppler evaluation in a “yin-yang” pattern; and a “to-and -fro” flow pattern on spectral Doppler evaluation of the pseudoaneurysm neck.
Pseudoaneurysms can cause pain, swelling, compression of adjacent structures, and can rupture [4,5,6]. As such, pseudoaneurysms require close follow-up to ensure spontaneous thrombosis and frequently require intervention. Primary surgical closure has historically been considered the standard management [5]. However, this is invasive, may require regional or general anesthesia, and carries a higher rate of morbidity compared with percutaneous techniques [7,8,9]. Given this, there has been a shift toward minimally invasive management of pseudoaneurysms. Endovascular repair utilizing a covered stent can be used to treat both pseudoaneurysms as well as arteriovenous fistulae, an additional complication of endovascular procedures; however, over time there is a risk of stent thrombosis and required repeat intervention [5, 10]. Ultrasound-guided compression, introduced in 1991, is a noninvasive technique, but is time-consuming, painful, may require moderate sedation, and has a success rate of only 75%, or less if the patient is on anticoagulation [3, 5, 11,12,13].
Since its description by Kang et al. in 1998, ultrasound-guided percutaneous thrombin injection has become the commonly preferred treatment method [14]. Technical success is achieved much more quickly than with ultrasound-guided compression, with thrombosis seen in just a few seconds with thrombin injection, as compared to 30–40 min in ultrasound-guided compression [11, 15]. The procedure is relatively safe, with a reported complication rate of approximately 2%, most frequently distal embolization of thrombin [3, 12, 16].
Ultrasound-guided thrombin injection is generally preferred in patients on anticoagulation, given the increased failure rate associated with ultrasound-guided compression and diminished enthusiasm for surgical repair while on systemic anticoagulation [3, 12, 13, 15]. Following cardiac catheterization, many patients are started on antiplatelet and/or anticoagulant therapy and it is possible that these patients may have lower success rates (incomplete thrombosis) following percutaneous thrombin injection in a femoral PSA. Previous studies have suggested that thrombocytopenia is associated with lower success rates following thrombin injection [17] but that administration of antiplatelet/anticoagulation agents do not preclude successful treatment [3, 13]; however, the data are limited.
The purpose of this study was to review our institutions’ experience with ultrasound-guided percutaneous thrombin injection of iatrogenic femoral artery pseudoaneurysms and evaluate our technical success rate and technique effectiveness rates (primary and secondary success). An additional goal was to identify any clinical or imaging factors that may affect successful ultrasound-guided percutaneous thrombin injection, in particular those pertaining to patient coagulopathies and administration of antiplatelet/anticoagulation agents.
Materials and methods
This was an IRB-approved, HIPAA-compliant retrospective review.
Patients
Our interventional services perform a high volume of arteriotomies per month. Local practice pattern dictates treatment of pseudoaneurysms, and the majority of pseudoaneurysms are treated with percutaneous thrombin injection at our institution. Through a search of our institutional medical record, 326 consecutive patients were identified who underwent ultrasound-guided percutaneous thrombin injection of an iatrogenic femoral artery pseudoaneurysm between March 2003 and January 2017. This included 138 male and 188 female patients, with a mean age of 68 years (range 13–95 years). Follow-up ultrasound evaluation was performed in 145 of these patients.
Diagnostic ultrasound
Standard protocol at our institution involves dedicated grayscale, color Doppler, and spectral Doppler evaluation of the femoral artery and femoral vein at three locations: superior to, at, and inferior to the puncture site. Measurements in three dimensions are obtained of any hematoma, pseudoaneurysm, and pseudoaneurysm neck, if visualized. The presence or absence of an AV fistula is documented. At our institution, the presence of an AV fistula is considered a contraindication to percutaneous thrombin injection.
Thrombin injection procedure
Pre-procedural sonographic evaluation is performed to confirm the presence of a pseudoaneurysm and to evaluate the anatomy of the pseudoaneurysm neck to confirm technical feasibility. Distal pulses of the affected extremity, including the dorsalis pedis and posterior tibial arteries, are evaluated prior to and immediately following the procedure.
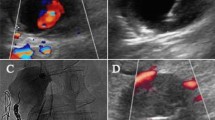
The procedure is performed utilizing sterile technique. Local anesthesia can be obtained via administration of 2% lidocaine (operator dependent). Bovine thrombin (1000 U/mL) is drawn up into a 1 mL syringe. A 22-gauge spinal needle is advanced into the pseudoaneurysm under ultrasound guidance. Thrombin is slowly injected under sonographic evaluation utilizing color Doppler until flow into the pseudoaneurysm ceases (Fig. 1).
A 76-year-old man with right femoral artery pseudoaneurysm after cardiac catheterization. a Color Doppler ultrasound image demonstrating a small pseudoaneurysm with “yin-yang” pattern of flow. b Grayscale ultrasound image demonstrating a needle tip within the pseudoaneurysm (arrow). c Color Doppler ultrasound image demonstrating lack of flow within the pseudoaneurysm at the completion of the procedure
The patient is then observed in the radiologic recovery unit or, if an inpatient, in their pre-existing hospital bed, with instructions to lie supine for a minimum of 6 h following the procedure and with arterial pulses rechecked periodically.
Procedural data
A single radiologist [BLINDED] performed a retrospective evaluation of 326 consecutive patients with an iatrogenic femoral pseudoaneurysm treated with thrombin injection via electronic medical record review. Recorded information included the number of lobes of the pseudoaneurysm; three-dimensional measurements of the pseudoaneurysm; neck width and length; and total volume of injected thrombin. The procedural note was reviewed for the presence of any immediate complications and for technical success of the procedure. Laboratory values obtained immediately prior to the procedure, including platelet count, activated partial thromboplastin time (aPTT), and international normalized ratio (INR), were recorded.
A single abdominal radiologist [BLINDED] reviewed the archived ultrasound images (both pre- and post-treatment and procedure) for all patients who underwent a follow-up ultrasound (n = 145). Confirmation of pseudoaneurysm measurements, neck length and width, technical success, and primary and secondary effectiveness rates were recorded.
Laboratory and clinical data
A single radiologist reviewed the electronic medical records for each patient who underwent a follow-up ultrasound (n = 145 patients). Height and weight were recorded and BMI was calculated where available in the electronic medical record (n = 93 patients). Sheath size was recorded where available (n = 93 patients). Other potential risk factors such as smoking history were unavailable. Record was made of any anticoagulation medications being administered at the time of thrombin injection, including aspirin, clopidogrel, heparin, warfarin, and enoxaparin. Technique effectiveness and follow-up as well as follow-up treatment for percutaneous thrombin injection failures were evaluated.
Statistical analysis
Statistical analysis was performed with Excel (version 2016, Microsoft) and R (version 3.3.2, The R Project for Statistical Computing) software. For all comparisons, p < 0.05 was considered as statistically significant. Results were summarized using descriptive statistics.
Technical success was defined as complete thrombosis (CT) (no residual flow within the pseudoaneurysm) at the time of initial thrombin injection. Technical failure was defined as incomplete thrombosis (IT) (any degree of residual flow within the pseudoaneurysm) at the time of initial thrombin injection. The primary effectiveness rate was defined by achieving complete pseudoaneurysm thrombosis at 24-h follow-up ultrasound. The secondary effectiveness rate was defined as achieving complete pseudoaneurysm thrombosis at 24-h follow-up ultrasound following repeat thrombin injection. Patients with follow-up ultrasound were evaluated as to having complete thrombosis versus incomplete thrombosis (successful vs. unsuccessful primary effectiveness). Student t test was used to compare continuous features of the CT and IT groups, including: number of pseudoaneurysm lobes; longest pseudoaneurysm dimension; pseudoaneurysm volume; neck width; neck length; volume of thrombin injected; and laboratory values including INR, aPTT, and platelet counts. The Chi-squared test was used to compare the proportions of CT and IT patients taking antiplatelet or anticoagulation medications.
Results
Technical success
Technical success occurred 98.2% (320/326) of procedures, with technical failure in the remaining 1.8% (6/326). Of the six patients with technical failure, four demonstrated near-complete thrombosis at the time of the initial procedure, underwent a repeat ultrasound-guided thrombin injection within 24 h, and achieved complete thrombosis of the pseudoaneurysm at the time of the second injection. In two of the six patients with technical failure, no change in pseudoaneurysm flow was identified at the time of the initial thrombin injection, and the decision was made by the primary operator in conjunction with the clinical service to pursue operative management of the pseudoaneurysm.
Technique effectiveness
Technique effectiveness was evaluated in patients who underwent follow-up ultrasound imaging (N = 145). Primary effectiveness (complete thrombosis at 24-h follow-up ultrasound) was seen in 74.5% of patients (108/145). There was no difference in patient sex, BMI, or sheath size between patients with complete thrombosis versus incomplete thrombosis on follow-up ultrasound. Fifty-four males and 54 females had complete thrombosis and 21 males and 16 females had incomplete thrombosis (p = 0.60). BMI and sheath data were available in 93 patients; mean BMI for patients with complete thrombosis was 30.5 kg/m2 (range 18.5–44.6) and 30.4 kg/m2 in patients with incomplete thrombosis (range 21.4–51.3) (p = 0.98). Average sheath size for the arteriotomy was 6 French for both groups (p = 0.88).
Of the 37 patients who did not achieve primary effectiveness based on incomplete thrombosis at 24-h follow-up ultrasound, 68% (25/37) underwent repeat thrombin injection. The secondary effectiveness rate of the repeat injection was 84.0% (21/25), with an overall effectiveness rate of 97.0% (129/133) (Table 1). Of these twenty-five patients who underwent repeat thrombin injection, 21 underwent two total thrombin injections with successful treatment in 18 cases. Four patients underwent a total of three injections with successful treatment in three cases. Treatment failure was seen in a total of 16% (4/25) of patients who underwent at least one additional thrombin injection, and all four ultimately underwent operative or IR intervention.
Of the 37 patients who did not achieve primary effectiveness, 32% (12/37) did not undergo repeat thrombin injection: 5 patients underwent operative treatment, 1 patient underwent percutaneous stent placement, 4 patients developed spontaneous thrombosis on subsequent follow-up, and 2 patients had no imaging follow-up (Table 1).
Imaging and procedure analysis
For patients who underwent follow-up ultrasound (N = 145), imaging and procedure factors associated with technique failure were evaluated, including volume of thrombin injected, number of pseudoaneurysm lobes, largest pseudoaneurysm dimension, and pseudoaneurysm volume. In patients in whom complete thrombosis was achieved (N = 108), a mean of 451 U thrombin was administered; in patients with incomplete thrombosis on follow-up ultrasound (N = 37), a mean of 719 U thrombin was administered (p = 0.005). The mean number of pseudoaneurysm lobes, the mean largest pseudoaneurysm dimension, and the mean pseudoaneurysm volume were not significantly different between patients with complete versus incomplete thrombosis (p > 0.344) (Table 2).
Laboratory analysis
For patients who underwent follow-up ultrasound (N = 145), laboratory values including INR, aPTT, and platelet count (available in 143 patients) showed no statistically significant difference between patients with CT versus IT (p > 0.088) (Table 3). Thrombocytopenia (platelet count < 150,000/μL) was present in a similar rate between patients with complete thrombosis (21.5%; 23/107) and incomplete thrombosis (20.0%; 6/36) (p = 0.699).
Anticoagulation
Medications were reviewed for patients who underwent follow-up ultrasound (N = 145). Medication records were not available for two patients. Antiplatelet therapy (aspirin and/or clopidogrel) was used in a similar proportion of patients in the complete thrombosis and incomplete thrombosis groups (p > 0.135) (Table 3). The rate of incomplete thrombosis was significantly higher in patients on anticoagulation therapy (warfarin, heparin, enoxaparin). For patients with incomplete thrombosis, 63.9% (23/36) of patients were on anticoagulation therapy, whereas only 35.5% (38/107) of patients with complete thrombosis were on anticoagulation therapy (p = 0.002) (Table 3).
Discussion
In this study, we have shown that ultrasound-guided percutaneous thrombin injection has a high technical success rate and high primary and secondary effectiveness rates for the treatment of iatrogenic femoral artery pseudoaneurysms. No imaging factors or pre-procedure laboratory values were associated with technique failure. Anticoagulation therapy was associated with technique failure, but antiplatelet therapy was not.
Ultrasound-guided percutaneous thrombin injection has been shown to have a high success rate for the treatment of femoral artery pseudoaneurysms. Our 98% technical success rate and 97% total effectiveness rate are similar to previously reported success rates of 90–100% technical success [6, 12, 15, 18, 19] and 95–99% total effectiveness [12,13,14,15, 19,20,21]. In our study, primary effectiveness (complete thrombosis at 24-h follow-up ultrasound) was observed in 74.5%, lower than the 85.7% success rate reported by Esterson et al.; however, our population of pseudoaneurysms were slightly larger and more complex (median largest dimension 2.9 cm vs. 2.3 cm and mean number of lobes 1.33 vs. 1.26) [17]. Our total effectiveness rate of 97% suggests that repeat thrombin injection can be used successfully to avoid the potential morbidity of surgical repair or stent placement in the majority of patients.
In our series, no imaging factor, including largest pseudoaneurysm dimension, total volume, or the number of pseudoaneurysm lobes was associated with technique failure. This is contrary to the results reported by Esterson et al., who found that pseudoaneurysms that measured 2 cm or larger were more likely to recur after treatment [17]. However, our findings are concordant with two other studies that did not find an association between pseudoaneurysm size and complete thrombosis [22, 23]. These findings suggest that larger pseudoaneurysms are amenable to thrombin injection and that pseudoaneurysm size is not a factor in predicting technical failure.
The higher rate of incomplete thrombosis seen in patients on anticoagulation (warfarin, heparin, enoxaparin) is in contrary to the success reported in prior studies [3, 13], however, this may be explained by their comparatively low sample size. As newer anticoagulants and antiplatelet agents are released, future work may need to investigate success of percutaneous thrombin injection in patients on these agents. Interestingly, we saw no difference in pseudoaneurysm recurrence based on laboratory parameters that are used to assess coagulation function, including INR and aPTT level [17]. These results suggest that the presence of anticoagulation therapy, not the surrogate coagulation parameter laboratory values, is important in predicting potential treatment failure and patients on anticoagulation therapy may require a follow-up ultrasound to confirm successful thrombosis of the pseudoaneurysm. As a retrospective study, we are unable to assess whether or not holding anticoagulation and the duration of the hold might affect treatment success.
No difference in technique effectiveness was seen in patients on antiplatelet therapy (aspirin, clopidogrel), suggesting that attempted thrombin injection should not be deterred based on a concern for technical failure in these patients. However, as previously mentioned, with the release of newer agents (such as ticagrelor or prasugrel), additional investigation may need to be done regarding success rates in patients on these agents. In addition, mean platelet values were similar for patients with complete versus incomplete pseudoaneurysm thrombosis, and thrombocytopenia was not identified as being associated with incomplete thrombosis. These findings differ from the results found by Esterson et al., who found that thrombocytopenia was weakly predictive of pseudoaneurysm recurrence [17].
There are several limitations to this study. It was a retrospective chart and imaging review, and some inpatient charts have been incompletely digitized with some information unavailable for review. Arteriotomies were performed by a variety of operators and it is unclear from the digitized records whether closure devices were utilized. In addition, the older portions of the medication administration record may have been incomplete, and complications may not have been systematically documented. Thrombin injections were performed by a variety of operators with varying experience. Finally, the total number of pseudoaneurysms (imaged and not treated; imaged and treated; and not imaged) is not currently known, and it is unclear how many procedures were declined due to potential contraindications.
In conclusion, imaging-guided percutaneous thrombin injection of iatrogenic femoral artery pseudoaneurysms has a high technical success rate as well as high primary and secondary effectiveness rates. No imaging factors of the pseudoaneurysm or laboratory values were associated with technique failure. Anticoagulation therapy was associated with technique failure; however, antiplatelet therapy was not, and laboratory investigations for coagulopathy were not associated with technique failure.
References
Katzenschlager R, Ugurluoglu A, Ahmadi A et al (1995) Incidence of pseudoaneurysm after diagnostic and therapeutic angiography. Radiology 195:463-466
Etemad-Rezai R, Peck DJ (2003) Ultrasound-guided thrombin injection of femoral artery pseudoaneurysms. Can Assoc Radiol J 54:118-120
Brophy DP, Sheiman RG, Amatulle P, Akbari CM (2000) Iatrogenic Femoral Pseudoaneurysms: Thrombin Injection after Failed US-guided Compression. Radiology 214:278-282
Tisi PV, Callam MJ (2013) Treatment for femoral pseudoaneurysms. Cochrane Database Syst Rev. https://doi.org/10.1002/14651858.CD004981.pub4:CD004981
Saad NE, Saad WE, Davies MG, Waldman DL, Fultz PJ, Rubens DJ (2005) Pseudoaneurysms and the role of minimally invasive techniques in their management. Radiographics : a review publication of the Radiological Society of North America, Inc 25 Suppl 1:S173-189
Kontopodis N, Tsetis D, Tavlas E, Dedes A, Ioannou CV (2016) Ultrasound Guided Compression Versus Ultrasound Guided Thrombin Injection for the Treatment of Post-Catheterization Femoral Pseudoaneurysms: Systematic Review and Meta-Analysis of Comparative Studies. European Journal of Vascular and Endovascular Surgery 51:815-823
Perler BA (1993) Surgical treatment of femoral pseudoaneurysm following cardiac catheterization. Cardiovasc Surg 1:118-121
Ricci MA, Trevisani GT, Pilcher DB (1994) Vascular complications of cardiac catheterization. Am J Surg 167:375-378
Lumsden AB, Miller JM, Kosinski AS et al (1994) A prospective evaluation of surgically treated groin complications following percutaneous cardiac procedures. The American surgeon 60:132-137
Thalhammer C, Kirchherr AS, Uhlich F, Waigand J, Gross CM (2000) Postcatheterization pseudoaneurysms and arteriovenous fistulas: repair with percutaneous implantation of endovascular covered stents. Radiology 214:127-131
Fellmeth BD, Roberts AC, Bookstein JJ et al (1991) Postangiographic femoral artery injuries: nonsurgical repair with US-guided compression. Radiology 178:671-675
Paulson EK, Nelson RC, Mayes CE, Sheafor DH, Sketch MH, Jr., Kliewer MA (2001) Sonographically guided thrombin injection of iatrogenic femoral pseudoaneurysms: further experience of a single institution. AJR Am J Roentgenol 177:309-316
Pezzullo JA, Dupuy DE, Cronan JJ (2000) Percutaneous injection of thrombin for the treatment of pseudoaneurysms after catheterization: an alternative to sonographically guided compression. AJR Am J Roentgenol 175:1035-1040
Kang SS, Labropoulos N, Mansour MA, Baker WH (1998) Percutaneous ultrasound guided thrombin injection: a new method for treating postcatheterization femoral pseudoaneurysms. J Vasc Surg 27:1032-1038
Paulson EK, Sheafor DH, Kliewer MA et al (2000) Treatment of iatrogenic femoral arterial pseudoaneurysms: comparison of US-guided thrombin injection with compression repair. Radiology 215:403-408
Morgan R, Belli AM (2003) Current treatment methods for postcatheterization pseudoaneurysms. Journal of vascular and interventional radiology : JVIR 14:697-710
Esterson YB, Pellerito JS (2017) Recurrence of Thrombin-Injected Pseudoaneurysms Under Ultrasound Guidance: A 10-Year Retrospective Analysis. Journal of ultrasound in medicine : official journal of the American Institute of Ultrasound in Medicine 36:1617-1624
Liau CS, Ho FM, Chen MF, Lee YT (1997) Treatment of iatrogenic femoral artery pseudoaneurysm with percutaneous thrombin injection. J Vasc Surg 26:18-23
Krueger K, Zaehringer M, Strohe D, Stuetzer H, Boecker J, Lackner K (2005) Postcatheterization pseudoaneurysm: results of US-guided percutaneous thrombin injection in 240 patients. Radiology 236:1104-1110
Shah KJ, Halaharvi DR, Franz RW, Jenkins Ii J (2011) Treatment of Iatrogenic Pseudoaneurysms Using Ultrasound-Guided Thrombin Injection over a 5-Year Period. Int J Angiol 20:235-242
Vlachou PA, Karkos CD, Bains S, McCarthy MJ, Fishwick G, Bolia A (2011) Percutaneous ultrasound-guided thrombin injection for the treatment of iatrogenic femoral artery pseudoaneurysms. European journal of radiology 77:172-174
Chen DH, Sammel AM, Jain P, Jepson NS (2015) Cardiologist operated ultrasound guided thrombin injection as a safe and efficacious first line treatment for iatrogenic femoral artery pseudoaneurysms. Heart Lung Circ 24:165-172
Olsen DM, Rodriguez JA, Vranic M, Ramaiah V, Ravi R, Diethrich EB (2002) A prospective study of ultrasound scan-guided thrombin injection of femoral pseudoaneurysm: a trend toward minimal medication. J Vasc Surg 36:779-782
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
Wendy Ehieli, Erol Bozdogan, Gemini Janas, Tracy Jaffe, Chad Miller, and Brian Allen have declare that they have no conflict of interest. Mustafa Bashir: Research support, Siemens Healthcare, GE Healthcare, NGM Bio, TaiwanJ Pharma, Madrigal Pharmaceuticals.
IRB statement
This retrospective study was approved by our institutional review board and was compliant with the Health Insurance Portability and Accountability Act. The requirement for written informed consent was waived.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Ehieli, W.L., Bozdogan, E., Janas, G. et al. Imaging-guided percutaneous thrombin injection for the treatment of iatrogenic femoral artery pseudoaneurysms. Abdom Radiol 44, 1120–1126 (2019). https://doi.org/10.1007/s00261-019-01923-6
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00261-019-01923-6