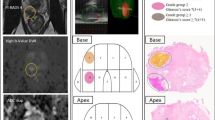
Abstract
Purpose
To determine the accuracy of in-bore transperineal 3-Tesla (T) magnetic resonance (MR) imaging-guided prostate biopsies for predicting final Gleason grades in patients who subsequently underwent radical prostatectomy (RP).
Methods
A retrospective review of men who underwent transperineal MR imaging-guided prostate biopsy (tpMRGB) with subsequent radical prostatectomy within 1 year was conducted from 2010 to 2015. All patients underwent a baseline 3-T multiparametric MRI (mpMRI) with endorectal coil and were selected for biopsy based on MR findings of a suspicious prostate lesion and high degree of clinical suspicion for cancer. Spearman correlation was performed to assess concordance between tpMRGB and final RP pathology among patients with and without previous transrectal ultrasound (TRUS)-guided biopsies.
Results
A total of 24 men met all eligibility requirements, with a median age of 65 years (interquartile range [IQR] 11.7). The median time from biopsy to RP was 85 days (IQR 50.5). Final pathology revealed Gleason 3 + 4 = 7 in 12 patients, 4 + 3 = 7 in 10 patients, and 4 + 4 = 8 in 2 patients. A strong correlation (ρ: +0.75, p < 0.001) between tpMRGB and RP results was observed, with Gleason scores concordant in 17 cases (71%). 16 of the 24 patients underwent prior TRUS biopsies. Subsequent tpMRGB revealed Gleason upgrading in 88% of cases, which was concordant with RP Gleason scores in 69% of cases (ρ: +0.75, p < 0.001).
Conclusion
Final Gleason scores diagnosed by tpMRGB at 3-T correlate strongly with final RP surgical pathology. This may facilitate prostate cancer diagnosis, particularly in patients with negative or low-grade TRUS biopsy results in whom clinically significant cancer is suspected or detected on mpMRI.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Prostate cancer is the most commonly diagnosed cancer in men, and the second most common cause of cancer death [1]. However, only a minority of cases lead to disease specific mortality and as a result there is an increasing concern for the overtreatment of prostate cancer [2, 3]. Traditionally, prostate biopsies are performed using transrectal ultrasound (TRUS) for guidance, which involves collection of 10–12 biopsy cores at the apex, base, and mid-gland regions of the prostate. The number of cores is generally based upon gland volume and systematic sampling of the entire gland is achieved by division of the gland into sextants or octants with extra cores taken in the transition zone. This method, which is not guided by the location of the suspected lesion, has the intrinsic drawbacks of non-targeted sampling, oversampling insignificant prostate tissue, and under-sampling regions of high clinical suspicion [4], leading to several well-recognized problems. On average, one third of TRUS biopsy pathologies are changed and upgraded when compared with final radical prostatectomy histopathology [5–7]. This has led to a significant rate of under-grading, which may lead to inappropriate treatment selection, a delay in necessary treatment, and worse patient outcomes [8, 9]. Furthermore, the high number of cores taken during TRUS increases the detection of clinically insignificant prostate cancer, potentially leading to unnecessary monitoring and treatment [10, 11].
The application of multiparametric magnetic resonance imaging (mpMRI), and its interpretation with PI-RADS v2 introduced in 2015 for the prostate, has presented a new approach to detection, diagnosis, and localization of prostate cancer [12–14]. The improved detection of clinically suspicious disease has led to multiple new approaches to prostate biopsy. There are several new techniques, with key differences in biopsy approach/route, navigational methods, and whether the patient is located in-bore or outside the magnet during the course of the procedure [11, 15–17]. Transperineal in-bore MR imaging-guided biopsy (tpMRGB), first described in 2001 [18], has been shown to be safe and feasible approach that offers several advantages over traditional techniques. It provided the ability to directly target the mpMRI-detected lesion and sample it under real-time MR guidance. This is a percutaneous approach, in which the biopsy needle pierces through the perineum parallel to the urethra, allowing for improved sampling of the anterior and apical prostate regions while decreasing the risk of sepsis and urethral damage [19–21]. Transperineal biopsy results have also been shown to improve cancer detection rates, reduce the risk of underestimating tumor grade, and decrease the false-negative results compared to TRUS-guided biopsies [22, 23]. Targeted in-bore biopsies allow for clear needle visualization during biopsy and most importantly, confirm with imaging the correct location of the needle in the suspicious lesion, thus improving accuracy and reducing the number of core biopsies needed [11, 24].
Gleason score, one of the best indicators of prostate cancer behavior, is a strong determinant of treatment selection. Gleason score of the biopsy sample helps guide many decisions in a given patient’s management, including the choice of the treatment from active surveillance to local ablations and radical prostatectomy, and whether or not to give concurrent hormonal or radiation therapy [25]. It also determines the extent of imaging needed to accurately stage the patient, and whether a lymph node dissection is indicated at time of RP. In the situations when RP is selected as the treatment option, the final Gleason score is determined from the analysis of the whole-gland specimen, and may disagree with the findings at the time of biopsy. RP Gleason score is an established prognostic factor for disease recurrence and prostate cancer specific death. Hence, accurate prediction of the final (RP) Gleason score is critical.
While several studies have demonstrated the ability of tpMRGB to detect clinically relevant prostate cancers [16, 26], these results have not been correlated to final histopathology as determined by subsequent radical prostatectomy. The goal of this study was to determine the accuracy of in-bore transperineal 3-Tesla (T) MR imaging-guided prostate biopsies for predicting final Gleason grades in patients who underwent radical prostatectomy.
Methods
Institutional review board approval was obtained for this retrospective study. Patient selection criteria for this study were (1) Pre-biopsy 3T endorectal coil mpMRI; (2) transperineal in-bore biopsy (tpMRGB) and enrollment in our on-going prospective study at our site; (3) radical prostatectomy within one year after tpMRGB; 4) no form of treatment received at the time between the tpMRGB procedure and RP.
Records of all men who underwent transperineal 3-T MR imaging-guided biopsy from 2010 to 2015 were obtained and reviewed for the specific eligibility criteria defined above. All patients had a baseline pre-biopsy MR scan using 3-T multiparametric MRI with endorectal coil (GE Signa HD, General Electric Healthcare, Milwaukee, WI & Medrad Inc., Warrendale, PA). Pre-biopsy and intraprocedural multiparametric MR imaging protocols and lesion identification were performed as previously described [16].
Patients were selected for biopsy if there was a suspicious prostate lesion to biopsy on mpMRI and there was a high degree of clinical suspicion for cancer, as determined by at least one of the following: an elevated prostate-specific antigen (PSA), prior negative TRUS-guided biopsy, or the inability to undergo transrectal biopsy due to rectal surgery. All tpMRGB were performed in a 70-cm wide-bore 3-T device (Siemens Magnetom Verio, Siemens Healthcare, Erlangen, Germany) by one of two interventional radiologists. Target lesions were identified using pre- and intraprocedural axial T2-weighted images and non-rigid registration [27, 28], and core needle biopsies were guided into the lesions using a standard template with a fixed 5-mm grid. Further details of the procedure, its setup, and results were previously described by Penzkofer et al. [16].
All pathology reports were reviewed to determine both biopsy and RP Gleason findings. Radical prostatectomy pathology was determined by the index lesion from standard pathologic processing. The primary outcome measure was concordance between MR-guided biopsy Gleason scores and radical prostatectomy final pathology Gleason scores. A subset analysis was performed on patients that underwent TRUS-guided biopsy within one year prior of tpMRGB.
Descriptive statistics were used to summarize the data and Spearman correlation was performed to assess the concordance between tpMRGB and final pathology among patients with and without previous transrectal ultrasound (TRUS)-guided biopsies. Statistical analysis was performed in STATA Version 11.2 StataCorp (College Station, TX 77845, USA), reporting only two-sided p-values at preset significant level of 0.05.
Results
On review of all patients in our tpMRGB database, there were 29 identified who initially met our eligibility criteria over the defined time period. There were 5 men subsequently found to be ineligible—1 had surgery more than 1 year after his in-bore biopsy and 4 did not have complete pathology reports—leaving 24 men who form this study group.
At the time of biopsy, the median patient age was 65 years (interquartile range [IQR] 11.7 years) and the median PSA was 8.7 ng/mL (IQR 8.9 ng/mL). Median time from biopsy to radical prostatectomy was 85 days (IQR 50.5 days). The majority of men, 17/24, had surgery within 100 days or less from the time of biopsy. The median prostate weight, as determined by final radical prostatectomy pathology, was 48.0 g (IQR 16.8 g). Of the 24 cases, 12 of the MR-targeted prostate lesions were located in the apex and 13 lesions were located anteriorly, with 6 cases located both anterior and apically.
MR-guided biopsy pathology revealed a Gleason score of 4 + 4 = 8 in 2 patients, 4 + 3 = 7 in 8 patients, 3 + 4 = 7 in 11 patients, 3 + 3 in 2 patients, and one case of benign findings. Gleason scores obtained using MR-guided biopsy strongly correlated with those obtained from the radical prostatectomy specimen (Spearman correlation coefficient ρ of +0.75, p < 0.001) (Fig. 1) and were unchanged in 71% of cases (n = 17). There was no significant difference in prostate weight or location between the concordant and discordant cases.
Correlation of Gleason’s scores of tpMRI biopsy and prostatectomy histopathology. Fitted plot of correlation between targeted transperineal MRI-guided prostate biopsy Gleason’s scores and prostatectomy histopathology Gleason scores shows very strong and significant positive correlation, ρ = 0.75, p < 0.001. rho correlation coefficient (ρ), p p-value, tpMRI Biopsy transperineal MRI-guided prostate biopsy, CI confidence interval
16 of the 24 patients also underwent TRUS biopsy up to one year prior to MR-guided biopsy (time between biopsies: 196.5, IQR 153.5 days). The TRUS pathology was benign in 6 cases and demonstrated Gleason scores of ≤3 + 3 in the remaining cases. There was no positive correlation between the TRUS-based Gleason scores and radical prostatectomy Gleason scores (Spearman correlation coefficient ρ of −0.20, p = 0.43) (Fig. 2), with all 16 cases discordant with final radical prostatectomy pathology. The subsequent tpMRGB of these patients resulted in Gleason scores upgrading in 88% of cases, which was concordant with radical prostatectomy Gleason scores in 69% of cases (Spearman correlation coefficient ρ of 0.75, p < 0.001).
Correlation of Gleason’s scores of TRUS biopsy and prostatectomy histopathology. Fitted plot of correlation between systematic transrectal ultrasound-guided prostate biopsy Gleason’s scores and prostatectomy histopathology Gleason scores shows insignificant negative correlation, ρ = −0.20, p = 0.43. rho correlation coefficient (ρ), p p-value, TRUS-guided biopsy transrectal ultrasound-guided prostate biopsy, CI confidence interval
Discussion
With the improvements in mpMRI imaging, namely the addition of diffusion imaging, prostate cancer localization and detection is becoming increasingly accurate [29, 30]. Vargas et al. demonstrated that in patients under active surveillance, mpMRI is very sensitive (87–98%) and specific (95–100%) in detecting clinically significant prostate cancer on confirmation biopsy [31]. Furthermore, mpMRI-guided biopsies have resulted in a reduction in the diagnosis of clinically insignificant cancer compared to standard biopsy [32]. Therefore, precise needle placement to properly assess cancer grade is of increasing importance for clinical management of prostate cancer patients. The utility of MR-guided prostate biopsies will continue to increase and its role in the clinical setting needs to be established.
There are several methods in which mpMRI-guided biopsies can be performed. Navigation can be with TRUS or MR imaging, the core needle path can be transrectal or transperineal, and samples can be taken either in the bore or outside the magnet [32, 33]. MR-TRUS fusion, in which previously acquired mpMR images are superimposed in real time onto TRUS images for guidance, has been gaining significant attention [34]. Siddiqui et al. have demonstrated that MR-TRUS-guided biopsies result in the detection of 30% more high-risk cancers compared to standard biopsy, and 17% fewer low-risk cancers. In addition, MR-TRUS fusion could predict whole-gland pathology after radical prostatectomy with a sensitivity of 77%, compared to 53% for TRUS [35]. However, existing data suggest MR-TRUS fusion does not detect 15–20% of all tumors, and further long-term data are needed to determine its clinical validity [34, 36].
Transperineal wide-bore 3-T multiparametric MR imaging-guided prostate biopsy offers several advantages to TRUS biopsies, including direct target identification, needle visualization, decreased rates of infection and bleeding, and better sampling of the anterior apical region [19, 37]. The anteroapical region has been shown to have a higher incidence of prostate cancer relative to other areas, and is increasingly difficult to sample with TRUS in large volume prostates [38]. Ouzzane et al. showed that 46% of anterior prostate cancers detected on mpMRI were missed by TRUS-guided biopsies [39]. Thus, improved sampling of suspicious lesions in these areas will likely increase detection rates.
Our study demonstrates a strong correlation between tpMRGB and final radical prostatectomy pathology (Fig. 1). While these findings suggest that tpMRGB may offer a more precise method to diagnose and appropriately treat men with prostate cancer compared to traditional non-target-guided TRUS sampling, 12.5% of cases were upgraded in final pathology from low- (summed Gleason <7) to high-risk cancer (summed Gleason ≥7) [40]. This could be secondary to higher grade disease outside the lesions of interest, which is in line with the limitations of mpMRI in detecting small, clinically significant prostate cancers [41, 42], intratumoral heterogeneity, or targeting errors while sampling the lesions of interest. Additionally, some degree of prostate cancer was noted outside the target lesions in most of the prostatectomy specimens. Thus, the tpMRGB approach would benefit from the combination with systematic biopsies, which agrees with prior studies [36]. In addition, we demonstrated that in the setting of negative or low-grade findings on TRUS and high clinical suspicion for prostate cancer, tpMRGB can accurately determine prostate cancer Gleason scores. Therefore, tpMRGB may serve as a useful diagnostic test for patients with TRUS findings that do not correlate with clinical suspicion.
Several limitations must be considered when interpreting the results of our study. The study had a small sample size (n = 24) and was conducted at a single center. Differences in clinical referrals, MR equipment, biopsy approaches, and operators may have an influence on the outcome in alternative settings. Additionally, radical prostatectomy specimens underwent standard pathological processing, not allowing for direct lesion comparison between tpMRGB and RP samples [43]. Another limitation is that PI-RADS v2 was not used in this retrospective study, as it was introduced after the time period of this study (2010–2015). Thus, the specific mpMRI criteria and ratings to determine target selection are beyond the scope of this study. The selection of patients for tpMRGB at our institution has been previously described [16]. In this study population, the patients were either biopsy naïve or with prior TRUS-guided biopsies. The latter only contained patients with either negative or clinically insignificant initial TRUS findings, which were discordant with other clinical variables. This resulted in a selection bias, which may limit the generalizability of our results to, for example, a screening population of biopsy naive men. Additionally, most of the patients had mpMRI findings of prostate cancer in the anterior or apical zone. This may have contributed to the large rate of upgrading seen between tpMRGB and TRUS-guided biopsy, although we did not observe any difference by location in the final pathology in our study. Thus, the overall ability of tpMRGB to detect clinically significant cancers compared to TRUS-guided biopsy is beyond the scope of this study and cannot be fully assessed. However, our results demonstrated a much higher correlation than seen in prior studies comparing traditional TRUS biopsies to radical prostatectomy results [5–7]. Additionally, there have been two recent studies, prospectively comparing the yield from TRUS and MR-guided biopsy, with one randomized trial demonstrating the superiority of MRGB for detection of clinically significant cancers [44]. Lastly, the length of time between biopsies and radical prostatectomy may have influenced the results; however, the majority had surgery within 100 days from the biopsy and most prostate cancers are slow growing so this is likely non-contributory [45].
In summary, transperineal wide-bore 3-T multiparametric MR imaging-guided prostate biopsy Gleason grading showed high concordance with the final pathology of prostate cancer; however, the approach may benefit from the addition of systematic biopsies to capture additional disease burden. This method may improve accuracy of determining prostate cancer grade, and can serve as a useful tool in high-risk patients with inconclusive findings on TRUS-guided biopsy.
References
Siegel R, Ma J, Zou Z, Jemal A (2014) Cancer statistics, 2014. CA Cancer J Clin 64(1):9–29
Hoffman KE, Niu J, Shen Y, et al. (2014) Physician variation in management of low-risk prostate cancer: a population-based cohort study. JAMA Intern Med 174(9):1450–1459
Moyer VA (2012) Screening for prostate cancer: U.S. Preventive Services Task Force recommendation statement. Ann Intern Med 157(2):120–134
Giannarini G, Briganti A, Crestani A, et al. (2015) Dismiss systematic transrectal ultrasound-guided and embrace targeted magnetic resonance imaging-informed prostate biopsy: is the paradigm ready to shift? Eur Urol 69(3):381–383
Griffin CR, Yu X, Loeb S, et al. (2007) Pathological features after radical prostatectomy in potential candidates for active monitoring. J Urol 178(3 Pt 1):860–863 (discussion 863)
Lellig E, Gratzke C, Kretschmer A, Stief C (2015) Final pathohistology after radical prostatectomy in patients eligible for active surveillance (AS). World J Urol 33(7):917–922
King CR, McNeal JE, Gill H, Presti JC (2004) Extended prostate biopsy scheme improves reliability of Gleason grading: implications for radiotherapy patients. Int J Radiat Oncol Biol Phys 59(2):386–391
Cookson MS, Fleshner NE, Soloway SM, Fair WR (1997) Correlation between Gleason score of needle biopsy and radical prostatectomy specimen: accuracy and clinical implications. J Urol 157(2):559–562
King CR (2000) Patterns of prostate cancer biopsy grading: trends and clinical implications. Int J Cancer 90(6):305–311
Shariat SF, Roehrborn CG (2008) Using biopsy to detect prostate cancer. Rev Urol 10(4):262–280
Robertson NL, Emberton M, Moore CM (2013) MRI-targeted prostate biopsy: a review of technique and results. Nat Rev Urol 10(10):589–597
Hegde JV, Mulkern RV, Panych LP, et al. (2013) Multiparametric MRI of prostate cancer: an update on state-of-the-art techniques and their performance in detecting and localizing prostate cancer. J Magn Reson Imaging 37(5):1035–1054
Futterer JJ, Heijmink SWTPJ, Scheenen TWJ, et al. (2006) Prostate cancer localization with dynamic contrast-enhanced MR imaging and proton MR spectroscopic imaging. Radiology 241(2):449–458
Yamauchi FI, Penzkofer T, Fedorov A, et al. (2015) Prostate cancer discrimination in the peripheral zone with a reduced field-of-view T2-mapping MRI sequence. Magn Reson Imaging 33(5):525–530
Nelson AW, Harvey RC, Parker RA, et al. (2013) Repeat prostate biopsy strategies after initial negative biopsy: meta-regression comparing cancer detection of transperineal, transrectal saturation and MRI guided biopsy. PLoS One 8(2):e57480
Penzkofer T, Tuncali K, Fedorov A, et al. (2015) Transperineal in-bore 3-T MR imaging-guided prostate biopsy: a prospective clinical observational study. Radiology 274(1):170–180
Valerio M, Donaldson I, Emberton M, et al. (2014) Detection of clinically significant prostate cancer using magnetic resonance imaging-ultrasound fusion targeted biopsy: a systematic review. Eur Urol 68(1):8–19
Hata N, Jinzaki M, Kacher D, et al. (2001) MR imaging-guided prostate biopsy with surgical navigation software: device validation and feasibility. Radiology 220(1):263–268
Chang DT, Challacombe B, Lawrentschuk N (2013) Transperineal biopsy of the prostate—is this the future? Nat Rev Urol 10(12):690–702
Bott SR, Henderson A, Halls JE, et al. (2006) Extensive transperineal template biopsies of prostate: modified technique and results. Urology 68(5):1037–1041
Grummet JP, Weerakoon M, Huang S, et al. (2014) Sepsis and ‘superbugs’: should we favour the transperineal over the transrectal approach for prostate biopsy? BJU Int 114(3):384–388
Barqawi AB, Rove KO, Gholizadeh S, et al. (2011) The role of 3-dimensional mapping biopsy in decision making for treatment of apparent early stage prostate cancer. J Urol 186(1):80–85
Taira AV, Merrick GS, Bennett A, et al. (2013) Transperineal template-guided mapping biopsy as a staging procedure to select patients best suited for active surveillance. Am J Clin Oncol 36(2):116–120
Hambrock T, Hoeks C, Hulsbergen-van de KC, et al. (2012) Prospective assessment of prostate cancer aggressiveness using 3-T diffusion-weighted magnetic resonance imaging-guided biopsies versus a systematic 10-core transrectal ultrasound prostate biopsy cohort. Eur Urol 61(1):177–184
Stangelberger A, Waldert M, Djavan B (2008) Prostate cancer in elderly men. Rev Urol 10(2):111–119
Kasivisvanathan V, Dufour R, Moore CM, et al. (2013) Transperineal magnetic resonance image targeted prostate biopsy versus transperineal template prostate biopsy in the detection of clinically significant prostate cancer. J Urol 189(3):860–866
Fedorov A, Fedorov A, Pursley J, et al. (2012) Image registration for targeted MRI-guided transperineal prostate biopsy. J Magn Reson Imaging 36(4):987–992
Oguro S, Tokuda J, Elhawary H, et al. (2009) MRI signal intensity based B-spline nonrigid registration for pre- and intraoperative imaging during prostate brachytherapy. J Magn Reson Imaging 30(5):1052–1058
Hambrock T, Hoeks C, Hulsbergen-van de KC, et al. (2011) Relationship between apparent diffusion coefficients at 3.0-T MR imaging and Gleason grade in peripheral zone prostate cancer. Radiology 259(2):453–461
Puech P, Potiron E, Lemaitre L, et al. (2009) Dynamic contrast-enhanced-magnetic resonance imaging evaluation of intraprostatic prostate cancer: correlation with radical prostatectomy specimens. Urology 74(5):1094–1099
Vargas HA, Akin O, Afaq A, et al. (2012) Magnetic resonance imaging for predicting prostate biopsy findings in patients considered for active surveillance of clinically low risk prostate cancer. J Urol 188(5):1732–1738
Moore CM, Robertson NL, Arsanious N, et al. (2013) Image-guided prostate biopsy using magnetic resonance imaging-derived targets: a systematic review. Eur Urol 63(1):125–140
Overduin CG, Futterer JJ, Barentsz JO (2013) MRI-guided biopsy for prostate cancer detection: a systematic review of current clinical results. Curr Urol Rep 14(3):209–213
Klein EA (2015) Prostate cancer: MR-TRUS fusion biopsy—defining a new standard. Nat Rev Clin Oncol 12(5):253–254
Siddiqui MM, Rais-Bahrami S, Turkbey B, et al. (2015) Comparison of MR/ultrasound fusion-guided biopsy with ultrasound-guided biopsy for the diagnosis of prostate cancer. Jama 313(4):390–397
Radtke JP, Schwab C, Wolf MB, et al. (2016) Multiparametric magnetic resonance imaging (MRI) and MRI-transrectal ultrasound fusion biopsy for index tumor detection: correlation with radical prostatectomy specimen. Eur Urol 70(5):846–853
Ficarra V, Martignoni G, Novella G, et al. (2006) Needle core length is a quality indicator of systematic transperineal prostate biopsy. Eur Urol 50(2):266–271
Lawrentschuk N, Haider MA, Daljeet N, et al. (2010) ‘Prostatic evasive anterior tumours’: the role of magnetic resonance imaging. BJU Int 105(9):1231–1236
Ouzzane A, Puech P, Lemaitre L, et al. (2011) Combined multiparametric MRI and targeted biopsies improve anterior prostate cancer detection, staging, and grading. Urology 78(6):1356–1362
Klotz L, Zhang L, Lam A, et al. (2010) Clinical results of long-term follow-up of a large, active surveillance cohort with localized prostate cancer. J Clin Oncol 28(1):126–131
Pepe P, Garufi A, Priolo G, Pennisi M (2016) Can MRI/TRUS fusion targeted biopsy replace saturation prostate biopsy in the re-evaluation of men in active surveillance? World J Urol 34(9):1249–1253
Russo F, Armando E, Giannini V, et al. (2016) Detection of prostate cancer index lesions with multiparametric magnetic resonance imaging (mp-MRI) using whole-mount histological sections as the reference standard. BJU Int 118(1):84–94
Fedorov A, Penzkofer T, Hirsch MS, et al. (2015) The role of pathology correlation approach in prostate cancer index lesion detection and quantitative analysis with multiparametric MRI. Acad Radiol 22(5):548–555
Panebianco V, Barchetti F, Sciarra A, et al. (2015) Multiparametric magnetic resonance imaging vs. standard care in men being evaluated for prostate cancer: a randomized study. Urol Oncol 33(1):17.e1–17.e7
Penney KL, Sesso LA, Mucci M, et al. (2013) Gleason grade progression is uncommon. Cancer Res 73(16):5163–5168
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Funding
This study was funded by NIH (Grant numbers R01 CA111288, P41 EB015898, and U01 CA151261).
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards. For this type of study formal consent is not required.
Informed consent
Statement of informed consent was not applicable since the manuscript does not contain any patient data.
Rights and permissions
About this article
Cite this article
Velez, E., Fedorov, A., Tuncali, K. et al. Pathologic correlation of transperineal in-bore 3-Tesla magnetic resonance imaging-guided prostate biopsy samples with radical prostatectomy specimen. Abdom Radiol 42, 2154–2159 (2017). https://doi.org/10.1007/s00261-017-1102-y
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00261-017-1102-y