Abstract
The “gastrografin challenge” has been used for decades in the evaluation of small bowel obstruction (SBO). This type of study involves enteric administration of a water-soluble contrast followed by serial abdominal radiographs. While its diagnostic role is well established, its therapeutic role remains controversial. Following an algorithm for gastrografin challenge cases can help with interpretation. An understanding of the appearance of diluted contrast in the small bowel, the concentrating effect of contrast in the colon, and knowledge of surgical history and anatomy is paramount for diagnosis. In this article, we review the approach to acute SBO and the use of gastrografin along with reviewing image interpretation of cases of partial and complete SBO. Gastrografin use in adynamic ileus along with other potential future uses is also discussed.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Background
Acute small bowel obstruction (SBO) is a major cause of morbidity and mortality and results in a significant use of healthcare resources. In the United States, adhesions related to prior intra-abdominal surgery are the most common cause of SBO. It is estimated that acute adhesive SBO accounts for 12–16% of hospital admissions for acute abdominal pain [1]. In the United States, 300,000 hospital admissions and 850,000 days of inpatient care per year can be attributed to acute adhesive SBO [1]. Adhesive SBO has been associated with mortality rates of 2–8% and as high as 25% if necessary surgical management is delayed [1]. Due to these high rates of mortality, particularly in the setting of delayed treatment, management of SBO creates a prognostic conundrum.
Classic teaching has described avoiding surgery in the patient who is hemodynamically stable with no signs of peritonitis, as surgical management may induce formation of further intra-abdominal adhesions, thus exacerbating the problem in the future. Instead, patients with SBO are typically managed expectantly with serial clinical abdominal exams, evaluating for signs of peritonitis, and close monitoring of vital signs.
Yet, there is a subset of patients for whom prompt surgery is essential to avoid serious morbidity and mortality. Clinical signs indicating the need for urgent operative intervention include leukocytosis, fever, and evidence of bowel strangulation. In modern practice, clinical findings are often supplemented with cross-sectional imaging with computed tomography to rule out conditions that may obviate the need for urgent surgery. Radiologic diagnoses prompting urgent surgical management include bowel ischemia, bowel perforation, closed loop obstruction, internal hernia, incarcerated external hernia, and small bowel volvulus.
In practice, it can be difficult to determine which patients can be managed expectantly compared to those that will require surgery. Due to the high rate of morbidity and mortality related to delay in surgical management, there is a need for a diagnostic tool to help predict which patients are more likely to require surgery. If that diagnostic tool could also be therapeutic, this potentially could help decrease the morbidity and mortality associated with adhesive SBO.
The gastrografin challenge
The concept of the “gastrografin challenge” can be traced to the roots of contrast-based radiographic evaluation of the bowel. The first published use of water-soluble iodine-containing contrast medium for evaluation of the gastrointestinal tract was described by Canada in 1955 [2]. Soon after, several others described the use of hypertonic contrast media to help promote bowel motility. For example in 1955, Epstein described using contrast media to determine position of bowel loops and to differentiate partial from complete SBO [3]. In 1969, Vest and Margulis showed the value of water-soluble, iodine-containing contrast media in differentiating post-operative adynamic ileus from mechanical obstruction [4].
Although several protocols for a gastrografin challenge have been described, the “challenge” involves enteric administration of 40–150 mL of a water-soluble contrast agent, typically gastrografin [5]. The patient is imaged with frontal abdominal radiographs between 4 and 24 h after contrast administration. The appearance of concentrating contrast in a patient’s colon within 4–24 h helps diagnose partial SBO and can be used to suggest that it is safe to proceed with non-operative management of the patient.
While this diagnostic utility of the gastrografin challenge is well established [5,6,7], the ability of enterically administered gastrografin to reduce the need for operative intervention in adhesive SBO remains a matter of controversy [5,6,7,8,9,10,11,12], with several recent studies reaching conflicting conclusions. A multicenter prospective study by Scotte et al. in 2017 showed that patients receiving gastrografin had no benefit in rate of operative intervention in uncomplicated acute SBO [8]. However, a 1998 study by Chen et al. found that 98% of patients responded to conservative therapy when contrast was in the colon within 24 h [6]. Likewise, a 2010 meta-analysis by Branco et al. as well as a 2016 meta-analysis by Ceresoli et al. both concluded that enteric administration of water-soluble contrast decreased the need for surgery and reduced the length of hospital stay [5, 7]. In all, while the literature seems to support a therapeutic role, it remains far from conclusive. A definitive trial for adhesive SBO is still lacking. In addition, based on our review of the literature, there is a paucity of literature concerning the use of gastrografin for management other conditions such as ileus, abdominal wall hernia, and Crohn’s disease.
Safety and proposed mechanism of action
Gastrografin has two active ingredients: sodium diatrizoate and meglumine diatrizoate [13]. Inactive ingredients include edetate disodium, polysorbate 80 (a wetting agent), saccharin sodium, simethicone, and sodium citrate [13]. The osmolarity of undiluted gastrografin is 1900 mOsm/L, six times that of extracellular fluid.
Allergic-like reactions from gastrografin have been reported. These are similar to those seen with intravenous contrast agents ranging from mild urticaria to severe airway compromise. Side effects of aspiration of gastrografin include pulmonary edema and pneumonitis.
The proposed mechanism for the therapeutic action of gastrografin involves stimulation of peristalsis and decreased bowel wall edema. The hyperosmolarity of gastrografin is hypothesized to promote fluid shifts into the bowel lumen. This has the dual effects of (1) increasing the pressure gradient across a site of obstruction and (2) decreasing bowel wall edema by shifting fluid from the intramural space to the intraluminal space. Polysorbate 80, a wetting agent, is thought to serve as a lubricant, promoting passage of bowel contents through a narrowed lumen.
Patients undergoing a gastrografin challenge should be thoroughly evaluated for emergent conditions prior to initiating the study and during the study to prevent potential morbidity and mortality because of delayed surgery. Clinical evaluation should focus on signs of peritonitis, bowel ischemia, and incarcerated/strangulated bowel-containing hernias. In patients who receive a CT scan, a thorough evaluation for signs of emergent conditions such as bowel perforation and bowel ischemia should be performed. Signs of bowel ischemia have previously been described and include: bowel wall thickening (> 3 mm), fluid in the mesentery, mesenteric edema, abnormal bowel wall enhancement (increased or decreased), occlusion of mesenteric vessels, engorged mesenteric veins, closed loop obstruction or volvulus, pneumatosis, as well as mesenteric venous gas and/or portal venous gas [14]. Of note, if patients require a CT exam after gastrografin has been administered, the utility of CT to detect alterations in wall enhancement, as well as the overall quality of the CT for any indication, may be markedly reduced due to the dense intraluminal contrast. Patients who do not receive a CT scan are typically imaged with abdominal radiographs, including either upright or decubitus films, to exclude intra-abdominal free air.
How we do it
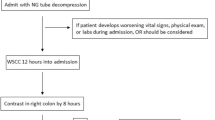
At our institution, the algorithm for a gastrografin challenge begins with evaluation for bowel ischemia or peritonitis. If there are any signs of peritonitis or bowel ischemia at any point during a gastrografin challenge, surgery should be considered.
Measures are taken to decrease the possibility of gastrografin aspiration. Placement of a transesophageal tube into the stomach, set on suction for at least 2 h, is required. It is recommended that the head of the bed be raised to at least 30 degrees. Frequency and timing of patient emesis is tracked in order to assess the likelihood of vomiting gastrografin. Although these measures may help to mitigate the possibility of aspiration, it is important to remain vigilant for aspiration risks such as in patients with higher-degree of obstruction, gastroparesis, and altered anatomy.
At our institution, 90–120 mL of gastrografin is instilled via the transesophageal tube at the patient’s bedside. If there is specific concern for aspiration, the gastrografin administration is monitored fluoroscopically to ensure that it is passing into the proximal small intestine. The transesophageal tube is subsequently clamped for 2 h. An abdominal radiograph is obtained 6–8 h following gastrografin administration. If no contrast is visible in the colon at 6–8 h, a repeat radiograph is performed at 24 h. If contrast is identified in the colon on either the 6- to 8-h or 24-h radiograph, the transesophageal tube is removed and the diet is slowly advanced beginning with sips of liquid and proceeding to full liquids within 24 h. If the patient can tolerate 800 mL of fluid intake without nausea or vomiting, they are discharged. If no contrast is seen in the colon on the 24-h radiograph, surgery is strongly considered. It is important to remember that throughout the study, regardless of imaging findings, patients should be routinely monitored for clinical signs of bowel ischemia or peritonitis which would necessitate surgery (Fig. 1).
Image interpretation
Most patients presenting with symptoms of SBO will undergo a CT scan or plain abdominal radiography. The role of the radiologist is to help diagnose SBO. It is important to evaluate for a focal transition point and to identify potential causes of obstruction such as a mass, inflammatory changes, or adhesions. Special attention should be directed towards imaging findings of bowel ischemia. Previous surgical history such as bowel resections or ostomy placements should be reviewed if available. The positioning of the transesophageal tubes should be assessed.
In interpretation of radiographs obtained moving through the algorithm of the gastrografin challenge, it is important to look at the distribution of contrast in the small and large bowel. There should be continued vigilance for ischemic changes such as bowel wall thickening, pneumatosis, portal venous air, and free intraperitoneal air/contrast. Evaluating the appearance of contrast distribution will diagnosis partial SBO, complete SBO or adynamic ileus as illustrated in the following cases.
Partial SBO
In the first case (Fig. 2), a CT of the abdomen revealed a SBO with a single transition point in a patient with no radiographic or clinical signs of ischemia. This suggested adhesions as the cause of the obstruction. With a gastrografin challenge initiated, partial SBO was diagnosed due to the presence of residual contrast in the small bowel and contrast throughout the colon on the 8-h radiograph. Based on this information, the clinical team began advancing the patient’s diet and bowel function returned.
A 48-year-old woman with prior abdominal surgery presented with symptoms of SBO. CT abdomen/pelvis (A) at time of presentation showed a single transition point (arrow) without evidence of bowel ischemia or bowel perforation. Radiograph obtained 8 h after gastrografin administration (B) showed residual contrast in the small bowel as well as contrast throughout the colon (arrowheads), consistent with a partial SBO. As a result, the patient’s diet was successfully advanced
In the second case (Fig. 3), since no contrast was identified in the colon on the 8-h radiograph and there were no signs/symptoms of peritonitis and/or ischemia, a follow-up radiograph was obtained at 24 h. Because contrast was present in the colon at 24 h, the patient’s diet was advanced, and bowel function returned. Note that contrast will appear denser in the colon than small bowel from the concentrating effect of water absorption in the colon.
A 64-year-old woman with multiple prior abdominal surgeries presented with SBO with a single transition point (arrow) on initial CT abdomen/pelvis (A). Radiograph obtained 8 h after gastrografin administration (B) showed contrast diluted within multiple distended loops of small bowel (stars). Radiograph obtained 24 h after gastrografin administration (C) showed contrast within the descending and sigmoid colon as well as the rectum (arrowheads). The patient had return of flatus and bowel movements 1 day after the study
In a third case (Fig. 4), partial SBO was diagnosed in a patient where prior knowledge of the surgical history was paramount. In such patients with a history of subtotal colectomy, attention should be focused on identifying dense contrast within the rectum.
A 28-year-old woman with a history of chronic constipation was diagnosed with delayed colonic transit and subsequently underwent a therapeutic subtotal colectomy with ileorectal anastomosis. 3 years after subtotal colectomy, she presented with nausea and vomiting. Initial abdominal radiograph (A) showed multiple stacked air-filled loops of small bowel particularly in the mid abdomen (arrows). Radiograph obtained 6 h post-gastrografin (B) showed concentrated contrast in the previously seen dilated small bowel loops (arrowheads). Radiograph obtained 22 h after gastrografin administration (C) showed contrast in the rectum (star)
Complete SBO
This case (Fig. 5) of a complete SBO was diagnosed with a gastrografin challenge. Following the algorithm, no contrast in the colon on the 6-h radiograph led to a follow-up 24-h radiograph which also showed no contrast in the colon. This suggested complete SBO.
A 77-year-old man with a history of inguinal hernia repair 3 years prior presented with symptoms of SBO. Non-contrast abdomen/pelvis CT (A) demonstrated a high grade SBO with a single transition point (arrow). 6 h after gastrografin administration (B), contrast is seen within loops of small bowel (stars). On the 24-h film, the contrast appears further diluted and no contrast has reached the colon (C). These findings are consistent with complete SBO. The patient was taken for an exploratory laparotomy with lysis of adhesions. Bowel function returned after surgery and the patient was discharged
In another case of a complete SBO (Fig. 6), the dilution of contrast within the already fluid-filled small bowel can make it difficult to appreciate the presence of contrast, particularly on the 24-h radiograph. Although consistent with complete SBO, this patient had a spontaneous resolution of symptoms prior to planned surgery. This could reflect the therapeutic action of gastrografin.
An 81-year-old man with a history of vertebroplasties for osteoporosis and prior abdominal surgeries presented with bilious emesis. Initial CT abdomen/pelvis (A) demonstrated several dilated fluid-filled loops of small bowel (arrows). Abdominal radiograph obtained 6 h after gastrografin ingestion (B) showed contrast in the stomach (arrowhead). By 24 h (C), gastrografin was diluted in the small bowel with no contrast seen in the colon (stars). This was consistent with a complete persistent SBO. Although scheduled for surgical lysis of adhesions for the morning after the 24-h radiograph, the patient had three bowel movements overnight and his symptoms resolved. This was presumed to be related to the therapeutic action of gastrografin
Adynamic ileus versus post-operative SBO
While standard abdominal radiographs are typically the first line in differentiating adynamic ileus from post-operative SBO, findings may be equivocal. In such cases, the gastrografin challenge has been used to differentiate suspected SBO from post-operative ileus (Fig. 7). Epstein described 14 post-operative cases in which a gastrografin challenge was used to assist in differentiating ileus from SBO [3]. In a series of 41 patients, Zer et al. showed the utility of a similar protocol to help differentiate adynamic post-operative ileus from SBO requiring surgery [15].
A 66-year-old man presented with bowel obstruction 3 days after posterior spinal fusion. Initial abdominal radiograph (A) shows numerous distended air-filled loops of small and large bowel throughout the abdomen (stars). Contrast is seen in the cecum (arrow) on the 6-h post-image (B) and in the rectum (arrowhead) on the 24-h post-image (C). NG tube was then removed; the diet was slowly advanced, and the patient was discharged. Findings are consistent with post-operative ileus
In a patient with a recent abdominal surgery presenting with obstipation and concern for possible complete obstruction, a gastrografin challenge can be helpful in diagnosing partial SBO, preventing the need for repeat surgery. (Figure 8).
A 58-year-old woman with a history of roux-en-Y gastric bypass performed 3 years prior presented at an outside institution with an internal hernia. Emergent exploratory laparotomy was performed—the efferent limb was divided and then re-anastomosed to reduce the internal hernia; a transesophageal tube was placed, and the patient was transferred to our institution. 2 days after surgery no flatus or bowel movement was noted. Initial radiograph (A) shows a non-obstructive bowel gas pattern. Radiograph obtained 12 h post-gastrografin (B) showed contrast throughout the colon (stars). Following gastrografin challenge, the patient had a bowel movement and abdominal pain resolved
In another case (Fig. 9) where post-operative ileus was diagnosed with contrast seen in the colon at 6 and 24 h, an interesting phenomenon was observed. The presence of a fine layer of contrast coating the colonic wall can give the illusion of seeing both sides of the bowel wall reminiscent of a Rigler sign. First reported by Dr. Rigler in 1941, the Rigler sign describes the visualization of both sides of a bowel wall on supine abdominal radiographs in cases of pneumoperitoneum [16]. It is important not to mistake this finding for intraperitoneal free air.
A 72-year-old woman presented with symptoms of bowel obstruction 3 days after laparoscopic cholecystectomy. Initial abdominal radiograph (A) showed a non-obstructive bowel gas pattern. 6 h radiographs (B) showed most of the contrast in the stomach (star), with contrast also identified in small bowel loops as well as the hepatic flexure (arrow). 24-h radiograph (C) has the illusion of seeing both sides of the bowel wall of the cecum and ascending colon reminiscent of a Rigler sign (arrowheads). However, this represents a fine layer of contrast coating the colonic wall rather than free air outlining the colon. The patient had multiple large bowel movements before the 24-h radiograph and was discharged
Knowledge of a patient’s post-surgical gastrointestinal anatomy is paramount in order to correctly interpret gastrografin challenge cases. Patients with total colectomies or diverting ileostomies will not opacity the colon. In this case (Fig. 10), the subtle linear opacity within the ileostomy bag could easily be missed.
A 40-year-old women with Crohn disease requiring multiple prior small bowel resections for recurrent mechanical SBO and diverting ileostomy for recurrent ileocolic disease presented with no output from her ileostomy. The patient refused placement of a transesophageal tube. Initial CT abdomen/pelvis (A, B) showed multiple dilated air-filled loops of small bowel (arrows) and an ileostomy in the right lower quadrant of the abdomen. Abdominal radiograph obtained 6 h after oral administration of gastrografin (C, D) shows contrast in the gastric fundus (arrowhead). There is a subtle region of increased density projecting over the soft tissues of the right lower quadrant, consistent with contrast in the ileostomy bag (star). Following the gastrografin challenge, the patient was able to tolerate small amounts of PO intake and had output from her ileostomy
Other uses
This case (Fig. 11) illustrates another indication for the gastrografin challenge which has not been extensively studied, but has been used at our institution. Leow et al. described a case of an obturator hernia which was managed non-operatively with the help of gastrografin challenge [17]. In our case of a large ventral hernia, the gastrografin challenge proved that contrast was able to pass distal to bowel in the hernia.
A 55-year-old woman with a history of large ventral hernia presented with abdominal pain, vomiting, and constipation. CT (A) revealed multiple loops of small bowel (arrowheads) as well as a portion of the transverse colon (arrow) within the hernia. An attempted 8-h radiograph obtained at 9 h after gastrografin administration (B) showed contrast throughout the colon including in the descending colon distal to the bowel contained in the ventral hernia (arrowhead). Bowel function returned after the gastrografin challenge
In an example of another potential use (Fig. 12), a gastrografin challenge helped the clinical team determine patient disposition. To our knowledge, the use of gastrografin challenge in cases of recurrent SBO in patients with Crohn’s disease has been described only once in the literature. In their series of 15 pediatric patients, Gorecki et al. describe one case of gastrografin challenge in a patient with Crohn’s disease [18]. In Crohn’s disease patients with active disease and recurrent symptoms, a gastrografin challenge may be diagnostic and potentially therapeutic precluding the need for a CT exam and reducing cumulative radiation dose.
A 38-year-old woman with a history of Crohn disease with ileocolic resection 2 years prior and multiple recurrent SBOs presented with another episode of SBO. Initial abdominal radiograph (A) shows dilated small bowel loops with air-fluid levels (arrowheads) consistent with a SBO. The patient refused a nasogastric tube. On AP abdominal film 6 h after the gastrografin challenge (B), contrast is appreciated in the stomach (star) and small bowel (asterisk). Marked improvement in the patient’s symptoms prompted a radiograph obtained 12 h after gastrografin administration (C) to expedite patient discharge. This showed contrast throughout the colon (arrows). The patient’s diet was rapidly advanced
Conclusion
While the therapeutic role of enterically administered gastrografin in resolving SBO remains a matter of controversy, the diagnostic role of the gastrografin challenge in the evaluation of adhesive SBO is well established. As our cases illustrate, by following a standardized approach, patients with adhesive SBO can be evaluated in a systematic way. Conservative management is usually appropriate in cases where contrast has reached the colon within 24 h. Surgery is likely warranted when no contrast is seen in the colon at 24 h. It is important to appreciate the appearance of diluted contrast in small bowel and the concentrating effect of contrast in the colon in order to identify where contrast is located. In addition, knowledge of surgical history prior to interpretation of abdominal radiographs is instrumental in predicting the expected location of gastrografin concentration. Vigilance for clinical and radiographic signs of bowel ischemia is critical for patient safety.
The gastrografin challenge has an emerging role beyond adhesive SBO. At our institution, we have seen it used for cases of post-operative ileus, abdominal wall hernia, and Crohn’s disease. Use of the gastrografin challenge has also been reported in the literature for abdominal/pelvic malignancy [19] and distal intestinal obstruction syndrome (DIOS) [20]. It may help replace repetitive CT use and reduce dose in patients with recurrent SBOs. In the future, the gastrografin challenge may also be used to expedite inpatient discharge for a wide variety of obstructive bowel pathology.
References
Catena F, Di Saverio S, Coccolini F, et al. (2016) Adhesive small bowel adhesions obstruction: evolutions in diagnosis, management and prevention? World J Gastrointest Surg 8:222. https://doi.org/10.4240/wjgs.v8.i3.222
Canada WJ (1954) Use of Urokon (sodium-3-acetylamino-2,4,6-triiodobenzoate) in roentgen study of the gastrointestinal tract. Radiology 64:867–873
Epstein BS (1955) Nonabsorbable water-soluble contrast mediums: Their use in diagnosis of intestinal obstruction. J Am Med Assoc 165:2–4
Margulis AR (1969) Contrast media the present status of water-soluble iodine-containing material in the examination of acute abdominal disease. Calif Med 110:193–199
Ceresoli M, Coccolini F, Catena F, et al. (2016) Water-soluble contrast agent in adhesive small bowel obstruction: a systematic review and meta-analysis of diagnostic and therapeutic value. Am J Surg 6:1114–1125. https://doi.org/10.1016/j.amjsurg.2015.06.012
Chen SC, Lin FY, Lee PH, et al. (1998) Water-soluble contrast study predicts the need for early surgery in adhesive small bowel obstruction. Br J Surg 85:1692–1694. https://doi.org/10.1046/j.1365-2168.1998.00919.x
Branco BC, Barmparas G, Schnüriger B, et al. (2010) Systematic review and meta-analysis of the diagnostic and therapeutic role of water-soluble contrast agent in adhesive small bowel obstruction. Br J Surg 97:470–478. https://doi.org/10.1002/bjs.7019
Scotté M, Mauvais F, Bubenheim M, Cossé C, Suaud L, Savoye-Collet C, et al. (2017) Use of water-soluble contrast medium (gastrografin) does not decrease the need for operative intervention nor the duration of hospital stay in uncomplicated acute adhesive small bowel obstruction? A multicenter, randomized, clinical trial (Adhesive Small. Surgery (United States) pp. 1315–1325. https://doi.org/10.1016/j.surg.2016.11.026
Zielinski MD, Haddad NN, Cullinane DC, et al. (2017) Multi-institutional, prospective, observational study comparing the Gastrografin challenge versus standard treatment in adhesive small bowel obstruction. J Trauma Acute Care Surg 83:47–54. https://doi.org/10.1097/TA.0000000000001499
Loftus T, Moore F, VanZant E, Bala T, Brakenridge S, Croft C, et al. (2015) A protocol for the management of adhesive small bowel obstruction. J Trauma Acute Care Surg 78: 13-9-21. https://doi.org/10.1097/ta.0000000000000491
Choi H-K, Chu K-W, Law W-L (2002) Therapeutic value of gastrografin in adhesive small bowel obstruction after unsuccessful conservative treatment: a prospective randomized trial. Ann Surg 236:1–6. https://doi.org/10.1097/01.SLA.0000018563.62559.95
Jaffar S, Qureshi S, Channa A, Maher M (2012) Role of orally administered gastrografin in small bowel obstruction after unsuccessful conservative treatment. Pakistan J Med Sci 28:936–939
Bracco Imaging. Gastrografin Safety Data Sheet. (2017) http://imaging.bracco.com/sites/braccoimaging.com/files/technica_sheet_pdf/us-en-2016-07-15-sds-Gastrografin.pdf
Paulson EK, Thompson WM (2015) Review of small-bowel obstruction: the diagnosis and when to worry. Radiology 275:332–342. https://doi.org/10.1148/radiol.15131519
Zer M, Kaznelson D, Feigenberg Z, Dintsman M (1977) The Value of Gastrografin in the Differential Diagnosis of Paralytic Ileus Versus Mechanical Intestinal Obstruction: Dis Colon Rectum. 20:573–579
Rigler LG (1941) Spontaneous pneumoperitoneum: a roentgenologic sign found in the supine position. Radiology 37:604–607
Leow JJ, How KY, Goh MH, Woon WWL, Low JK (2014) Non-operative management of obturator hernia in an elderly female. Hernia 18:431–433. https://doi.org/10.1007/s10029-012-1036-9
Gorecki W, Krysta M, Bysiek A, Wojciechowski P, Wyrobek L (2007) Gastrografin challenge test for the management of subileus in children. Przegl Lek 64:53–55
Khasawneh MA, Eiken PW, Srvantstyan B, Bannon MP, Zielinski MD (2013) Use of the Gastrografin challenge in patients with a history of abdominal or pelvic malignancy. Surg (United States) 154:769–776. https://doi.org/10.1016/j.surg.2013.07.002
O’Halloran SM, Gilbert J, McKendrick OM, Carty HML, Heaf DP (1986) Gastrografin in acute meconium ileus equivalent. Arch Dis Child 61:1128–1130. https://doi.org/10.1136/adc.61.11.1128
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Funding
No funding was received for this study.
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
This article does not contain any studies with human participants or animals performed by any of the authors.
Informed consent
Statement of informed consent was not applicable since the manuscript does not contain any patient data.
Rights and permissions
About this article
Cite this article
D’Agostino, R., Ali, N.S., Leshchinskiy, S. et al. Small bowel obstruction and the gastrografin challenge. Abdom Radiol 43, 2945–2954 (2018). https://doi.org/10.1007/s00261-018-1591-3
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00261-018-1591-3